ABSTRACT
Flurbiprofen, a potent nonsteroidal anti-inflammatory drug, is widely used for relief of pain in patients suffering from rheumatic diseases, migraine, sore throat and primary dysmenorrheal. However, this drug has many gastrointestinal side effects produced by its oral administration, such as gastric bleeding and peptic ulcer. These effects were responsible for non-compliance among patients, which ultimately results in treatment failure. The physicochemical properties of flurbiprofen, make it a suitable candidate for transdermal drug delivery, which can overcome the drawbacks of oral administration. In this sense, microemulsions have been proved to increase the cutaneous absorption of lipophilic drugs when compared to conventional drug delivery systems. The purpose of this study was to formulate and characterize gel based microemulsions, for topical delivery of flurbiprofen. Different gel bases, containing microemulsion and hydro-alcoholic solution of flurbiprofen, were developed and compared. In vitro study showed that gels containing microemulsion had a higher permeation rate than those containing hydro-alcoholic solutions. Additionally, formulation of Carbopol-I (microemulsion) showed higher percent of inhibition of inflammation than others bases. Further, skin irritation study demonstrated that Carbopol-I was none irritating. Flurbiprofen microemulsion incorporated on Carbopol-I showed physicochemical, in vitro and in vivo characteristics suitable for the development of alternative transdermal delivery formulation.
Key words:
anti-inflammatory study; flurbiprofen; gels; In vitro permeation; microemulsion
RESUMO
O flurbiprofeno, potente anti-inflamatório não esteroidal, é amplamente utilizado no alívio da dor de pacientes que possuem doenças reumáticas, enxaqueca, dores de garganta e dismenorreia primária. Entretanto, apresenta muitos efeitos gastrointestinais indesejáveis quando administrado por via oral como hemorragia gástrica e úlcera péptica. Esses efeitos foram responsáveis por baixa adesão entre os pacientes, o que resulta no comprometimento do tratamento. As propriedades físico-químicas do flurbiprofeno fazem dele um candidato promissor para a administração transdérmica, que pode eliminar os inconvenientes causados durante a administração oral. Neste sentido, provou-se que as microemulsões aumentam a absorção cutânea de fármacos lipofílicos quando comparadas aos sistemas de liberação convencionais. O objetivo desse estudo foi desenvolver e caracterizar géis bases contendo microemulsões de flurbiprofeno. Diferentes géis bases contendo microemulsão e solução hidroalcoólica de flurbiprofeno foram desenvolvidos e comparados. Os estudos in vitroindicaram que os géis contendo microemulsão apresentaram velocidade de permeação superior ao géis contendo as soluções hidroalcoólicas. Adicionalmente, a formulação de Carbopol -I (microemulsão) apresentou maior capacidade de inibição da inflamação em relação às outras bases. Além disso, o estudo demonstrou que formulação de Carbopol -I (microemulsão) não ocasionou irritações cutâneas. Desta forma, o gel base de Carbopol -I contendo microemulsão de flurbiprofeno apresentou características físico-químicas, desempenho in vitro e in vivo adequados para o desenvolvimento de formulação alternativas de liberação transdérmica.
Palavras-chave:
estudo de ação anti-inflamatória; flurbiprofeno; géis; permeação In vitro ; microemulsões
INTRODUCTION
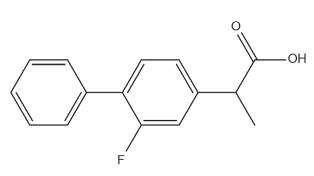
Flurbiprofen is used in inflammation, rheumatic diseases and mild to moderate pain of migraine, sore throat and also in primary dysmenorrheal ( British National Formulary 2006BNF - BRITISH NATIONAL FORMULARY. 2006. 52, BMJ Publishing Group., Schachtel et al. 2014Schachtel B, Aspley s, Shephard a, Shea t, Smith g, Sanner k, Savino l, Rezuke j and Schachtel e. 2014. Onset of action of a lozenge containing flurbiprofen 8.75mg: a randomized, double-blind, placebo-controlled trial with a new method for measuring onset of analgesic activity. Pain 155: 2422-2428.). Many gastrointestinal side effects caused due to the presence of free carboxylic acid group ( Figure 1), are produced by its oral administration, such as dyspepsia, cramping, gastric bleeding and peptic ulcer, causing non-compliance among patients, which ultimately results in treatment failure ( Mishra et al. 2008Mishra A, Ravichandran V, Jain PK, Dixit VK and Agrawal RK. 2008. Synthesis, characterization and pharmacological evaluation of amide prodrugs of flurbiprofen. J Braz Chem Soc 19: 89-100.).
The physicochemical properties of flurbiprofen, i.e., lipophilicity, low molecular weight and short elimination half-life make it a suitable candidate for transdermal drug delivery, which can overcome the drawbacks of oral administration ( Fang et al. 2003Fang J, Hwang T and Leu Y. 2003. Effect of enhancers and retarders on percutaneous absorption of flurbiprofen from hydrogels. Int J Pharm 250: 313-325.). However, it's very low aqueous solubility hinders the skin permeation. Thus, it is imperative to develop novel drug delivery systems aimed to improve its skin permeation and therapeutic compliance.
In this sense, microemulsions have been proven to increase the cutaneous absorption of lipophilic drugs when compared to conventional vehicles ( Nisha et al. 2011Nisha GS, Vaishali P, Geeta R, Prabhakar P, Harish NM and Marina K. 2011. Formulation and evaluation of self micro-emulsifying drug delivery system of carbamazepine. Int J Res Pharm Sci 2: 162-169.). Microemulsions are fluid, transparent, thermodynamically stable oil and water systems, stabilized by a surfactant usually in conjunction with a cosurfactant that could be a short-chain alcohol, amine, or other weakly amphiphilic molecule. An interesting characteristic of microemulsions is that the diameter of the droplets is in the range of 100-1000 A°, whereas the diameter of droplets in a kinetically stable macroemulsion is 5000 A°. The small droplet size allows the microemulsion to act as carriers for drugs that are poorly soluble in water ( Zhu et al. 2009Zhu W, Guo C and Yu A. 2009. Microemulsion based hydrogel formulation of penciclovir for topical delivery. Int J Pharm 378: 152-158.). The suggested method of preparation of microemulsions is as follows: the surfactant, oil and water are mixed, to form a milky emulsion, and titrated with a fourth component, the co-surfactant, until the mixture becomes clear. If more oil is added to a water/oil (w/o) system, the system becomes cloudy, but the addition of more co-surfactant again gives a clean transparent emulsion ( Betageri and Prabhu 2007Betageri G and Prabhu S. 2007. Encyclopedia of pharmaceutical technology. In: Semisolid Preparations. USA: Informa Healthcare, 4370 p., Fouad et al. 2013Fouad SA, Basalious EB, El-Nabarawi MA and Tayel SA. 2013. Microemulsion and poloxamer microemulsion-based gel for sustained transdermal delivery of diclofenac epolamine using in-skin drug depot: In vitro/in vivo evaluation. Int J Pharm 453: 569-578.).
In the present study, the polymer based gel containing microemulsion or hydro-alcoholic solution were formulated using Carbopol 934P and Xanthan gum as gelling agents and were characterized on the basis of pH, viscosity, conductivity and thermodynamic stability study. In vitropermeation through excised rabbit skin and in vivo anti-inflammatory activity were also investigated. Furthermore 934P based gel containing microemulsion was evaluated for skin irritation.
MATERIALS AND METHODS
The following materials were received and used: Flurbiprofen (Hamaz, Pakistan), Oleic acid (Merck, Germany), Tween 20 (Fisher Scientific, UK) Propylene glycol (Merck, Germany), Ethanol (Merck, Germany), Potassium dihydrogen orthophosphate (Merck, Germany), Sodium hydroxide pellets (Merck, Germany), Xanthan Gum (CCL Lahore), Carbopol 934P (Wilson, Islamabad), Triethanol amine (Merck, Germany), Methyl and Propyl paraben (Merck, Germany), Water Soluble Tetrazolium-1 (Dojindo Laboratories, Kumamoto, Japan), Zymosan A (Sigma Chemicals, St Louis, MO, USA).
PREPERATION OF MICROEMULSION AND HYDRO-ALCOHOLIC SOLUTION OF FLURBIPROFEN
Flurbiprofen loaded microemulsion obtained by a simplex lattice experiment design in our previous study was comprised of 5% (w/w) flurbiprofen, 5% (w/w) oleic acid, 46% (w/w) Tween 20:ethanol (2:1) and 44% (w/w) water ( Idress et al. 2011Idress MA, Rahman NU, Ahmad S, Ali MY, Ahmad I. 2011. Enhance transdermal delivery of flurbiprofen via microemulsions: effects of different types of surfactants and cosurfactants. DARU J Pharm Sci 6: 433-439.). The preparation method was as follows, Oil and Smix (surfactant + cosurfactant, 2:1) were mixed vigorously under constant stirring. A known amount of flurbiprofen was added to this oily phase and mixed until completely dissolved. Then an appropriate amount of distilled water was added slowly under constant stirring at room temperature. The microemulsions were stored at room temperature ( Chen et al. 2006Chen H, Chang X, Du D, Li J, Xu H and Yang X. 2006. Microemulsion based hydrogel formulation of ibuprofen for topical delivery. Int J Pharm 315: 52-58.).
For hydro-alcoholic solution, flurbiprofen was dissolved in ethanol and mixed well until completely dissolved. Then distilled water was added in alcoholic drug solution to form hydro-alcoholic solution under gentle mixing.
PREPERATION OF CARBOPOL AND XANTHAN GUM BASED GELS CONTAINING MICROEMULSION AND HYDRO-ALCOHOLIC SOLUTION
The first step consisted of Carbopol gel bases containing 0.70, 0.80, 0.90, 1.00, 1.10 and 1.20% w/w were prepared by gradually dissolving Carbopol 934P in specific amounts of distilled water, at room temperature, under constant stirring. Triethanol amine was added to adjust the pH between 6 to 7. From the different bases, that containing Carbopol 1% (w/w) was found to have optimum viscosity and was thus selected for further studies.
Xanthan gel bases containing 1.10, 1.20, 1.30, 1.40, 1.50 and 1.60% were prepared by first dissolving the gum in specific amounts of distilled water at room temperature under constant stirring. The methyl and propyl paraben were then added as preservatives. From the different bases, those containing 1% (w/w) and 1.5% (w/w) of Carbopol 934P and Xanthan gum respectively, were found to have optimum viscosity and selected for further studies.
In second step microemulsion or hydro-alcoholic solution of flurbiprofen were added separately to the 1% (w/w) Carbopol 934P and 1.5% (w/w) Xanthan gum to make Carbopol 934P and Xanthan gum based gels and mixing was done at room temperature.
CHARACTERIZATION OF GEL BASES AND MICROEMULSION
Brookfield RVDV III ultra, Programmable Rheometer (Brookfield Engineering Laboratories, Middleboro, MA) was used to determine the viscosities by using ULA (Ultra Low Adaptor) under a temperature of 25 °C. Conductometer WTW Cond 197i (Weilhein, Germany) was used to determine the conductivities ( σ) under a temperature of 25 °C ( Djordjevic et al. 2005Djordjevic L, Primorac M and Stupar M. 2005. In vitro release of diclofenac diethyl amine from caprylocaproyl macrogolglycerides based microemulsions. Int J Pharm 296: 73-79.). pH meter (WTW inolab, Germany) was used to measure the pH values under a temperature of 25 °C. The analyses were performed in triplicate.
THERMODYNAMIC STABILITY TESTS
The selected formulations of Carbopol and Xanthan were kept for 3 months at 40 °C and centrifuged at 10,000 rpm for 15 min to check physical stability. Furthermore, these formulations were also evaluated at 25 °C for pH changes, visual clarity, viscosity, conductivity and assessment of drug using UV assay.
IN VITROSKIN PERMEATION RELEASE RATE
Skin samples were obtained from the dorsal region of rabbit after removing hair with razor ( Chen et al. 2006Chen H, Mou D, Du D, Chang X, Zhu D and Liu J. 2007. Hydrogel thickened microemulsion for topical adminis¬tration of drug molecule at an extremely low concentration. Int J Pharm 341: 78-84.). Heat separation was used to remove epidermis and scalpel was used to remove subcutaneous fat ( Shah et al. 2006Shah S, Rabbani M and Amir F. 2006. Effect of urea on topical absorption of diclofenac diethylamine through hairless rabbit skin. J Res Sci 17: 165-171., Pellet et al. 1997Pellet M, Watkinson A, Brain K and Hadgraft J. 1997. Synergism between supersaturation and chemical enhancement in permeation through human skin. In: Brain KR, James VJ and Walter KA (Eds), Perspectives in Percutaneous Penetration. Cardiff: STS Publishing, p. 86.). Diffusion cells of Vertical Franz type (PermeGear, Bethlehem, PA) were used with diffusional surface area of 1.767 cm2. The receptor compartment, with capacity of 12 ml, contained the phosphate buffer solution (PBS) at 7.4 pH. The skin was placed between the donor and receptor compartments of the cell ( Ozguney et al. 2006Ozguney I, Karasulu H, Kantarci G, Sozer S, Guneri T and Ertan G. 2006. Transdermal delivery of diclofenac sodium through rat skin from various formulations. AAPS Pharm Sci Tech 7: 88.). Water bath and a peristaltic pump were used to maintain the temperature of the receptor solution at 37° C. Teflon-coated magnet bar was used for stirring. The concentration of the selected formulations was 1.0 g that was applied to donor compartment. After pre-determined time intervals (0 to 24 hours), 1ml of the sample was removed from the receptor compartment for UV determination and immediately replaced with an equal quantity of fresh PBS. There was no interference of the components. These dose conditions are infinite ( Roessler et al. 2001Roessler B, Wu H, Ramachandran C and Weinar N. 2001. Topical transport of hydrophilic compounds using water in oil nano-emulsions. Int J Pharm 220: 63-75.).
The cumulative amount of flurbiprofen was plotted as a function of time through excised rabbit skin. The permeation release rate of flurbiprofen was calculated from the slope and intercept of the straight line by plotting the amount of flurbiprofen permeated versus the time in steady state conditions. Permeation coefficient was calculated by dividing the flux by the flurbiprofen concentration of the donor compartment.
ANTI-INFLAMMATORY ACTIVITY ASSAY
The method for the isolation of human neutrophils was established ( Kantarci et al. 2007Kantarci G, Ozguney I, Karasulu Y, Arzik S and Guneri T. 2007. Comparison of different water/oil microemulsions containing diclofenac sodium: preparation, characterization, release rate and skin irritation studies. AAPS Pharm Sci Tech 8: 91.). The anti-inflammatory activity of polymer based gel formulations was assayed ( Siddiqui et al. 1995Siddiqui R, English D and Cui Y. 1995. Phorbal ester induced priming of superoxide degeneration by phosphatidic acid stimulated neutrophils and granule free neutrophil cytoplasts. J Leukoc Biol 58: 189-195.). In this in vitrostudy, Opsonised Zymosan A was used to activate the neutrophils. These activated neutrophils were used for the reduction of water soluble tetrazolium salt. The anti-inflammatory activity was determined by using Carbopol and Xanthan gum based gels containing microemulsion or hydro-alcoholic solution, water soluble tetrazolium salt in concentration of 250 µm and Modified Hank's Solution (MHS) of pH 7.4 in concentration of 200 µl containing 1.0-104 neutrophils/ml. The control consisted of water soluble tetrazolium salt, buffer and neutrophils. The Opsonised Zymosan A (15mg/ml) was added to initiate a reaction of test compounds. Absorbance was taken at 450 nm. The formula for percent of inhibition is given below. The software used was EZ-FIT Windows-based software.
% Inhibition = 100 - {(Optical density of formulation / Optical density of control) ×100}.
IN VIVOSKIN IRRITATION STUDY
The screened Carbopol 934P based gel containing microemulsion was applied to the inner arms of 10 human volunteers aged between 21-27 years, by placing gel on gauze dressing (1×1 cm2). Stretch adhesive tape was used for fixing the gel on the inner arm. The reading was taken after 8 h ( OECD 2004OECD - Organization for Economic Co-operation and Development. 2004. Series on testing and assessment. Guidance document for the conduct of skin absorption studies.).
STATISTICAL ANALYSIS
Two-way analysis of variance (ANOVA) was used to measure the skin permeation release rate. For the study of skin irritation, statistical paired sample t-test was used at the level of P= 0.05. These tests were performed by SPSS 12.0 software.
ETHICAL STANDARDS
Ethical approval for different bases containing flurbiprofen microemulsion studies was granted by the Islamia University of Bahawalpur, Pakistan and Institutional ethical committee. The informed consent was filled by the patients before the test.
RESULTS
CHARACTERIZATION OF GEL BASES AND MICROEMULSION
A number of gel bases were prepared by using different concentrations of Carbopol 934P and Xanthan gum. The viscosities of Carbopol 934P (0.70-1.20% w/w) and Xanthan gum (1.10-1.60% w/w) gel bases were in the range of 307-1522 cp and 151-460 cp respectively. The conductivities of Carbopol 934P (0.70-1.20% w/w) and Xanthan gum (1.10-1.60% w/w) gel bases were in the range of 322-1293 and 405-1360 microsiemens/cm respectively. The pH values of Carbopol 934P (0.70-1.20% w/w) and Xanthan gum (1.10-1.60% w/w) gel bases were in the range of 6.00-6.11 and 6.13-6.25 respectively. The Carbopol 934P and Xanthan gum gel bases of 0.7% and 1.10% (w/w) respectively had high fluidity while the Carbopol 934P and Xanthan gum gel bases of 1.20% and 1.60% (w/w) respectively had high viscosity.
THERMODYNAMIC STABILITY STUDY
As the Carbopol 934P and Xanthan gum gel bases of 1.00% and 1.50% respectively had suitable fluidity and viscosity therefore, these were selected for further studies. Stability study showed that the selected gel bases containing microemulsion (Carbopol-I and Xanthan-I) and gel bases containing hydro-alcoholic solution (Carbopol-II and Xanthan-II) were physically stable. The formulations were stable at 40° C and no phase separation and degradation of flurbiprofen were observed during the 3 months study. The viscosity, conductivity and pH of these gel formulations and the microemulsion after thermodynamic stability test are shown in Table I.
Physicochemical parameters of microemulsions, Carbopol 934P and Xanthan gum based gels containing microemulsions and hydro-alcoholic solution after 3 months at 40 °C. All measurements were done at 25 °C.
IN VITROPERMEATION SKIN RELEASE STUDY
The cumulative amount of flurbiprofen after 24 h of applying microemulsion, Carbopol-I, Xanthan-I, Carbopol-II and Xanthan-II was 572.01, 476.40, 436.62, 135.59 and 132.61 µg/cm2 respectively. The in vitroskin permeation showed a steady increase in concentration of flurbiprofen in the receptor chamber with time ( Figure 2). The permeation release rate was highest for microemulsion and lowest for both Carbopol-II and Xanthan-II.
Comparison of Permeation profile of Flurbiprofen through excised rabbit skin of Microemulsion and; Carbopol 934P and Xanthan gum based gel containing microemulsion and hydro-alcoholic solution. Mean ± S.D. (n = 3).
The transdermal flux was calculated from permeation release rate and the permeation coeffi cient was calculated from transdermal flux values. Then the results were analyzed by two-way ANOVA with level of significance of 0.05. The transdermal flux of microemulsion, Carbopol-I, Xanthan-I, Carbopol-II and Xanthan-II was 18.75, 15.72, 9.80, 4.76 and 2.70 µg/cm2/h respectively after 24 hour. In the same way, the permeability coefficient of microemulsion, Carbopol-I, Xanthan-I, Carbapol-II and Xanthan-II was 0.38, 0.31, 0.19, 0.09 and 0.05 µg/cm2/h respectively after 24 h ( Table II).
Permeation parameters of Carbopol 934P and Xanthan Gum based gels containing microemulsions and hydro-alcoholic solutions. Mean ± S.D. (n = 3).
The permeation release rate of microemulsion, Carbopol-I, Xanthan-I, Carbopol-II and Xanthan-II were measured by two-way ANOVA with level of significance of 0.05 and the measured value of P was less than 0.05.
ANTI-INFLAMMATORY STUDY
The results of anti-inflammatory study of Carbopol-I, Xanthan-I, Carbopol-II and Xanthan-II are given in Table III. The percent of inhibition of inflammation was highest for gel bases containing the microemulsion and lowest for both containing hydro-alcoholic solution.
Percent of inhibition of inflammation of polymer based gel containing microemulsion (Carbopol-I and Xanthan-I) and hydro-alcoholic solution of flurbiprofen (Carbopol-II and Xanthan-II).
IN VIVOSKIN IRRITATION STUDY
Erythema values before and after the application of Carbopol-I were noted and are given in Table IV. Statistical pair sample t-test was used to analyze the results, with level of significance of 0.05 and p=0.05. No significant increase in erythema values was observed after 8 h of application.
Erythema values before (control) and after the application of Carbopol-I on 10 human volunteers.
DISCUSSION
Carbopol 934P and Xanthan gum was used to prepare gel bases in different concentrations. In preparing gels, the color of the final formulation was turned white. This was caused by dehydration of some of the ingredients like surfactant and co-surfactant of microemulsion, which results in dissociation of polymers from hydrated state.
Incorporation of polymers into microemulsion and addition of microemulsion into the gel bases were used to formulate polymer based gel. In direct addition of polymer to the microemulsion, more time was consumed for the swelling of polymer. A non-homogeneous gel containing microemulsion was formed due to incomplete swelling of polymer and formation of small agglomerates. The gel base was first prepared in which the whole amount of polymer was completely swelled in aqueous phase. The known amount of microemulsion was mixed gradually into the gel base. Complete swelling with no agglomerates was observed due to complete swelling of polymer ( Tan and Berridge 2000Tan A and Berridge V. 2000. Superoxide produced by activated neutrophils efficiently reduces the tetrazolium salt, WST-1 to produce a soluble formazan: a simple colorimetric assay for measuring respiratory burst activation and for screening anti-inflammatory agents. J Immunol Methods 238: 50-68.).
The Carbopol 934P and Xanthan gum gel bases of 1.00 and 1.50% (w/w) respectively had suitable fluidity and viscosity for the topical delivery system. Thus, these gel bases were used for the preparation of gels containing microemulsion or hydro-alcoholic solution of flurbiprofen. The high conductivity indicated Oil/Water (O/W) microemulsion and the pH values near 7 could be resulted in less irritation to skin. The intracellular and transcellular micropathways play a vital role in the permeation of drug through stratum corneum. The intracellular pathway has higher percutaneous absorption than that of transcellular. The physicochemical properties of microemulsion and stratum corneum were studied to check the permeability enhancing ability of microemulsion and efficiency for percutaneous absorption. The polar and lipid pathways are changed when oily phase of microemulsion containing drug enter the stratum corneum of the skin. The oily phase of microemulsion can interact with skin in a number of ways. The oily phase of microemulsion containing drug can directly partition into the lipids of stratum corneum. This changes the bilayer structure of skin. The intracellular volume of stratum corneum can be increased by the oily phase of microemulsion, which in turn changes the interfacial structure. The permeation of hydrophobic drug can be increased by disruption of lipid layers caused by swelling of proteins (Chen et al. 2007).
Carbopol-I showed intermediate permeation release rate between microemulsion and Xanthan-I. Release rate of microemulsion is higher than Carbopol-I which is due to the presence of oleic acid and ethanol in microemulsion. This in turn reduce the barrier function of the stratum corneum and enhance the permeability coefficient of flurbiprofen through skin. The results showed that the addition of polymer to microemulsion decrease the permeability of flurbiprofen by increasing the viscosity and transform from microemulsion to the lamellar structure or a highly ordered microstructure ( Hire et al. 2007Hire N, Gudsoorkar V, Bhise K, Upasani C, Nandgude T and Dalvi H. 2007. Microparticulate drug delivery system for topical administration of itraconazole. Asian J Pharm 1: 83-88., Trotta 1999Trotta M. 1999. Influence of phase transformation on indomethacin release from microemulsion. J Control Release 60: 399-405., Peltota et al. 2003Peltota S, Kiesvaara J and Suhonen T. 2003. Microemulsion for topical delivery of estradiol. Int J Pharm 254: 99-107.). Additionally, the permeation release rate of microemulsion, Carbopol-I, Xanthan-I, Carbopol-II and Xanthan-II showed that the transdermal flux of these formulations are significantly different from each other (ANOVA, P<0.05). It is clear from all the formulations that the transdermal flux of microemulsion and polymer based gels containing microemulsion was greater than that of polymer based gels containing hydro-alcoholic solution.
In relation to anti-inflammatory study, a significant increase in the percent of inhibition of inflammation was observed for gel bases containing microemulsion (Carbopol-I and Xanthan-I) as compared to gel bases containing hydro-alcoholic solution (Carbopol-II and Xanthan-II). Moreover, droplet sizes of microemulsion used in both Carbopol-I and Xanthan-I was smaller and thus provides more opportunities for drug transferring into the skin improving its transdermal flux and pharmacologic activity ( Khurana et al. 2013Khurana S, Jain N K and Bedi PM. 2013. Nanoemulsion based gel for transdermal delivery of meloxicam: physico-chemical, mechanistic investigation. Life Sci 92: 383-392., Okur et al. 2014OKUR NÜ, YAVAŞOĞLU A and KARASULU HY. 2014. Preparation and evaluation of micro-emulsion formula¬tions of naproxen for dermal delivery. Chem Pharm Bull 62: 135-143.).
Although microemulsion had higher permeation release rate, but its use is limited due to having low pH which might produce skin irritation and comparatively less adherence to the skin. Therefore, adherence and pH of microemulsion could be increased by incorporation of Carbopol 934P. Therefore, the formed Carbopol 934P based gel containing microemulsion (Carbopol-I) had pH in the range of 6-7 which is non-irritating and more adhering to the skin to produce intermediate permeation release rate with sustained effect. Carbopol-I had higher transdermal flux and percent of inhibition of inflammation as compared to Xanthan-I. Consequently, Carbopol-I was finally selected for in vivoskin irritation study. No significant difference in the values of erythema before and after the application of Carbopol-I was observed. Moreover, no apparent change on the surface of the skin (t-test, p=0.05) was noticed that confirmed non-irritating effect of Carbopol-I to the skin of all volunteers.
CONCLUSIONS
The present study showed that Carbopol 934P based gel containing microemulsion of flurbiprofen had optimum thermodynamic stability, potential for in vitropermeation release rate and inhibition of inflammation. These properties can facilitate development of a cutting-edge strategy for trans dermal delivery of flurbiprofen using innovative formulations of polymer based gel containing microemulsion.
ACKNOWLEDGMENTS
We are thankful to Faculty of Pharmacy, The Islamia University Bahawalpur for providing financial assistance.
REFERENCES
- Betageri G and Prabhu S. 2007. Encyclopedia of pharmaceutical technology. In: Semisolid Preparations. USA: Informa Healthcare, 4370 p.
- BNF - BRITISH NATIONAL FORMULARY. 2006. 52, BMJ Publishing Group.
- Chen H, Chang X, Du D, Li J, Xu H and Yang X. 2006. Microemulsion based hydrogel formulation of ibuprofen for topical delivery. Int J Pharm 315: 52-58.
- Chen H, Mou D, Du D, Chang X, Zhu D and Liu J. 2007. Hydrogel thickened microemulsion for topical adminis¬tration of drug molecule at an extremely low concentration. Int J Pharm 341: 78-84.
- Djordjevic L, Primorac M and Stupar M. 2005. In vitro release of diclofenac diethyl amine from caprylocaproyl macrogolglycerides based microemulsions. Int J Pharm 296: 73-79.
- Fang J, Hwang T and Leu Y. 2003. Effect of enhancers and retarders on percutaneous absorption of flurbiprofen from hydrogels. Int J Pharm 250: 313-325.
- Fouad SA, Basalious EB, El-Nabarawi MA and Tayel SA. 2013. Microemulsion and poloxamer microemulsion-based gel for sustained transdermal delivery of diclofenac epolamine using in-skin drug depot: In vitro/in vivo evaluation. Int J Pharm 453: 569-578.
- Hire N, Gudsoorkar V, Bhise K, Upasani C, Nandgude T and Dalvi H. 2007. Microparticulate drug delivery system for topical administration of itraconazole. Asian J Pharm 1: 83-88.
- Idress MA, Rahman NU, Ahmad S, Ali MY, Ahmad I. 2011. Enhance transdermal delivery of flurbiprofen via microemulsions: effects of different types of surfactants and cosurfactants. DARU J Pharm Sci 6: 433-439.
- Kantarci G, Ozguney I, Karasulu Y, Arzik S and Guneri T. 2007. Comparison of different water/oil microemulsions containing diclofenac sodium: preparation, characterization, release rate and skin irritation studies. AAPS Pharm Sci Tech 8: 91.
- Khurana S, Jain N K and Bedi PM. 2013. Nanoemulsion based gel for transdermal delivery of meloxicam: physico-chemical, mechanistic investigation. Life Sci 92: 383-392.
- Mishra A, Ravichandran V, Jain PK, Dixit VK and Agrawal RK. 2008. Synthesis, characterization and pharmacological evaluation of amide prodrugs of flurbiprofen. J Braz Chem Soc 19: 89-100.
- Nisha GS, Vaishali P, Geeta R, Prabhakar P, Harish NM and Marina K. 2011. Formulation and evaluation of self micro-emulsifying drug delivery system of carbamazepine. Int J Res Pharm Sci 2: 162-169.
- OECD - Organization for Economic Co-operation and Development. 2004. Series on testing and assessment. Guidance document for the conduct of skin absorption studies.
- OKUR NÜ, YAVAŞOĞLU A and KARASULU HY. 2014. Preparation and evaluation of micro-emulsion formula¬tions of naproxen for dermal delivery. Chem Pharm Bull 62: 135-143.
- Ozguney I, Karasulu H, Kantarci G, Sozer S, Guneri T and Ertan G. 2006. Transdermal delivery of diclofenac sodium through rat skin from various formulations. AAPS Pharm Sci Tech 7: 88.
- Pellet M, Watkinson A, Brain K and Hadgraft J. 1997. Synergism between supersaturation and chemical enhancement in permeation through human skin. In: Brain KR, James VJ and Walter KA (Eds), Perspectives in Percutaneous Penetration. Cardiff: STS Publishing, p. 86.
- Peltota S, Kiesvaara J and Suhonen T. 2003. Microemulsion for topical delivery of estradiol. Int J Pharm 254: 99-107.
- Roessler B, Wu H, Ramachandran C and Weinar N. 2001. Topical transport of hydrophilic compounds using water in oil nano-emulsions. Int J Pharm 220: 63-75.
- Schachtel B, Aspley s, Shephard a, Shea t, Smith g, Sanner k, Savino l, Rezuke j and Schachtel e. 2014. Onset of action of a lozenge containing flurbiprofen 8.75mg: a randomized, double-blind, placebo-controlled trial with a new method for measuring onset of analgesic activity. Pain 155: 2422-2428.
- Shah S, Rabbani M and Amir F. 2006. Effect of urea on topical absorption of diclofenac diethylamine through hairless rabbit skin. J Res Sci 17: 165-171.
- Siddiqui R, English D and Cui Y. 1995. Phorbal ester induced priming of superoxide degeneration by phosphatidic acid stimulated neutrophils and granule free neutrophil cytoplasts. J Leukoc Biol 58: 189-195.
- Tan A and Berridge V. 2000. Superoxide produced by activated neutrophils efficiently reduces the tetrazolium salt, WST-1 to produce a soluble formazan: a simple colorimetric assay for measuring respiratory burst activation and for screening anti-inflammatory agents. J Immunol Methods 238: 50-68.
- Trotta M. 1999. Influence of phase transformation on indomethacin release from microemulsion. J Control Release 60: 399-405.
- Zhu W, Guo C and Yu A. 2009. Microemulsion based hydrogel formulation of penciclovir for topical delivery. Int J Pharm 378: 152-158.
Publication Dates
-
Publication in this collection
15 Sept 2015 -
Date of issue
Sept 2015
History
-
Received
06 Nov 2013 -
Accepted
18 May 2015