ABSTRACT
To describe potential intraspecific variation in phosphorus incorporation in two strains of Phaeodactylum tricornutum (Bohlin), Ub3 and Ub7, alkaline phosphatase (AP) activity was evaluated via enzyme-labeled fluorescence assay. Analysis using the probe ELF-97(r) provides individual evaluation, and therefore can determine the nutritional status of inorganic phosphorus in phytoplanktonic cells. Bioassays compared the control treatment to both phosphate-enriched and phosphate-depleted treatments by varying only the phosphate concentration in the media. The P. tricornutum strains exhibited differences in their development when incubated in the phosphate-enriched media. The development of the Ub7 strain differed by exhibiting "luxury uptake" and utilization of organic phosphorus, and the alkaline phosphatase analysis indicated limitations of this clone under such conditions. The Ub7 strain showed higher AP activity, when compared to Ub3, in the P-enriched condition. P. tricornutum presented increases in AP activity and low variation in Surface/Volume ratio, by increasing biovolume and its maximum linear dimension, as strategies for phosphate incorporation. Our results highlight intraspecific differences in alkaline phosphatase activity, and hence differences in the incorporation of organic phosphorus, as the tested species regulated enzymatic activity under different external phosphate concentrations.
Keywords
alkaline phosphatase; auxotrophy; marine diatom; organic phosphorus incorporation; phosphate limitation
Introduction
Biochemical characteristics of microalgae are dependent on taxonomy as well as on individual physiological characteristics and their response to light and nutrient availability. For example, the same concentration of a nutrient could mean repletion for one species and nutritional stress for another. Internal concentrations of proteins, nucleic acids, phospholipids, phosphorus reserves and nitrogen depend on cell nutritional history and physiological and taxonomic differences (Falkowski 2000Falkowski PG. 2000. Rationalizing elemental ratios in unicellular algae. Journal of Phycology 36: 3-6. ; Vance et al. 2003Vance CP, Uhde-Stone C, Allan DL. 2003. Phosphorus acquisition and use: critical adaptations by plants for securing a nonrenewable resource. New Phytologist 157: 423-447.). One way to assess differences in nutrient incorporation within and among species is by studying the activity of a specific enzyme.
Phosphorus (P) is a limiting nutrient in some freshwater and coastal marine ecosystems (Gregoracci et al. 2012Gregoracci GB, Nascimento JR, Cabral AS, et al. 2012. Structuring of bacterioplankton diversity in a large tropical bay. PLoS ONE 7(2): e31408. doi: 10.1371/journal.pone.0031408.
https://doi.org/10.1371/journal.pone.003...
; Maitra et al. 2015Maitra N, Manna SK, Samanta S, et al. 2015. Ecological significance and phosphorus release potential of phosphate solubilizing bacteria in freshwater ecosystems. Hydrobiologia 745: 69-83. ). According to Strojsová et al. (2003Strojsová A, Vrba J, Nedoma J, Komárková J, Znachor P. 2003. Seasonal study of extracellular phosphatase expression in the phytoplankton of a eutrophic reservoir. European Journal of Phycology 38: 295-306.) and Reynolds (2006Reynolds CS. 2006. The ecology of phytoplankton. 1st. edn. New York, Cambridge University Press. ), the enzymatic production of phosphatases, which hydrolyze dissolved organic compounds, is an important physiological trait that allows some species to endure conditions of limited phosphate. Alkaline phosphatase (AP) is an extracellular enzyme expressed by a wide variety of phytoplankton species in response to the condition of limited phosphorus. Highly stable in seawater, it is a membrane-associated enzyme that catalyzes the hydrolysis of phosphorus-containing organic compounds such as phosphate esters (Fitzgerald & Nelson 1966Fitzgerald CP, Nelson T. 1966. Extractive and enzymatic analyses for limiting or surplus phosphorus in algae. Journal of Phycology 2: 32-37. ; Graziano et al. 1996 Graziano LM, La Roche J, Geider RJ. 1996. Physiological response to phosphorus limitation in batch and steady state cultures of Dunaliella tertiolecta (Chlorophyta): a unique stress protein as an indicator of phosphate deficiency. Journal of Phycology 32: 825-838. ; Dyhrman 2005Dyhrman ST. 2005. Ectoenzymes in Prorocentrum minimum. Harmful Algae 4: 619-27.; Pandey & Parveen 2011Pandey VD, Parveen S. 2011. Alkaline phosphatase activity in cyanobacteria: Physiological and ecological significance. Indian Journal of Fundamental and Applied Life Sciences 4: 295-303.), to obtain orthophosphate, a form of inorganic phosphorus directly available to organisms (Pandey & Parveen 2011Pandey VD, Parveen S. 2011. Alkaline phosphatase activity in cyanobacteria: Physiological and ecological significance. Indian Journal of Fundamental and Applied Life Sciences 4: 295-303.). In other words, alkaline phosphatase facilitates the use of dissolved organic phosphorus (DOP) by phytoplankton when dissolved inorganic phosphorus (DIP) is limited in the environment (Lin et al. 2011Lin X, Zhang H, Huang B, Lin S. 2011. Alkaline phosphatase gene sequence and transcriptional regulation by phosphate limitation in Amphidinium carterae (Dinophyceae). Journal of Phycology 47: 1110-1120.). The activity of alkaline phosphatase is related to different phosphorus concentrations (Hernández et al. 2002Hernández I, Niell FX, Whitton BA. 2002. Phosphatase activity of benthic marine algae. An overview. Journal of Applied Phycology 14: 475-487.), with it being produced when cells are experiencing phosphate deprivation or it being suppressed in conditions of sufficient phosphate (Kuenzler & Perras 1965Kuenzler EJ, Perras JP. 1965. Phosphatases in marine algae. Biology Bulletin 128: 271-284. ; Fitzgerald & Nelson 1966; Grainger 1989Grainger SLJ. 1989. Filament structure and phosphatase activity in the Rivulariaceae. PhD Thesis, Durham University, United Kingdom. ; Vance et al. 2003Vance CP, Uhde-Stone C, Allan DL. 2003. Phosphorus acquisition and use: critical adaptations by plants for securing a nonrenewable resource. New Phytologist 157: 423-447.).
Many phytoplankton species have increased alkaline phosphatase (AP) activity in P-limited environments, and so its activity can be used as an indicator of phosphorus deficiency in these organisms (Rengefors et al. 2001Rengefors K, Pettersson K, Blenckner T, Anderson DM. 2001. Species-specific alkaline phosphatase activity in freshwater SPRING phytoplankton: application of a novel method. Journal of Plankton Research 23: 435-443.). Previous studies have observed an increase in AP activity under conditions of depletion of inorganic P in Chlorella pyrenoidosa, Scenedesmus dimorpha, Microcystis aeruginosa and Aphanizomenon flos-aquae (Beardall et al. 2001Beardall J, Young E, Roberts S. 2001. Approaches for determining phytoplankton nutrient limitation. Aquatic Sciences 63: 44-69. ); Alexandrium fundyense, Amphidinium sp. and Isochrysis galbana (González-Gil et al. 1998 González-Gil S, Keafer BA, Jovine RVM, Aguilera A, Lu S, Anderson DM. 1998. Detection and quantification of alkaline phosphatase in single cells of phosphorus-starved marine phytoplankton. Marine Ecology Progress Series 164: 21-35. ); and Phaeodactylum tricornutum (i.e. Lin et al. 2013Lin HY, Shih CI, Liu HC, et al. 2013. Identification and characterization of an extracellular alkaline phosphatase in the marine diatom Phaeodactylum tricornutum. Marine Biotechnology 15: 425-436. ; Yang et al. 2014Yang ZK, Zheng JW, Niu YF, Yang WD, Liu JS, Li HY. 2014. Systems-level analysis of the metabolic responses of the diatom Phaeodactylum tricornutum to phosphorus stress. Environmental Microbiology 16: 1793-1807.). On the other hand, Marco & Orus (1988Marco E, Orus MI. 1988. Variation in growth and metabolism with phosphorus-nutrition in 2 cyanobacteria. Journal of Plant Physiology 132: 339-344. ) observed that the cyanobacteria Trichodesmium sp. and Anabaena sp., under similar conditions of P limitation, showed no significant increase in the production of AP. These findings demonstrate that each species responds differently to the different environmental conditions of either sufficient or depleted nutrients (Rengefors et al. 2001Rengefors K, Pettersson K, Blenckner T, Anderson DM. 2001. Species-specific alkaline phosphatase activity in freshwater SPRING phytoplankton: application of a novel method. Journal of Plankton Research 23: 435-443.).
This study evaluates the incorporation of phosphorus in two clones of Phaeodactylum tricornutum in order to describe that allow these organisms to out-compete other individuals in conditions of phosphate deprivation, and to be outcompeted in conditions of replete phosphate.
Materials and methods
Sterilization and pre-experimental preparation
Glass vessels were cleaned by immersing them in a 10% chloridric acid bath for 24 hours, rinsed with abundant distilled water, dried in an area protected from dust and autoclaved (30 minutes, 121o C), according to the protocols described in Kawachi & Noel (2005Kawachi M, Noel MH. 2005. Sterilization and sterile technique. In: Andersen R. (ed.) Algal culturing techniques. Burlington, Elsevier. p. 65-82. ). Plastic vessels that could not be autoclaved were cleaned as described above and then sterilized under UV radiation (260 nm) for 10 minutes (Kawachi & Noel 2005Kawachi M, Noel MH. 2005. Sterilization and sterile technique. In: Andersen R. (ed.) Algal culturing techniques. Burlington, Elsevier. p. 65-82. ).
The seawater used in all experimental treatments and as a control was collected with a Van Dorn bottle from coastal waters (southeastern Brazil near Ilha Grande- RJ), filtered through a 150-μm mesh to remove microzooplankton and through 0.45 and 0.22 μm cartridges (Milipore(r)), to eliminate phytoplankton and bacterioplankton. The water was then sterilized by UV radiation and autoclaved prior to use in the experiments.
Organism and culture conditions
The unicellular diatom Phaeodactylum tricornutum (Bacillariophyceae; strains Ub3 and Ub7 available at the Instituto Oceanográfico, Universidade de São Paulo Marine Microorganisms Culture Collection) used in this study was isolated from coastal waters in southeastern Brazil (Ubatuba, state of São Paulo). These non-axenic strains have been maintained in the Marine Microalgae Culture Collection of the Faculdade de Oceanografia, Universidade do Estado do Rio de Janeiro in Guillard F/2 medium (Guillard & Ryther 1962Guillard RRL, Ryther JH. 1962. Studies of marine planktonic diatoms: I Cyclotella nana Husted, and Detonula confervacea (Cleve) Gran. Canadian Journal of Microbiology 8: 229-239. ) for many generations. Prior to experimentation, cells were batch-cultured in 500 mL Erlenmeyer flasks containing 250 mL of enriched seawater (34 salinity) with F/2 medium until the end of the log growth phase (5-8 days). The cells were then washed by centrifugation (three times, 2000 x g for 10 minutes) in control seawater and remained for 12 hours in 500 ml of this water for starvation. During the experiments, cultures were exposed to 80 μE.m-2.s-1 PAR provided by fluorescent lamps (Sylvania cool white tubes, 40 W), under a 12:12 h light:dark cycle and a temperature of 20º C (± 0.5° C). Cultures were manually shaken two times a day. Cell growth was followed by direct microscopic cell counts of culture subsamples, using a Sedgewick Rafter Counting Cell, after treating with acidic Lugol's solution, and by optical density. Cultures were set at an initial cell density of about 1.0 x 103 cells.mL-1 and inoculated with three treatments by the addition of silica: 1) Control - control water enriched with Guillard F/2 medium (36 μM); 2) F (Phosphate-enriched) - treatment with F/2 enriched with phosphate at medium proportion of F (72 μM); and 3) F/8 (Phosphate-depleted) - treatment with F/2 under phosphate deprivation at medium proportion of F/8 (4.5 μM) (Guillard & Ryther 1962Guillard RRL, Ryther JH. 1962. Studies of marine planktonic diatoms: I Cyclotella nana Husted, and Detonula confervacea (Cleve) Gran. Canadian Journal of Microbiology 8: 229-239. ). Bioassays were performed in triplicate within 15 days, and cell number was used to calculate the growth rate of all treatments.
Alkaline phosphatase incorporation assays
Alkaline phosphatase activity of the tested microalgae was determined using an ELF-97 endogenous phosphatase detection kit (Molecular Probes, E-6601(r)) following the method of Gonzalez-Gil et al. (1998) González-Gil S, Keafer BA, Jovine RVM, Aguilera A, Lu S, Anderson DM. 1998. Detection and quantification of alkaline phosphatase in single cells of phosphorus-starved marine phytoplankton. Marine Ecology Progress Series 164: 21-35. , as modified by Dyhrman & Palenik (2001)Dyhrman ST, Palenik B. 2001. A single cell immunoassay for phosphate stress in the dinoflagellate Prorocentrum minimum. Journal of Phycology 37: 400-410. and Skelton et al. (2006) Skelton HM, Parrow MW, Burkholder JM. 2006. Phosphatase activity in the heterotrophic dinoflagellate Pfiesteria shumwayae. Journal of Plankton Research 5: 395-406. . Aliquots of 5 mL of each culture were removed during late exponential (12 day culture) and stationary (15 day culture) growth phases, fixed with 1 mL of 70% ethanol for 30 minutes and washed by centrifugation at 2000 x g for 10 minutes. The supernatant was then aspirated with the aid of a vacum pump.
Prior to use, the ELF-97(r) substrate was diluted in ELF detection buffer at a ratio of 1:20 and filtered through a 0.2 μm cartridge (Milipore(r)); 100 μL of this solution was added to each sample, which were then incubated at 20 °C for 30 minutes. Following incubation, the cells were washed and centrifuged (2000 x g for 10 minutes) in 1 mL solution of 10 mM phosphate buffered saline (PBS) for five times, resuspended in 1 mL PBS and filtered (3 µm black membranes- Poretics(r)). Slides of the resulting product were prepared for observation under an epifluorescence microscope.
The microalgae were analyzed to determine the enzymatic activity of alkaline phosphatase (fluorescence of ELF-97(r)) using a Nikon(r) epifluorescence microscope (model Eclipse E200), equipped with a mercury lamp of 100W and an epifluorescence filter device with excitation at 360 ± 40 nm and emission at 535 ± 50 nm (# 31060v2; Chroma Technology Corp., Rockingham, VT).
Qualitative analysis involved counts of total cells and cells with ELFA precipitate (termed labeled cells). Images were obtained with the aid of a digital camera (Canon(r)) attached to the epifluorescence microscope.
Cell volume
Cell volume and surface area were estimated at the end of the experiment (stationary phase) by measuring 40 cells randomly chosen from each clone and each treatment, according to the formulation proposed by Sun & Liu (2003Sun J, Liu D. 2003. Geometric models for calculating cell biovolume and surface area for phytoplankton. Journal of Plankton Research 25: 1331-1346. ), using an inverted optical microscope.
Phosphate analysis
The phosphate concentration of each treatment was analyzed during the beginning of the experiment (time zero), and during late exponential (12 day culture) and stationary (15 day culture) growth phases, using FIA (Flow Injection Analysis - FOSS Tecator FiaStar 5000 analyzer-FOSS analytical, Hillerod, Denmark). Analyses were performed according to the specifications and recommendations of Note AP 5201 (ISO 13395: 1996-revision 2, detection limit 0.05 μmol.L-1).
Functional Traits
Half-saturation constant (Ks - μM)
The clones of P. tricornutum were grown in continuous cultures (1L) in F/2 medium (Guillard & Ryther 1962Guillard RRL, Ryther JH. 1962. Studies of marine planktonic diatoms: I Cyclotella nana Husted, and Detonula confervacea (Cleve) Gran. Canadian Journal of Microbiology 8: 229-239. ). The experiment was repeated three times, and the data were considered as triplicates. Cells (106 cells.L-1) were maintained in suspension using a magnetic stirrer at 30 rpm in the culture conditions described in item 2.2. The population reached the steady state between 10 and 12 days, when cell concentration varied less than 5 %. Until reaching the steady state, cell density data was used to calculate growth rates.
Upon reaching steady state, 10 µM of phosphate (final concentration) was added to the continuous culture. After this pulse, 5 mL subsamples were taken every 15 minutes for 10 hours (time experimentally determined by Riegman et al. 2000Riegman R, Stolte W, Noordeloos AAM, Slezak D. 2000. Nutrient uptake and alkaline phosphatase (EC 3:1:3:1) activity of Emiliania huxleyi (Prymnesiophyceae) during growth under N and P limitation in continuous cultures. Journal of Phycology 36: 87-96. ), and filtered through a 0.2 µm membrane (Acrodisc, Gelman Sciences(r)). This experiment was designed to determine the rate of cellular uptake (Vt) at a given time (t) by the maximum rate of incorporation established immediately after the pulse of 10 µM phosphate:
Vt = Vmax 1− ∆Q Q max t
Where Vt (which is equal to 𝑥−1 × 𝑑𝑆 𝑑𝑡 ; where 𝑥 is the cell concentration and S is the external nutrient concentration) is the cellular uptake rate at time t. Vmax is the maximum incorporation rate measured at t = 0; ΔQ is the amount of phosphate incorporated at time t, and Qmax t is the maximum amount of phosphate that cells can incorporate after the pulse at a given time t. The half saturation constant (ks) was estimated by ks = 1 2 × 𝑉 𝑚𝑎𝑥 (Riegman & Mur 1986Riegman R, Mur LR. 1986. Phytoplankton growth and phosphate uptake (for P limitation) by natural phytoplankton populations from the Loosdrecht lakes (The Netherlands). Limnology and Oceanography 31: 983-988.).
Cell quota (Q - μM)
The phosphate ate was obtained by subtracting the final concentration of phosphorus from its initial concentration in the medium during the stationary phase, since this latter phase is with nutrient limitation and exhibited maximum cell growth.
Intracellular phosphorus ([P] int. - pg P.Cell-¹):
Intracellular phosphorus was calculated by normalizing the cell quota per total cellular density in the steady state.
Statistical analysis
The results were analyzed by a Nonparametric Kruskal-Wallis ANOVA and Median Test, followed by Multiple comparisons of mean ranks for all groups, with a significance level of α = 0.05, using Statsoft Statistica 7.0 software.
Results and discussion
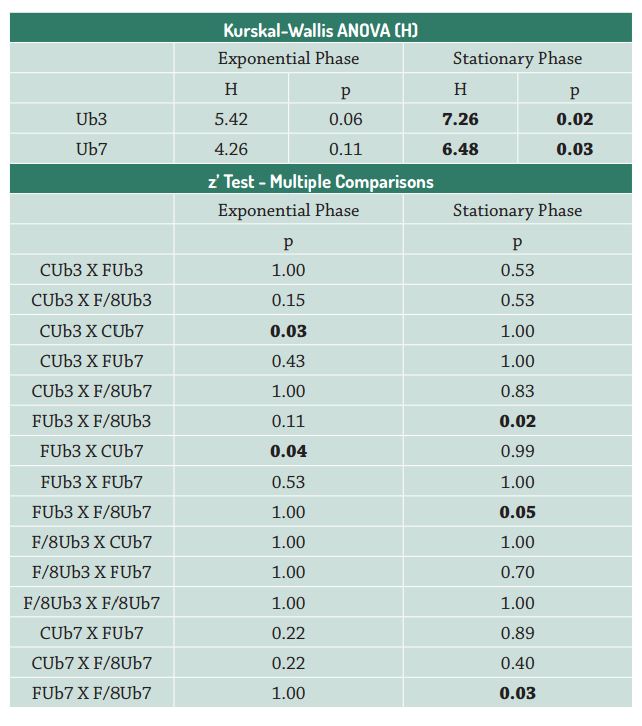
The growth of the Phaeodactylum tricornutum strains Ub3 and Ub7 under laboratory conditions showed a lag phase of 4 days. After this time, cells grew actively up to the stationary phase with the Ub3 clone having higher growth rates in all treatments than the Ub7 strain (Tab. 1), with the exception of the F/8 treatment in which both grew at similar rates and achieved similar densities (Fig. 1A-B).
The highest densities of Ub3 were observed at the end of the exponential growth phase (10-13 days) in the control (4.1 x 106 ± 2.1 x 105 cells.mL-1) and the F (4.0 x 106 ± 1.4 x 105 cells.mL-1) treatment (Fig. 1A). During the same period, the densities of Ub7 in the F treatment and the control were similar (3.0 x 106 ± 7.0 x 104 cells.mL-1) (Fig. 1B). Although phytoplankton adjust their growth rates to changes in environmental conditions, variation in maximum cell densities may be explained by differences in experimental conditions, such as the phosphate concentration of the culture media (Harris 1978Harris GP. 1978. Photosynthesis, productivity and growth: The physiological ecology of phytoplankton. Archiv für Hydrobiologie-BeiheftErgebnisse der Limnologie 10: 1-171. ).
Growth of Phaeodactylum tricornutum strains Ub3 (A) and Ub7 (B) in Control (C), F and F/8 treatments.
In the literature, P. tricornutum is frequently reported as an opportunistic species with fast growth (Falciatore et al. 1999Falciatore A, Casotti R, Leblanc C, Abrescia C, Bowler C. 1999. Transformation of Nonselectable Reporter Genes in Marine Diatoms. Marine Biotechnology 1: 239-251.; Martino et al. 2007Martino A, Meichenin A, Shi J, Pan K, Bowler C. 2007. Genetic and phenotypic characterization of Phaeodactylum tricornutum (bacillariophyceae) accessions. Journal of Phycology 43: 992 -1009.; Desbois et al. 2010Desbois AP, Walton M, Smith VJ. 2010. Differential antibacterial activities of fusiform and oval morphotypes of Phaeodactylum tricornutum (Bacillariophyceae). Journal of the Marine Biological Association of the United Kingdom 90: 769-774.), as was observed in the present study (see growth curves in Fig. 1A-B). This species reaches the stationary phase from the forth to the seventh day of growth, with maximum cell density varying from 104 to 107 cells.mL-1. As with growth rate, maximum cell density can vary considerably among strains of the same species under the same culture conditions (i.e. Aidar et al. 1991Aidar E, Ehrlich R, Asano CS, Sigaud TCS. 1991. Variação da composição química do melo de cultura e da bioquímica celular de Phaeodactylum tricornutum (Bohlin), em cultivos estanques. Boletim do Instituto Oceanográfico 39: 131-139. ; Voltolina et al. 1998Voltolina D, Nieves M, Navarro G, Oliva T, Peraza D. 1998. The importance of acclimation for the evaluation of alternative media for microalga growth. Aquacultural Engineering 19: 7-15.; Foster et al. 2008Foster S, Thomson D, Maher W. 2008. Uptake and metabolism of arsenate by anexic cultures of the microalgae Dunaliella tertiolecta and Phaeodactylum tricornutum. Marine Chemistry 108: 172-183.; Ohse et al. 2008Ohse S, Derner RB, Ozório RA, et al. 2008. Crescimento de microalgas em sistema autotrófico estacionário. Biotemas 21: 7-18. ; Lin et al. 2013Lin HY, Shih CI, Liu HC, et al. 2013. Identification and characterization of an extracellular alkaline phosphatase in the marine diatom Phaeodactylum tricornutum. Marine Biotechnology 15: 425-436. ).
Both strains of P. tricornutum had lower growth rates when subjected to P-depleted conditions. Specific growth rates observed in the tested strains were similar to those described by Gaeta (1985Gaeta SA. 1985. Comparação das respostas de crescimento e fotossíntese de três clones de Phaeodactylum tricornutum Bohlin. PhD Thesis, Universidade de São Paulo, Brazil. ), who reported growth rates of 1.13 ± 0.06 d-1 and 1.39 ± 0.08 d-1 in phosphate-enriched media (105 μM and 210 μM), and by Lin et al. (2013Lin HY, Shih CI, Liu HC, et al. 2013. Identification and characterization of an extracellular alkaline phosphatase in the marine diatom Phaeodactylum tricornutum. Marine Biotechnology 15: 425-436. ), who observed rates of 0.8 d-1in non-limited P-conditions.
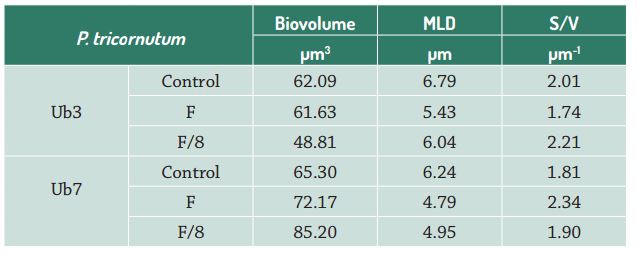
Concerning biovolume, the Ub3 strain presented a lower biovolume and a surface/volume ratio (S/V) similar to that of the Ub7 strain, as was expected for an organism developing in a P-depleted medium (Tab. 2). However, the highest changes were observed in the Ub7 strain, with maximum biovolume in the P-depleted treatment, despite having a S/V ratio similar to the control (Tab. 2). This variation among treatments is probably related to the growth phase, since measurements were performed in the stationary phase with the cells limited by nutrients (Timmermans & Wagt 2010Timmermans KR, Wagt B. 2010. Variability in cell size, nutrient depletion, and growth rates of the Southern Ocean diatom Fragilariopsis kerguelensis (Bacillariophyceae) after prolonged iron limitation. Journal of Phycology 46: 497-506.). The two strains of P. tricornutum adapted to low phosphorus concentrations by increasing, as observed in Ub7 strain, or decreasing, as employed by Ub3 strain, biovolume, and slightly altering S/V ratios. Thus, the Ub7 strain could better exploit the absorption of this nutrient in the depleted treatment since increased biovolume and maintenance of S/V ratio are related to absorption and intracellular phosphorus storage (luxury uptake).
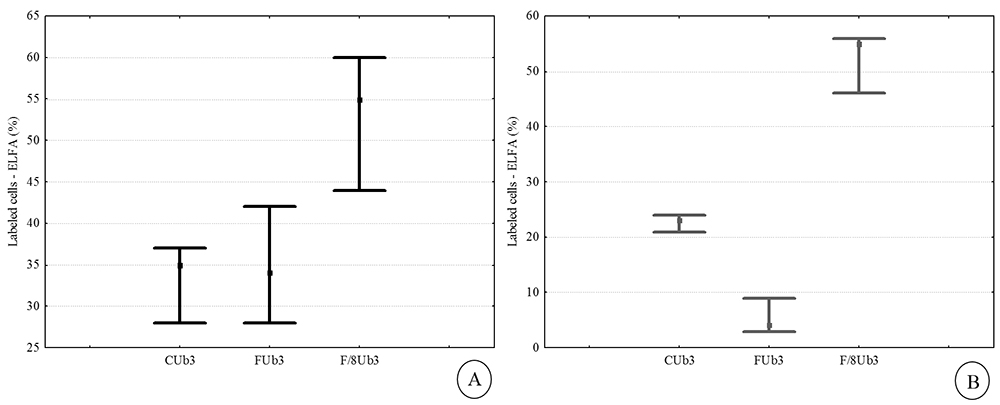
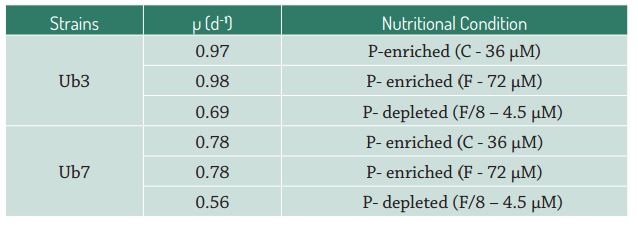
The activity of alkaline phosphatase in the two strains of P. tricornutum exhibited different responses for incorporating phosphorus into their cells under different conditions of phosphate availability. The Ub3 strain exhibited higher enzymatic activity in the P-limited treatment (Fig. 2A-B), although the difference was statistically significant only during the stationary growth phase (p < 0.05; Tab. 3), indicating that AP activity is higher in cells under conditions of low phosphate concentrations. These results agree with previous studies (González-Gil et al. 1998 González-Gil S, Keafer BA, Jovine RVM, Aguilera A, Lu S, Anderson DM. 1998. Detection and quantification of alkaline phosphatase in single cells of phosphorus-starved marine phytoplankton. Marine Ecology Progress Series 164: 21-35. ; Beardall et al. 2001Beardall J, Young E, Roberts S. 2001. Approaches for determining phytoplankton nutrient limitation. Aquatic Sciences 63: 44-69. ; Dyhrman & Palenik 2001Dyhrman ST, Palenik B. 2001. A single cell immunoassay for phosphate stress in the dinoflagellate Prorocentrum minimum. Journal of Phycology 37: 400-410.; Moser et al. 2010Moser GAO, Tocci BRC, Richard EC, Barrera-Alba JJ. 2010. Avaliação da incorporação de fósforo em microalgas marinhas através da técnica de marcadores enzimáticos fluorescentes. Paraty, Resumos do XIII Congresso Brasileiro de Ficologia, p. 51. ; Yang et al. 2014Yang ZK, Zheng JW, Niu YF, Yang WD, Liu JS, Li HY. 2014. Systems-level analysis of the metabolic responses of the diatom Phaeodactylum tricornutum to phosphorus stress. Environmental Microbiology 16: 1793-1807.), since this microalgae is able to use a strategy of organic phosphorus incorporation when the inorganic form of this nutrient is limited (Rengefors et al. 2001Rengefors K, Pettersson K, Blenckner T, Anderson DM. 2001. Species-specific alkaline phosphatase activity in freshwater SPRING phytoplankton: application of a novel method. Journal of Plankton Research 23: 435-443.; Nedoma et al. 2003Strojsová A, Vrba J, Nedoma J, Komárková J, Znachor P. 2003. Seasonal study of extracellular phosphatase expression in the phytoplankton of a eutrophic reservoir. European Journal of Phycology 38: 295-306.). On the other hand, the control and F treatment (P-replete conditions) for Ub7 exhibited greater proportions of labeled cells in the exponential growth phase compared to the P-limited treatment (Fig. 3A), results also found for diatoms of a freshwater reservoir and hypereutrophic lakes (Strojsová et al. 2003Strojsová A, Vrba J, Nedoma J, Komárková J, Znachor P. 2003. Seasonal study of extracellular phosphatase expression in the phytoplankton of a eutrophic reservoir. European Journal of Phycology 38: 295-306.; Cao et al. 2005Cao XY, Strojsová A, Znachor P, et al. 2005. Detection of extracellular phosphatases in natural spring phytoplankton of a shallow eutrophic lake (Donghu, China). European Journal of Phycology 40: 251-258.). However, despite the increase in labeled cells, these treatments did not differ significantly from each other (p > 0.05; Tab. 3), indicating phosphate limitation for the Ub7 strain in all tested conditions. At stationary growth phase, the enzymatic activity was similar to that observed for the Ub3 strain, with a greater number of labeled cells being found in the P-depleted treatment (p < 0.05; Tab. 3). It is noteworthy that the phosphate cell quota in the Ub7 strain, in both treatments, was lower than those of the Ub3 strain (Tab. 4), which suggests increased uptake of phosphate by this algae.
Percentage of labeled cells (ELFA) of Phaeodactylum tricornutum strain Ub3 in Control (C), F and F/8 treatments, at late exponential (A) and stationary (B) growth phases; (■) Median; ( ) 25% - 75%.
Percentage of labeled cells (ELFA) of Phaeodactylum tricornutum strain Ub7 in Control (C), F and F/8 treatments, at late exponential (A) and stationary (B) growth phases; (■) Median; ( ) 25% - 75%.
Intraspecific variation in AP activity in P. tricornutum has been previously reported, hence this microalgae is used worldwide in bioassays of algal growth potential (Yang et al. 2014Yang ZK, Zheng JW, Niu YF, Yang WD, Liu JS, Li HY. 2014. Systems-level analysis of the metabolic responses of the diatom Phaeodactylum tricornutum to phosphorus stress. Environmental Microbiology 16: 1793-1807. and references therein). Lin et al. (2013Lin HY, Shih CI, Liu HC, et al. 2013. Identification and characterization of an extracellular alkaline phosphatase in the marine diatom Phaeodactylum tricornutum. Marine Biotechnology 15: 425-436. ) observed that P. tricornutum growing in a starved medium had intense AP activity from the fourth to the seventh day of incubation, and that AP mRNA expression was abundant. Additionally, Yang et al. (2014)Yang ZK, Zheng JW, Niu YF, Yang WD, Liu JS, Li HY. 2014. Systems-level analysis of the metabolic responses of the diatom Phaeodactylum tricornutum to phosphorus stress. Environmental Microbiology 16: 1793-1807. found two genes responsible for encoding alkaline phosphatases in P. tricornutum, which exhibit sharp rises in their transcription when limited by phosphorus. In such conditions, the increase in AP activity may be related to the incorporation of organic phosphorus. In contrast, Ruiz et al. (1997Ruiz RG, Hernández I, Lucena J, Niell FX. 1997. Preliminary studies on the significance of alkaline phosphatase activity in the diatom Phaeodactylum tricornutum Bohlin. Scientia Marina 61: 517-525. ) found that P. tricornutum growing in phosphate concentrations ranging from 2.5 to 50 μM, exhibited no significant differences in alkaline phosphatase activity. However, when the microalgae were incubated at concentrations higher than 50 μM, a large decrease in enzymatic activity was observed after four days until it ceased its production.
The activity of AP seems to be controlled by the availability of inorganic phosphorus for some, but not all, phytoplankton species, suggesting that enzyme activity is not a universal measure of phosphorus requirement (Rengefors et al. 2001Rengefors K, Pettersson K, Blenckner T, Anderson DM. 2001. Species-specific alkaline phosphatase activity in freshwater SPRING phytoplankton: application of a novel method. Journal of Plankton Research 23: 435-443.). In the experiments conducted by Marco & Orus (1988Marco E, Orus MI. 1988. Variation in growth and metabolism with phosphorus-nutrition in 2 cyanobacteria. Journal of Plant Physiology 132: 339-344. ), the cyanobacteria Trichodesmium sp. and Anabaena sp. exhibited no significant increase in AP in limited concentrations of inorganic phosphorus, while Keenan & Auer (1974Keenan JD, Auer MT. 1974. The influence of phosphorus luxury uptake on algae bioassays. Journal of the Water Pollution Control Federation 46: 532-542. ), detected no AP activity in Microcystis aeruginosa and Selenastrum capricornutum. Experiments conducted using P-starved Microcystis aeruginosa cells indicated that colonial morphotypes grow in low P levels, while unicellular morphotypes consumed more P and had higher alkaline phosphatase activity in P-depleted conditions (Shen & Song 2007Shen H, Song L. 2007. Comparative studies on physiological responses to phosphorus in two phenotypes of bloom-forming Microcystis. Hydrobiologia 592: 475-486. ).
An inverse relationship between the intracellular concentration of phosphorus, cell quota (Q) and the phosphate content of the medium is well established (Kuenzler & Ketchum 1962Kuenzler EJ, Ketchum BH. 1962. Rate of phosphorus uptake by Phaeodactylum tricornutum. Biology Bulletin 123: 134-145. ; Fitzgerald & Nelson 1966Fitzgerald CP, Nelson T. 1966. Extractive and enzymatic analyses for limiting or surplus phosphorus in algae. Journal of Phycology 2: 32-37. ; Hernández et al. 1993Hernández I, Fernández JA, Niell FX. 1993. Influence of phosphorus status on the seasonal variation of alkaline phosphatase activity in Porphyra umbilicalis (L.) Kützing. Journal of Experimental Marine Biology and Ecology 173: 181-196.; Ruiz et al. 1997Ruiz RG, Hernández I, Lucena J, Niell FX. 1997. Preliminary studies on the significance of alkaline phosphatase activity in the diatom Phaeodactylum tricornutum Bohlin. Scientia Marina 61: 517-525. ). Kuenzler & Ketchum (1962)Kuenzler EJ, Ketchum BH. 1962. Rate of phosphorus uptake by Phaeodactylum tricornutum. Biology Bulletin 123: 134-145. , found that cells of P. tricornutum growing in high concentrations of phosphate (80 μM) exhibited a high cell quota after a long period of nutrient accumulation, while cells incubated under limiting conditions were capable of removing almost all of the nutrients from the media before significant cell multiplication occurred. As culture growth continues, the intracellular concentration of phosphorus declined in the limited treatment (8 μM) indicating that P. tricornutum had become phosphorus deficient and stopped multiplying.
The intracellular concentration of phosphorus of the Ub7 strain observed in this study ranged from 0.6 to 4.7 pg P. Cell-1 in P-depleted (F/8) and P-replete (F) treatments, respectively (Tab. 4). Nevertheless, the control for both strains showed intracellular P concentrations (~2.0 pg P. Cell-1) similar to those reported by Kuenzler & Ketchum (1962Kuenzler EJ, Ketchum BH. 1962. Rate of phosphorus uptake by Phaeodactylum tricornutum. Biology Bulletin 123: 134-145. ) when phosphate depletion limited cell growth (8µM). It should also be noted that in the present work, the highest cell quota and intracellular concentration of phosphorus were observed in the Ub7 strain in the P-replete condition (Tab. 4), suggesting that this strain possesses a large capacity to store phosphorus, which has also been described for P. tricornutum by Terry et al. (1983Terry KL, Hirata J, Laws EA. 1983. Light-limited growth of two strains of the marine diatom Phaeodactylum tricornutum Bohlin: chemical composition, carbon partitioning and the diel periodicity of Physiological processes. Journal of Experimental Marine Biology and Ecology 68: 209-227. ) and diatoms and other organisms of a natural community (Domingues et al. 2011Domingues RB, Anselmo TP, Barbosa AB, Sommer U, Galvão HM. 2011. Nutrient limitation of phytoplankton growth in the freshwater tidal zone of a turbid, Mediterranean estuary. Estuarine Coastal and Shelf Science 91: 282-297.; 2015Domingues RB, Guerra CC, Barbosa B, Galvão HM. 2015. Are nutrients and light limiting summer phytoplankton in a temperate coastal lagoon? Aquatic Ecology 49: 127-146.).
The two strains of P. tricornutum exhibited different phosphate half-saturation constants (Ks). The Ks value estimated for Ub7 was twice that of Ub3, indicating lower affinity for phosphate, which can be further enhanced throughout growth. These results indicate that under P-enriched conditions (72 μM) the Ub7 strain of P. tricornutum has a greater ability to store intracellular phosphorous. However, this phosphate pool is not incorporated into the maintenance of cellular metabolism in the same proportion that it is in Ub3, since lower cell densities were observed in all treatments (Fig. 1B).
According to Monod (1949Monod J. 1949. The growth of bacterial cultures. Annual Review Microbiology 3: 371-394.), higher Ks values are correlated with a lower affinity for a specific nutrient by microorganisms, as well as lower specific growth rate. Thus, when nutrient concentration is low in a culture medium, the growth rate becomes limited and dependent on this nutrient. Different competitive strategies for phosphate are possible (Sommer 1984Sommer U. 1984. The paradox of the plankton: Fluctuations of phosphorus availability maintain diversity of phytoplankton in flow-through cultures. Limnology and Oceanography 29: 633-636.). One possibility is that species with smaller Ks values have greater affinity for absorption of nutrients providing an advantage in nutrient limited environments. In this sense, the lowest value of Ks and higher cell densities observed in Ub3 suggest a strategy to more effectively exploit the availability of phosphate, especially in the limited treatment. On the other hand, species with high nutrient absorption and lower growth rates favor nutrient storage inside the cells. This excessive consumption ("luxury uptake"), which refers to the absorption and storage of phosphorous at levels greater than those required for immediate growth, could favor these species during periods of reduced availability of nutrients (Keenan & Auer 1974Keenan JD, Auer MT. 1974. The influence of phosphorus luxury uptake on algae bioassays. Journal of the Water Pollution Control Federation 46: 532-542. ; Mackey et al. 2012Mackey KRM, Mioni CE, Ryan JP, Paytan A. 2012. Phosphorus cycling in the red tide incubator region of Monterey Bay in response to upwelling. Frontiers in Microbiology 3: 1-14.; Wasmund et al. 2014Wasmund N, Nausch G, Hansen A. 2014. Phytoplankton succession in an isolated upwelled Benguela water body in relation to different initial nutrient conditions. Journal of Marine Systems 140: 163-174.; Domingues et al. 2015Domingues RB, Guerra CC, Barbosa B, Galvão HM. 2015. Are nutrients and light limiting summer phytoplankton in a temperate coastal lagoon? Aquatic Ecology 49: 127-146.). This particular uptake was shown by the Ub7 clone and evidenced by it having the highest half-saturation constant and higher intracellular concentrations of phosphorus in the P-enriched treatment. Consequently, cell division may proceed for a certain period, without subsequent nutrient uptake (Kuenzler & Ketchum 1962Kuenzler EJ, Ketchum BH. 1962. Rate of phosphorus uptake by Phaeodactylum tricornutum. Biology Bulletin 123: 134-145. ), since phosphate reserves can sustain growth for several generations in the absence of phosphorus in the medium (Fitzgerald & Nelson 1966Fitzgerald CP, Nelson T. 1966. Extractive and enzymatic analyses for limiting or surplus phosphorus in algae. Journal of Phycology 2: 32-37. ). The prompt absorption of nutrients is a common strategy by which phytoplankton exploit the nutrient resources available in oligotrophic waters (Goldman & Glibert 1982Goldman JC, Glibert PM. 1982. Comparative rapid ammonium uptake by four species of marine phytoplankton. Limnology and Oceanography 27: 814-827. ), while the absorption rate may increase up to 100 times in cells limited by phosphorus (Brown et al. 1978Brown EJ, Harris RF, Koonce JF. 1978. Kinetics of phosphate uptake by aquatic microorganisms: deviation from a simple Michaelis-Menten equation. Limnology and Oceanography 23: 26-43.).
In accordance with Aidar et al. (1991Aidar E, Ehrlich R, Asano CS, Sigaud TCS. 1991. Variação da composição química do melo de cultura e da bioquímica celular de Phaeodactylum tricornutum (Bohlin), em cultivos estanques. Boletim do Instituto Oceanográfico 39: 131-139. ), the highest phosphate absorption rates occurred during the lag phase of cell growth, indicating that phosphate absorption occurred immediately. However, significant increase in biomass only occurred late in the exponential phase. These results demonstrate that the processes of absorption and assimilation are different, and their indicator rates do not occur simultaneously (Goldman & Glibert 1983Goldman JC, Glibert PM. 1983. Kinetics of inorganic nitrogen uptake by phytoplankton. In: Capone DG, Carpenter EJ. (eds.) Nitrogen in the marine environment. Cambridge, Academic Press. p. 233-274. ; Collos 1986Collos Y. 1986. Time-Iag algal growth dynamics: biological constraints on primary production in aquatic environments. Marine Ecology and Progress Series 33: 193-206.). For many microalgae, the processing of absorbing nutrient metabolism into biomass is not instantaneous and requires a particular period of time that varies among species. For some organisms, the lag phase of cell division is so extensive that absorption and growth are not on the same time scale. These different scales have important implications for interpreting competition among species for limiting nutrients (Collos 1986Collos Y. 1986. Time-Iag algal growth dynamics: biological constraints on primary production in aquatic environments. Marine Ecology and Progress Series 33: 193-206.).
Experimental results show that cells in conditions of phosphorus deficiency can achieve higher absorption rates than cells in conditions of phosphate saturation. In those deficiency circumstances, P. tricornutum are able to absorb from 8 to 16 times as much as the minimum cell quota, by converting phosphate into intracellular polyphosphate reserves to support three to four generations in conditions of phosphate depletion (Yao et al. 2011Yao B, Xi B, Hu C, Huo S, Su J, Liu H. 2011. A model and experimental study of phosphate uptake kinetics in algae: Considering surface adsorption and P-stress. Journal of Environmental Sciences 23: 189-198.). The isolated strains of the same species may reflect significant genetic variability that is reflected in variability of characters of cellular physiology (Gallagher 1980Gallagher JC. 1980. Population genetics of Skeletonema costatum (Bacillariophyceae) in Narragansett Bay. Journal of Phycology 16: 464-474. ; 1982Gallagher, J.C., 1982. Physiological variation and electrophoretic banding patterns of genetically different seasonal populations of Skeletonema costatum (Bacillariophyceae). Journal of Phycology 18: 148-162. ; Huang et al. 2011Huang A, He L, Wang G. 2011. Identification and characterization of microRNAs from Phaeodactylum tricornutum by high-throughput sequencing and bioinformatics analysis. BioMed Central Genomics 12: 337. doi: 10.1186/1471-2164-12-337.
https://doi.org/10.1186/1471-2164-12-337...
; Yang et al. 2014Yang ZK, Zheng JW, Niu YF, Yang WD, Liu JS, Li HY. 2014. Systems-level analysis of the metabolic responses of the diatom Phaeodactylum tricornutum to phosphorus stress. Environmental Microbiology 16: 1793-1807.). This agrees with our results, since Ub3 and Ub7 both showed different responses in relation to absorption and phosphate assimilation, as shown by their absorption rates (Tab. 4).
Additionally, Yang et al. (2014Yang ZK, Zheng JW, Niu YF, Yang WD, Liu JS, Li HY. 2014. Systems-level analysis of the metabolic responses of the diatom Phaeodactylum tricornutum to phosphorus stress. Environmental Microbiology 16: 1793-1807.) analyzed transcriptional changes in P. tricornutum under phosphorus stress and found two genes encoding phosphatases, and 11 encoding phospholipases involved in certain key genes for adaptation to P limitation. The sequencing of the same species by Huang et al. (2011)Huang A, He L, Wang G. 2011. Identification and characterization of microRNAs from Phaeodactylum tricornutum by high-throughput sequencing and bioinformatics analysis. BioMed Central Genomics 12: 337. doi: 10.1186/1471-2164-12-337.
https://doi.org/10.1186/1471-2164-12-337...
, revealed 13 novel microRNAs (miRNAs) that perform specific regulatory functions in metabolism related to the synthesis of fatty acids and the urea cycle under nitrogen and silica limitation. When restricted to low iron concentrations, P. tricornutum employed metabolic rearrangements to acclimatize to the restricted nutrient levels (Allen et al. 2008 Allen AE, La Roche J, Maheswari U, et al. 2008. Whole-cell response of the pennate diatom Phaeodactylum tricornutum to iron starvation. Proceedings of the National Academy of Sciences of the United States of America 105: 10438-10443. ). According to Terry et al. (1983Terry KL, Hirata J, Laws EA. 1983. Light-limited growth of two strains of the marine diatom Phaeodactylum tricornutum Bohlin: chemical composition, carbon partitioning and the diel periodicity of Physiological processes. Journal of Experimental Marine Biology and Ecology 68: 209-227. ), the physiological differences between strains of P. tricornutum may be greater than those found among different species of microalgae. In Gaeta (1985Gaeta SA. 1985. Comparação das respostas de crescimento e fotossíntese de três clones de Phaeodactylum tricornutum Bohlin. PhD Thesis, Universidade de São Paulo, Brazil. ), statistical differences found in physiological parameters, such as growth rate, productivity and carbon assimilation, and the polymorphism shown by the clones, endorse the existence of different physiological strains of the same species (P. tricornutum). Furthermore, Martino et al. (2007Martino A, Meichenin A, Shi J, Pan K, Bowler C. 2007. Genetic and phenotypic characterization of Phaeodactylum tricornutum (bacillariophyceae) accessions. Journal of Phycology 43: 992 -1009.) analyzed intraspecific genetic diversity of 10 strains and found indications of four different genotypes that exhibited distinct phenotypic characteristics related to the physiological differences of the morphotypes of P. tricornutum.
Many species of microalgae are able to obtain phosphorus from phosphate esters to sustain growth in an environment restricted by orthophosphate, and different algae differ in the presence and location of phosphatases (González-Gil et al. 1998 González-Gil S, Keafer BA, Jovine RVM, Aguilera A, Lu S, Anderson DM. 1998. Detection and quantification of alkaline phosphatase in single cells of phosphorus-starved marine phytoplankton. Marine Ecology Progress Series 164: 21-35. ; Dyhrman & Palenik 1999Dyhrman ST, Palenik B. 1999. Phosphate stress in cultures and field populations of the dinoflagellate Prorocentrum minimum detected by a single-cell alkaline phosphatase assay. Applied and Environmental Microbiology 65: 3205-3212.; Rengefors et al. 2001Rengefors K, Pettersson K, Blenckner T, Anderson DM. 2001. Species-specific alkaline phosphatase activity in freshwater SPRING phytoplankton: application of a novel method. Journal of Plankton Research 23: 435-443.). The incorporation of organic nutrients by P. tricornutum is widely known, with alkaline phosphatase activity consistently bound to the cells (Kuenzler & Perras 1965Kuenzler EJ, Perras JP. 1965. Phosphatases in marine algae. Biology Bulletin 128: 271-284. ) and the plasma membrane as the site of greater specificity of PA (Flynn et al. 1986Flynn KJ, Opic H, Syrett PJ. 1986. Localization of the alkaline phosphatase and 5'nucleotidase activities of the diatom Phaeodactylum tricornutum. Journal of General Microbiology 132: 289-298.). Likewise, in this study, the greatest ELFA precipitation was observed around the cells of P. tricornutum and attached to the plasma membrane (Fig. 4B).
Phaeodactylum tricornutum strain Ub7, at stationary growth phase (epifluorescence microscopy; 1000x). Cells without alkaline phosphatase activity, cultured under treatment control (A) and with alkaline phosphatase activity, cultured under Phosphate-depleted (treatment F/8) conditions (B). Scale 50 µm.
Conclusions
Despite Phaeodactylum tricornutum usually having greater enzymatic activity in conditions of phosphate depletion, intraspecific variation was observed.
The Ub7 strain displays deficiency in phosphate incorporation, even in P-enriched conditions when there was excessive consumption of phosphate, alkaline phosphatase activity and use of P-organic. Through the use of organic phosphorus, P. tricornutum, in exponential growth phase, is capable of storing a higher internal concentration of phosphorus, however, this storage does not reflect an increase of cell density in the stationary phase, showing a probable imbalance between the absorption and assimilation of this nutrient.
The differences observed between the strains may be the result of a higher capacity for absorbing the nutrient by P. tricornutum Ub7, specified by the excessive consumption, or to different physiological conditions of the cells that interfere with their metabolic and chemical composition relations. Perhaps the incorporation of organic phosphorus and the modification of cell volume and length are mechanisms used by Ub7 to increase the orthophosphate cell quota, even in P-enriched conditions.
Batch cultures undergo continual changes in their chemical composition, especially with regard to nutrient concentration, which are reflected in metabolism and cellular biochemistry and, consequently, changes in surface/volume ratio and other physiological interactions that occur during different growth stages of a microalgae.
Acknowledgements
The authors are grateful to Phosphite APQ1-FAPERJ, CAPES and to LaGOM (Laboratório de Geoquímica Orgânica Marinha).
References
- Aidar E, Ehrlich R, Asano CS, Sigaud TCS. 1991. Variação da composição química do melo de cultura e da bioquímica celular de Phaeodactylum tricornutum (Bohlin), em cultivos estanques. Boletim do Instituto Oceanográfico 39: 131-139.
- Allen AE, La Roche J, Maheswari U, et al. 2008. Whole-cell response of the pennate diatom Phaeodactylum tricornutum to iron starvation. Proceedings of the National Academy of Sciences of the United States of America 105: 10438-10443.
- Beardall J, Young E, Roberts S. 2001. Approaches for determining phytoplankton nutrient limitation. Aquatic Sciences 63: 44-69.
- Brown EJ, Harris RF, Koonce JF. 1978. Kinetics of phosphate uptake by aquatic microorganisms: deviation from a simple Michaelis-Menten equation. Limnology and Oceanography 23: 26-43.
- Cao XY, Strojsová A, Znachor P, et al. 2005. Detection of extracellular phosphatases in natural spring phytoplankton of a shallow eutrophic lake (Donghu, China). European Journal of Phycology 40: 251-258.
- Collos Y. 1986. Time-Iag algal growth dynamics: biological constraints on primary production in aquatic environments. Marine Ecology and Progress Series 33: 193-206.
- Desbois AP, Walton M, Smith VJ. 2010. Differential antibacterial activities of fusiform and oval morphotypes of Phaeodactylum tricornutum (Bacillariophyceae). Journal of the Marine Biological Association of the United Kingdom 90: 769-774.
- Domingues RB, Anselmo TP, Barbosa AB, Sommer U, Galvão HM. 2011. Nutrient limitation of phytoplankton growth in the freshwater tidal zone of a turbid, Mediterranean estuary. Estuarine Coastal and Shelf Science 91: 282-297.
- Domingues RB, Guerra CC, Barbosa B, Galvão HM. 2015. Are nutrients and light limiting summer phytoplankton in a temperate coastal lagoon? Aquatic Ecology 49: 127-146.
- Dyhrman ST. 2005. Ectoenzymes in Prorocentrum minimum. Harmful Algae 4: 619-27.
- Dyhrman ST, Palenik B. 1999. Phosphate stress in cultures and field populations of the dinoflagellate Prorocentrum minimum detected by a single-cell alkaline phosphatase assay. Applied and Environmental Microbiology 65: 3205-3212.
- Dyhrman ST, Palenik B. 2001. A single cell immunoassay for phosphate stress in the dinoflagellate Prorocentrum minimum. Journal of Phycology 37: 400-410.
- Falciatore A, Casotti R, Leblanc C, Abrescia C, Bowler C. 1999. Transformation of Nonselectable Reporter Genes in Marine Diatoms. Marine Biotechnology 1: 239-251.
- Falkowski PG. 2000. Rationalizing elemental ratios in unicellular algae. Journal of Phycology 36: 3-6.
- Fitzgerald CP, Nelson T. 1966. Extractive and enzymatic analyses for limiting or surplus phosphorus in algae. Journal of Phycology 2: 32-37.
- Flynn KJ, Opic H, Syrett PJ. 1986. Localization of the alkaline phosphatase and 5'nucleotidase activities of the diatom Phaeodactylum tricornutum. Journal of General Microbiology 132: 289-298.
- Foster S, Thomson D, Maher W. 2008. Uptake and metabolism of arsenate by anexic cultures of the microalgae Dunaliella tertiolecta and Phaeodactylum tricornutum. Marine Chemistry 108: 172-183.
- Gaeta SA. 1985. Comparação das respostas de crescimento e fotossíntese de três clones de Phaeodactylum tricornutum Bohlin. PhD Thesis, Universidade de São Paulo, Brazil.
- Gallagher JC. 1980. Population genetics of Skeletonema costatum (Bacillariophyceae) in Narragansett Bay. Journal of Phycology 16: 464-474.
- Gallagher, J.C., 1982. Physiological variation and electrophoretic banding patterns of genetically different seasonal populations of Skeletonema costatum (Bacillariophyceae). Journal of Phycology 18: 148-162.
- Goldman JC, Glibert PM. 1982. Comparative rapid ammonium uptake by four species of marine phytoplankton. Limnology and Oceanography 27: 814-827.
- Goldman JC, Glibert PM. 1983. Kinetics of inorganic nitrogen uptake by phytoplankton. In: Capone DG, Carpenter EJ. (eds.) Nitrogen in the marine environment. Cambridge, Academic Press. p. 233-274.
- González-Gil S, Keafer BA, Jovine RVM, Aguilera A, Lu S, Anderson DM. 1998. Detection and quantification of alkaline phosphatase in single cells of phosphorus-starved marine phytoplankton. Marine Ecology Progress Series 164: 21-35.
- Grainger SLJ. 1989. Filament structure and phosphatase activity in the Rivulariaceae. PhD Thesis, Durham University, United Kingdom.
- Graziano LM, La Roche J, Geider RJ. 1996. Physiological response to phosphorus limitation in batch and steady state cultures of Dunaliella tertiolecta (Chlorophyta): a unique stress protein as an indicator of phosphate deficiency. Journal of Phycology 32: 825-838.
- Gregoracci GB, Nascimento JR, Cabral AS, et al. 2012. Structuring of bacterioplankton diversity in a large tropical bay. PLoS ONE 7(2): e31408. doi: 10.1371/journal.pone.0031408.
» https://doi.org/10.1371/journal.pone.0031408 - Guillard RRL, Ryther JH. 1962. Studies of marine planktonic diatoms: I Cyclotella nana Husted, and Detonula confervacea (Cleve) Gran. Canadian Journal of Microbiology 8: 229-239.
- Harris GP. 1978. Photosynthesis, productivity and growth: The physiological ecology of phytoplankton. Archiv für Hydrobiologie-BeiheftErgebnisse der Limnologie 10: 1-171.
- Hernández I, Fernández JA, Niell FX. 1993. Influence of phosphorus status on the seasonal variation of alkaline phosphatase activity in Porphyra umbilicalis (L.) Kützing. Journal of Experimental Marine Biology and Ecology 173: 181-196.
- Hernández I, Niell FX, Whitton BA. 2002. Phosphatase activity of benthic marine algae. An overview. Journal of Applied Phycology 14: 475-487.
- Huang A, He L, Wang G. 2011. Identification and characterization of microRNAs from Phaeodactylum tricornutum by high-throughput sequencing and bioinformatics analysis. BioMed Central Genomics 12: 337. doi: 10.1186/1471-2164-12-337.
» https://doi.org/10.1186/1471-2164-12-337. - Kawachi M, Noel MH. 2005. Sterilization and sterile technique. In: Andersen R. (ed.) Algal culturing techniques. Burlington, Elsevier. p. 65-82.
- Keenan JD, Auer MT. 1974. The influence of phosphorus luxury uptake on algae bioassays. Journal of the Water Pollution Control Federation 46: 532-542.
- Kuenzler EJ, Ketchum BH. 1962. Rate of phosphorus uptake by Phaeodactylum tricornutum. Biology Bulletin 123: 134-145.
- Kuenzler EJ, Perras JP. 1965. Phosphatases in marine algae. Biology Bulletin 128: 271-284.
- Lin HY, Shih CI, Liu HC, et al. 2013. Identification and characterization of an extracellular alkaline phosphatase in the marine diatom Phaeodactylum tricornutum. Marine Biotechnology 15: 425-436.
- Lin X, Zhang H, Huang B, Lin S. 2011. Alkaline phosphatase gene sequence and transcriptional regulation by phosphate limitation in Amphidinium carterae (Dinophyceae). Journal of Phycology 47: 1110-1120.
- Mackey KRM, Mioni CE, Ryan JP, Paytan A. 2012. Phosphorus cycling in the red tide incubator region of Monterey Bay in response to upwelling. Frontiers in Microbiology 3: 1-14.
- Maitra N, Manna SK, Samanta S, et al. 2015. Ecological significance and phosphorus release potential of phosphate solubilizing bacteria in freshwater ecosystems. Hydrobiologia 745: 69-83.
- Marco E, Orus MI. 1988. Variation in growth and metabolism with phosphorus-nutrition in 2 cyanobacteria. Journal of Plant Physiology 132: 339-344.
- Martino A, Meichenin A, Shi J, Pan K, Bowler C. 2007. Genetic and phenotypic characterization of Phaeodactylum tricornutum (bacillariophyceae) accessions. Journal of Phycology 43: 992 -1009.
- Monod J. 1949. The growth of bacterial cultures. Annual Review Microbiology 3: 371-394.
- Moser GAO, Tocci BRC, Richard EC, Barrera-Alba JJ. 2010. Avaliação da incorporação de fósforo em microalgas marinhas através da técnica de marcadores enzimáticos fluorescentes. Paraty, Resumos do XIII Congresso Brasileiro de Ficologia, p. 51.
- Naedoma J, Satrojsová A, Varba J, Kaomárková J, Saimek K. 2003. Extracellular phosphatase activity of natural plankton studied with ELF97 phosphate: fluorescence quantification and labelling kinetics. Environmental Microbiology 5: 462-472.
- Ohse S, Derner RB, Ozório RA, et al. 2008. Crescimento de microalgas em sistema autotrófico estacionário. Biotemas 21: 7-18.
- Pandey VD, Parveen S. 2011. Alkaline phosphatase activity in cyanobacteria: Physiological and ecological significance. Indian Journal of Fundamental and Applied Life Sciences 4: 295-303.
- Rengefors K, Pettersson K, Blenckner T, Anderson DM. 2001. Species-specific alkaline phosphatase activity in freshwater SPRING phytoplankton: application of a novel method. Journal of Plankton Research 23: 435-443.
- Reynolds CS. 2006. The ecology of phytoplankton. 1st. edn. New York, Cambridge University Press.
- Riegman R, Mur LR. 1986. Phytoplankton growth and phosphate uptake (for P limitation) by natural phytoplankton populations from the Loosdrecht lakes (The Netherlands). Limnology and Oceanography 31: 983-988.
- Riegman R, Stolte W, Noordeloos AAM, Slezak D. 2000. Nutrient uptake and alkaline phosphatase (EC 3:1:3:1) activity of Emiliania huxleyi (Prymnesiophyceae) during growth under N and P limitation in continuous cultures. Journal of Phycology 36: 87-96.
- Ruiz RG, Hernández I, Lucena J, Niell FX. 1997. Preliminary studies on the significance of alkaline phosphatase activity in the diatom Phaeodactylum tricornutum Bohlin. Scientia Marina 61: 517-525.
- Shen H, Song L. 2007. Comparative studies on physiological responses to phosphorus in two phenotypes of bloom-forming Microcystis. Hydrobiologia 592: 475-486.
- Skelton HM, Parrow MW, Burkholder JM. 2006. Phosphatase activity in the heterotrophic dinoflagellate Pfiesteria shumwayae. Journal of Plankton Research 5: 395-406.
- Sommer U. 1984. The paradox of the plankton: Fluctuations of phosphorus availability maintain diversity of phytoplankton in flow-through cultures. Limnology and Oceanography 29: 633-636.
- Strojsová A, Vrba J, Nedoma J, Komárková J, Znachor P. 2003. Seasonal study of extracellular phosphatase expression in the phytoplankton of a eutrophic reservoir. European Journal of Phycology 38: 295-306.
- Sun J, Liu D. 2003. Geometric models for calculating cell biovolume and surface area for phytoplankton. Journal of Plankton Research 25: 1331-1346.
- Terry KL, Hirata J, Laws EA. 1983. Light-limited growth of two strains of the marine diatom Phaeodactylum tricornutum Bohlin: chemical composition, carbon partitioning and the diel periodicity of Physiological processes. Journal of Experimental Marine Biology and Ecology 68: 209-227.
- Timmermans KR, Wagt B. 2010. Variability in cell size, nutrient depletion, and growth rates of the Southern Ocean diatom Fragilariopsis kerguelensis (Bacillariophyceae) after prolonged iron limitation. Journal of Phycology 46: 497-506.
- Vance CP, Uhde-Stone C, Allan DL. 2003. Phosphorus acquisition and use: critical adaptations by plants for securing a nonrenewable resource. New Phytologist 157: 423-447.
- Voltolina D, Nieves M, Navarro G, Oliva T, Peraza D. 1998. The importance of acclimation for the evaluation of alternative media for microalga growth. Aquacultural Engineering 19: 7-15.
- Wasmund N, Nausch G, Hansen A. 2014. Phytoplankton succession in an isolated upwelled Benguela water body in relation to different initial nutrient conditions. Journal of Marine Systems 140: 163-174.
- Yang ZK, Zheng JW, Niu YF, Yang WD, Liu JS, Li HY. 2014. Systems-level analysis of the metabolic responses of the diatom Phaeodactylum tricornutum to phosphorus stress. Environmental Microbiology 16: 1793-1807.
- Yao B, Xi B, Hu C, Huo S, Su J, Liu H. 2011. A model and experimental study of phosphate uptake kinetics in algae: Considering surface adsorption and P-stress. Journal of Environmental Sciences 23: 189-198.
Publication Dates
-
Publication in this collection
25 Aug 2016 -
Date of issue
Jul-Sep 2016
History
-
Received
28 Apr 2016 -
Accepted
21 July 2016