Abstracts
BACKGROUND: Arterial Hypertension (AH) is an aggravating condition for Metabolic Syndrome (MS), as well as being aggravated by it. Menopause can make hypertension treatment more difficult, as it favors the worsening of MS components. Although there is evidence that exercise training reduces blood pressure, whether menopause and SM affect the exercise-induced benefits is yet to be elucidated. OBJECTIVE: To compare the effects of aerobic training on blood pressure in non-menopausal and menopausal women with MS METHODS: A total of 44 women were recruited and divided into four groups: non-menopausal control (NMC: 39.5 ± 3.6 years, n = 11); menopausal control (MC: 54.9 ± 5.9 years, n = 12), non-menopausal aerobics (NMA: 43.1 ± 6.8 years, n = 11) and menopausal aerobics (MA: 52.1 ± 5 years, n = 10). The exercise groups performed aerobic training for three months, five times a week, at an intensity between 60% and 70% of heart rate reserve. The resting blood pressure and blood pressure response after 60 minutes of exercise were measured before and after the training period. The two-way ANOVA test was used, considering a p value < 0.05. RESULTS: The training program resulted in a decrease in abdominal fat, blood glucose and improved VO2 max. Compared to pre-intervention values, Systolic Blood Pressure (SBP) and Diastolic Blood Pressure (DBP) did not change after training in NMC, MC, MA and NMA groups (p > 0.05). CONCLUSION: Three months of aerobic training improved MS components, but did not alter resting blood pressure or the BP response after an acute exercise session in women with MS.
Exercise; blood pressure; women; menopause; metabolic X syndrome
FUNDAMENTO: A Hipertensão arterial (HA) é uma condição tanto agravante quanto agravada pela Síndrome Metabólica (SM). A menopausa pode tornar o tratamento da hipertensão mais difícil porque é uma condição que favorece a piora nos componentes da SM. Embora existam evidências de que o treinamento com exercícios físicos reduza a pressão arterial, se as condições da menopausa e da SM afetam os benefícios induzidos pelo exercício é algo ainda não evidenciado. OBJETIVO: Comparar os efeitos do treinamento aeróbio na pressão arterial entre mulheres com SM não menopausadas e menopausadas. MÉTODOS: Foram recrutadas 44 mulheres divididas em quatro grupos experimentais: controle não menopausada (CNM: 39,5 ± 1,1 anos, n = 11); controle menopausada (CM: 54,9 ± 1,7 anos, n = 12); aeróbio não menopausada (ANM: 43,1 ± 2,1 anos, n = 11) e aeróbio menopausada (AM: 52,1 ± 1,6 anos, n = 10). Os grupos de exercício realizaram treinamento aeróbio durante três meses, cinco vezes por semana, com intensidade entre 60% e 70% da frequência cardíaca de reserva. A pressão arterial de repouso e a resposta pressórica clínica após 60 minutos de exercício foram medidas antes e após o período treinamento. O teste de ANOVA de dois fatores foi usado, considerando p < 0,05. RESULTADOS: O programa de treinamento resultou em redução da gordura abdominal, glicemia e melhora do VO2 máx. Em comparação aos valores pré-intervenção, Pressão Arterial Sistólica (PAS) e Pressão Arterial Diastólica (PAD) não se alteraram após o treinamento nos grupos CNM, CM, ANM e AM (p > 0,05). CONCLUSÃO: Três meses de treinamento aeróbio melhora componentes da SM, mas não altera a pressão arterial de repouso, nem a resposta pressórica aguda após uma sessão de exercício aeróbio em mulheres com SM.
Exercício; pressão arterial; mulheres; menopausa; síndrome metabólica
Aerobic training does not alter blood pressure in menopausal women with metabolic syndrome
Aluísio Henrique Rodrigues de Andrade LimaI; Henrique Eduardo CoutoI; Glêbia Alexa CardosoI; Lidiane Tavares ToscanoI; Alexandre Sérgio SilvaI; Maria Paula Gonçalves MotaII
IUniversidade Federal da Paraíba, João Pessoa, PB - Brazil
IIUniversidade de Trás-os-Montes e Alto Douro2 - Portugal
Correspondência
ABSTRACT
BACKGROUND: Arterial Hypertension (AH) is an aggravating condition for Metabolic Syndrome (MS), as well as being aggravated by it. Menopause can make hypertension treatment more difficult, as it favors the worsening of MS components. Although there is evidence that exercise training reduces blood pressure, whether menopause and SM affect the exercise-induced benefits is yet to be elucidated.
OBJECTIVE: To compare the effects of aerobic training on blood pressure in non-menopausal and menopausal women with MS.
METHODS: A total of 44 women were recruited and divided into four groups: non-menopausal control (NMC: 39.5 ± 1.1 years, n = 11); menopausal control (MC: 54.9 ± 1.7 years, n = 12), non-menopausal aerobics (NMA: 43.1 ± 2.1 years, n = 11) and menopausal aerobics (MA: 52.1 ± 1.6 years, n = 10). The exercise groups performed aerobic training for three months, five times a week, at an intensity between 60% and 70% of heart rate reserve. The resting blood pressure and blood pressure response after 60 minutes of exercise were measured before and after the training period. The two-way ANOVA test was used, considering a p value < 0.05.
RESULTS: The training program resulted in a decrease in abdominal fat, blood glucose and improved VO2 max. Compared to pre-intervention values, Systolic Blood Pressure (SBP) and Diastolic Blood Pressure (DBP) did not change after training in NMC, MC, MA and NMA groups (p > 0.05).
CONCLUSION: Three months of aerobic training improved MS components, but did not alter resting blood pressure or the BP response after an acute exercise session in women with MS.
Keywords: Exercise; blood pressure; women; menopause; metabolic syndrome
Introduction
Among the different metabolic changes resulting from menopause, the prevalence of Metabolic Syndrome (MS) has been documented. This prevalence increases from 13.8% in the premenopausal period to 60% in the postmenopausal period1-2. Similar behavior is observed regarding Systemic Arterial Hypertension (SAH), which increases on average from 32.1% to 60% in these periods3-4, regardless of the presence of MS5.
The mechanisms associated with increased prevalence of MS and hypertension in menopause are yet to be fully understood, but some factors seem to influence this response, such as estrogen deficiency, lipid profile alterations, endothelial dysfunction, decreased parasympathetic tone and increase in renin-angiotensin system activity, oxidative stress and body adiposity in this population6-10.
Physical exercise has been considered a non-pharmacological tool in the treatment of SAH11-12. However, cardiometabolic alterations resulting from menopause can directly interfere with the mechanisms by which exercise reduces blood pressure (BP). BP decrease after a training period has been attributed to a reduction in cardiac output, of sympathetic tone to the heart13, of circulating levels of noradrenaline14 and increased production and/or bioavailability of nitric oxide15. However, these pathways are impaired in postmenopausal women with MS.
Some studies have shown that after an aerobic training intervention period, there is no decrease in BP in healthy postmenopausal women, with overweight and high BP16-18. However, other studies have shown BP reduction in healthy postmenopausal19 and postmenopausal hypertensive women20-22. These studies did not clarify the influence of menopause on the effects of exercise on BP, as the researchers did not use a control group to compare BP responses20-22. Only one study compared women in the periods before and after menopause19, identifying a significant decrease in systolic blood pressure after training only in the group of postmenopausal women. As the women were healthy, it remains unclear whether MS together with menopause can interfere with BP responses to exercise training.
Thus, the objective of the study was to analyze the influence of an aerobic training program on the BP response in premenopausal and postmenopausal women with MS.
Methods
Study Subjects
Patients with MS recruited from public hospitals and private clinics in the city of Iguatu (state of Ceará, Brazil) were invited to participate in this study. The inclusion criteria consisted of (a) presence of at least three of the following components of MS: high systolic blood pressure, hypertriglyceridemia, increased fasting glucose, reduced HDL lipoproteins and elevated waist circumference23, (b) age between 40 and 55 years (c) to be in the premenopausal (normal menstrual cycle) or postmenopausal period (menstrual cycle cessation for more than one year), (d) to be physically inactive. Participants were considered sedentary when they reported not being physically active individuals, those who did not perform physically active work and those who reported walking less than 20 minutes a day in daily dislocations.
The exclusion criteria included patients entering menopause, those with evidence of heart disease, including ischemic heart disease, kidney disease, hypo- or hyperthyroidism and cancer, as well as smokers, those who consumed alcohol (more than three doses of alcohol a day), patients who used supplements and medications (fibrates, statins, exogenous insulin or hypoglycemic agents, beta-blockers, and antiarrhythmics), and pregnant women. Forty-four women were considered eligible for the study. They were randomly distributed using a number-generating software program to select a random sample (www.randomizer.org), in non-menopausal control group (NMC - 39.5 ± 1.1 years, n = 11), menopausal control (MC - 54.9 ± 1.7 years, n = 12), non-menopausal aerobics (NMA - 43.1 ± 2.1 years, n = 11) and menopausal aerobics (MA - 52.1 ± 1.6 years, n = 10).
This study was approved by the Ethics Committee on Human Research of Universidade Federal do Ceará, under protocol nº 42111/2011. Each patient was informed of the risks and benefits involved in the study and signed an informed consent form, according to Resolution 196/96 of the National Health Council.
Study Design
Initially, the volunteers underwent blood sample collection for subsequent analysis of lipid profile and glucose levels, as well as anthropometric measurements. The women from groups NMA and MA underwent a training program with aerobic exercises for three months. Blood pressure measurements were carried out at rest before and after the training period. Moreover, at the first and last training sessions, blood pressure measurements were performed before the exercise and for 60 minutes in the recovery period to determine BP response to acute exercise.
Pre-Participation Evaluations
All subjects underwent a progressive treadmill exercise test with continuous ECG monitoring. Only those who showed no signs of ischemia or arrhythmia and whose physicians gave their consent for study participation were enrolled. Subsequently, they underwent nutritional assessment and were advised to maintain their usual dietary habits. Each month, new nutritional assessments were performed with the objective of monitoring the nutritional behavior throughout the study. These evaluations were performed using a food frequency questionnaire, following the proposal of Block et al.24. We considered the responses regarding the women's usual monthly food consumption. Calculations were carried out using AVANUTRI software, release 4.0 (Avanutri & Nutrição Serviços de Informática, Três Rios-RJ-Brazil).
One week before and 48 hours after the training protocols, submaximal one-mile tests were carried out (1,609 km) as well as ten-repetition maximum (10RM) tests for assessment of aerobic capacity and strength, respectively.
Training Protocol
The participants underwent a 12-week training protocol with five weekly sessions. Initially, they underwent an adjustment period in the first week of training, with three sessions of 20 minutes of exercise at 50% of MHR, according to the protocol proposed by Karvonen et al.25, with a 48-hour interval between sessions. From the second week on, the training volume increased to 30 minutes at 60% of MHR and progressed to 60 minutes in the following three weeks. From the fifth week on, the training session lasted 60 minutes, which was maintained until the end of the program. At this phase, the intensity increased from 60% to 70% of MHR. The intensity of all sessions was adjusted according to the subjective perception of effort (SPE) reported by participants, so that they maintained the effort between 11 and 14 in the Borg scale.
Blood Pressure Measurements
A Missouri aneroid sphygmomanometer (Embu, Brazil) with a 2-millimeter mercury precision, previously calibrated against a mercury column was used only for the purpose of gathering research data during the study. The measurements of resting BP were performed according to the procedures proposed by the VI Brazilian Guidelines on Hypertension (2010), observing all procedures related to food, bladder emptying, previous physical exercise. Measurements were performed with subjects in the sitting position, with legs uncrossed and after a resting period of at least 10 minutes, with the use of antihypertensive medications when the individual was hypertensive. Initially, the pressure was measured in both upper limbs, and using the auscultatory method employing phases I and V of Korotkoff sounds to identify the values of systolic and diastolic blood pressure, respectively. Blood pressure was measured in each arm until three consecutive values were obtained with a difference of less than 5 mmHg. When differences > 5 mmHg were found between the upper limbs, the arm with the highest BP value was chosen for the remainder of the study. When there was no difference, the right arm was chosen for future measurements.
Women were considered hypertensive when they had a previous diagnosis of SAH or were considered hypertensive at the clinical appointment during which the exercise test was performed, as well as those who were already using antihypertensive medication, regardless of the BP value found during the study. We considered the possibility of BP values ≥ 140/90 mmHg in women considered normotensive, and these would undergo a new medical assessment. However, that was not observed.
To determine the chronic response, BP was measured before the intervention period and after three months of training, which occurred 48 hours after the last session of the training protocol. BP measurements were performed on the same days of the week and at the same time of day.
To determine the acute response, measurements were performed after ten minutes of rest, immediately after the exercises, and at the 10, 20, 30, 40, 50 and 60-minute recovery period after exercise in the first and last sessions of the three-month training protocol. All these measurements were performed with the patient in the sitting position.
Blood sample collection and assessment of lipoproteins, triglycerides and glucose levels
Blood samples containing 6 mL of blood from the antecubital vein were collected 24 hours before the intervention period. Three milliliters of blood were placed in tubes containing EDTA and gently homogenized by inversion. The remaining 3 mL were placed in tubes without anticoagulant. Then, they were centrifuged at 1500 rpm for 20 minutes. The plasma or serum was separated, placed in Eppendorf tubes and frozen at -20 °C until the analysis was performed. All these analyses were carried out using a Labtest commercial kit (Minas Gerais, Brazil), following the manufacturer's recommendations.
Statistical Analysis
Given the experimental nature of this study, the number of subjects that was calculated for each group was based on sample power, as proposed by Eng26. For that purpose, previous data on how exercise affects the systolic blood pressure were used. The variation promoted by the exercise was 17 mmHg for a standard deviation of 10 mmHg. Adopting a statistical power of 0.80 and a confidence level of 0.05, it was estimated that the minimum size for each group was 14 women.
The normality and homogeneity of data variance were analyzed by the Shapiro-Wilks and Levene's tests, respectively. One-way ANOVA for repeated measures was used to compare the pre-intervention differences between groups (NMC, MC, MA and NMA). To analyze the changes in cardiovascular measures at rest before and after the intervention period was used two-way ANOVA, with the established groups (NMC, MC, MA and NMA) and moments (pre- and post-intervention) being considered as factors. Delta data (post - pre) measured after performing an experimental session before and after the intervention period were compared between groups by two-way ANOVA for repeated measures. For all analyses, a value of p < 0.05 was adopted as statistically significant and post-hoc comparisons were performed by the Newman-Keuls test, when necessary. Data are presented as mean ± standard error.
Results
Baseline characteristics of patients are shown in Table 1. The postmenopausal women were older than premenopausal ones. However, the group was characterized as belonging to the same age range (middle-age) and none of the women were older than 55. The four groups had borderline BMI between overweight and grade I obesity and all had a waist circumference level above the normal range. The aerobic capacity was classified as regular and levels of blood glucose, lipoproteins and triglycerides were close to the maximum reference values for all groups. Eight women from the NMC group and seven from the NMA group used oral contraceptives. None of the postmenopausal menopausal women used hormonal replacement therapy.
The groups had similar dietary habits at baseline. According to the given dietary advice, none of the groups modified dietary intake during the study period, so there was no intergroup differences regarding nutritional intake in the assessments. As a result, the four groups remained statistically similar during and after the intervention process. Food intake data from the first and last assessment (immediately before and after the study) are shown in Table 1.
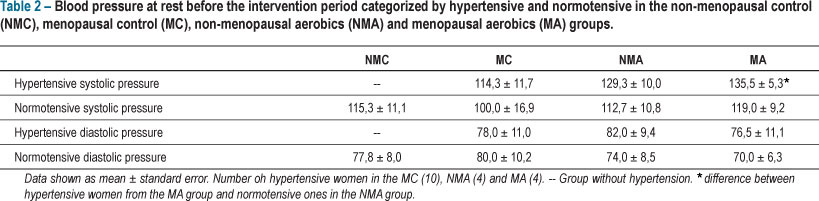
None of the women from the NMC group were hypertensive, whereas of the 11 volunteers from the NMA group, four were hypertensive. Hypertension was more prevalent in MC group, where six of the 12 women were hypertensive, whereas four of the 10 postmenopausal women who remained in the MA group were hypertensive. Regardless of the presence of hypertension, BP values at rest before the start of the training program were similar between normotensive and hypertensive women, except for the BP values observed in the hypertensive women from the MA group, which were greater than in the normotensive ones from the NMA group. Table 2 shows a comparison of BP values of hypertensive and normotensive women from all groups.
The 12 weeks of training promoted aerobic capacity improvement in the women who were involved with the training program, so that these women completed the intervention period with significantly better VO2max when compared with their initial values and the ones observed in their counterparts who remained sedentary, as shown in Table 3.
This improvement in aerobic capacity was accompanied by a decrease in BMI, waist circumference and blood glucose (Figure 1).
The BP values at rest before and after three months of intervention are shown in Figure 2. The training program was ineffective in promoting changes in the pre-intervention BP values in premenopausal or postmenopausal women. That occurred in spite of the evident increase in aerobic capacity in response to training in the groups submitted to exercise training.
The effect of the training program on acute blood pressure response to a single exercise session is shown in Figure 3. It shows the greatest reduction in blood pressure within 60 minutes of recovery that followed the exercise sessions performed before and after the training protocol. Compared to pre-intervention values, the SBP and DBP deltas did not change after training in either group.
Discussion
The main finding of this study was that an aerobic training program promotes body fat reduction and improves aerobic capacity, but does not reduce blood pressure, acute or chronically, in women with MS, regardless of their menopausal status.
The decrease in blood pressure after a training period, either acute or chronically, has been demonstrated in normotensive27-28 and hypertensive29-30 individuals. In postmenopausal women, it was observed a reduction of 18 and 10 mmHg in systolic and diastolic values, respectively, after six months of training at 50% of MHR, with three weekly sessions 24. However, this study did not have a control group, so that the effects of menopause on the benefits of exercise were not elucidated.
In another study of postmenopausal women, Figueroa et al.31 observed a reduction in BP after 12 weeks of training, but this protocol consisted of a combination of aerobic and resistance exercises, which differs from our study in which women performed aerobic exercises only. Meanwhile, Cardoso et al.32 demonstrated that a protocol of aerobic exercises, as performed in this study, prevent ambulatory increases in blood pressure typically seen in women who underwent hormonal replacement therapy with oral estrogens. Although the results of our study differ from previous studies, it should be noted that while in this study we used the aerobic exercises, another was in combination with resistance training whereas no reduction of the PA, but no increase in pressure in postmenopausal replacement with estrogens. These differences in methodology and subjects' characteristics may explain the differences in our results.
On the other hand, there have been studies with similar results to ours. In the study by Arsenault et al.16, there was no change in BP, even with a decrease in BMI and waist circumference in women with high blood pressure who underwent six months of aerobic exercise training, performed three to four times a week, at 50% of the VO2max. Meanwhile, our study failed to promote BP reduction even after improving the aerobic capacity of the volunteers. Yoshizawa et al.18 observed that in spite of the decrease in BMI and VO2 improvement, there was no change in systolic and diastolic blood pressure after a period of eight weeks of aerobic training performed four to five days a week, with an intensity of 60% -75% of MHR. Despite this evidence, neither of the studies showed an association between menopause and MS in the studied women.
BP decrease after a period of aerobic training has been demonstrated in women with MS. In a recent study, Mujica et al.33 observed that a four-month exercise period, performed three times per week, lasting 60 minutes, of which intensity increased progressively from 40% to 80% of MHR between the first and last month's training, decreased BP levels in women with MS. Corroborating these results, another study34 observed that two months of training divided in 180 minutes a week associated with a reduced-calorie diet was enough to reduce the BP of patients with MS.
Given the absence of the hypotensive effect in this study, it can be assumed that the training protocol would not have been effective in promoting this hypotensive response. However, the training program of the present study was performed so that there was increased overload, both in volume and intensity in the second, third, fourth and fifth weeks of training. From the fifth to twelfth weeks, the duration of training was maintained at 60 minutes, with five weekly sessions. This is an appropriate volume-intensity ratio according to the current guidelines for the treatment of hypertension35-36. In fact, this training protocol resulted in significant increase in VO2 max and total and central body fat reduction in the study volunteers.
It can also be assumed that the sample size was responsible for the lack of the hypotensive effect. However, we believe that there was no lack of power in the statistical analysis of this study. Initially, when performing the analyses, we realized that the p values were distant from the adopted significance (p < 0.05). Thus, even if new individuals were added to the sample, probably these would not be enough to change the results. In addition, a recent study32, which used a design similar to that of the present study and assessed BP showed no significant differences with only nine subjects in one of the assessed groups.
Even the hypertensive women had pre-intervention BP values < 140/90 mmHg. It is well-known that the potential of exercise to promote blood pressure reduction is influenced by pre-exercise BP values37. Therefore, this is one more reason we can list to explain the absence of blood pressure reduction after the administered training program.
One limitation of this study was the fact that it was not possible to eliminate the volunteers who were using oral contraceptives. To apply this as an exclusion criterion would have reduced the size of the non-menopausal groups by approximately 75%. Nevertheless, when considering the experimental and control groups of menopausal women (who had contraceptive use as an exclusion criterion), the results do not change in relation to the non-menopausal groups. Furthermore, the two non-menopausal groups had users and non-users of oral contraceptives.
As it was observed for the chronic effect of exercise on blood pressure, a single session did not promote acute blood pressure reduction after the exercise, even though the phenomenon of hypotension has been well documented after aerobic exercises in menopausal38 and non-menopausal39 women. The study training protocol did not affect Post-Exercise Hypotension (PEH), either. These data corroborate other previous studies, in which no differences were found in the magnitude of PEH between different training situations40.
Menopausal women with MS have increased cardiovascular risk2 and interventions aimed at reducing this risk should be used in this population. Although the training protocol of the present study did not cause significant alterations in blood pressure, body fat decreased in the menopausal and non-menopausal groups who exercised, as well as and glucose level reduction in the non-menopausal group. These variables are two components of the MS. Therefore, this study shows that a training program can reduce body fat in women with MS, regardless of whether or not they are menopausal. On the other hand, the data seem to indicate that menopause can interfere with metabolic responses to exercise. However, although this study is indicative, it is not enough to evaluate the hypothesis that menopause influences cardiometabolic responses to exercise.
Conclusion
The results of this study indicated that a 12-week protocol of aerobic training can promote improvement in MS components, but this was not accompanied by chronic reduction in blood pressure levels or presence of acute hypotension in the first moments after a session of this type of exercise. Although glycemia was improved only in menopausal women who exercised, this study is not yet sufficient to answer whether the metabolic alterations of menopause can interfere with cardiometabolic responses to exercise in women with MS.
Potential Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Sources of Funding
There were no external funding sources for this study.
Study Association
This article is part of the thesis of doctoral submitted by Glêbia Alexa Cardoso, from Universidade Federal da Paraíba.
References
- 1. Janssen I, Powell LH, Crawford S, Lasley B, Sutton-Tyrrell K. Menopause and the metabolic syndrome: the Study of Women's Health Across the Nation. Arch Intern Med. 2008 ;168(14):1568-75.
- 2. Pandey S, Srinivas M, Agashe S, Joshi J, Galvankar P, Prakasam CP, et al. Menopause and metabolic syndrome: a study of 498 urban women from western India. J Midlife Health. 2010;1(2):63-9.
- 3. Zanchetti A, Facchetti R, Cesana GC, Modena MG, Pirrelli A, Sega R. Menopause-related blood pressure increase and its relationship to age and body mass index: the SIMONA epidemiological study. J Hypertens. 2005;23(12):2269-76.
- 4. Taddei S. Blood pressure through aging and menopause. Climacteric. 2009;12(Suppl 1):36-40.
- 5. Coylewright M, Reckelhoff JF, Ouyang P. Menopause and hypertension: an age-old debate. Hypertension. 2008;51(4):952-9.
- 6. Taddei S, Virdis A, Ghiadoni L, Mattei P, Sudano I, Bernini G, et al. Menopause is associated with endothelial dysfunction in women. Hypertension. 1996;28(4):576-82.
- 7. Phillips GB, Jing T, Heymsfield SB. Does insulin resistance, visceral adiposity, or a sex hormone alteration underlie the metabolic syndrome? Studies in women. Metabolism. 2008; 57(6):838-44.
- 8. Farag NH, Bardwell WA, Nelesen RA, Dimsdale JE, Mills PJ. Autonomic responses to psychological stress: the influence of menopausal status. Ann Behav Med. 2003 ;26(2):134-8.
- 9. Menozzi R, Cagnacci A, Zanni AL, Bondi M, Volpe A, Del Rio G. Sympathoadrenal response of postmenopausal women prior and during prolonged administration of estradiol. Maturitas. 2000;34(3):275-81.
- 10. Rosano GM, Rillo M, Leonardo F, Pappone C, Chierchia SL. Palpitations: what is the mechanism, and when should we treat them? Int J Fertil Womens Med. 1997;42(2):94-100.
- 11. Brum PC, Forjaz CLdM, Tinucci T, Negrão CE. Adaptações agudas e crônicas do exercício físico no sistema cardiovascular. Rev paul Educ Fís. 2004;18:21-31.
- 12. Zanesco A, Antunes E. Effects of exercise training on the cardiovascular system: pharmacological approaches. Pharmacol Ther. 2007;114(3):307-17.
- 13. Gava NS, Veras-Silva AS, Negrao CE, Krieger EM. Low-intensity exercise training attenuates cardiac beta-adrenergic tone during exercise in spontaneously hypertensive rats. Hypertension. 1995;26(6 Pt 2):1129-33.
- 14. Urata H, Tanabe Y, Kiyonaga A, Ikeda M, Tanaka H, Shindo M, et al. Antihypertensive and volume-depleting effects of mild exercise on essential hypertension. Hypertension. 1987;9(3):245-52.
- 15. Graham DA, Rush JW. Exercise training improves aortic endothelium-dependent vasorelaxation and determinants of nitric oxide bioavailability in spontaneously hypertensive rats. J Appl Physiol. 2004;96(6):2088-96.
- 16. Arsenault BJ, Cote M, Cartier A, Lemieux I, Despres JP, Ross R, et al. Effect of exercise training on cardiometabolic risk markers among sedentary, but metabolically healthy overweight or obese post-menopausal women with elevated blood pressure. Atherosclerosis. 2009;207(2):530-3.
- 17. Riesco E, Aubertin-Leheudre M, Maltais ML, Audet M, Dionne IJ. Synergic effect of phytoestrogens and exercise training on cardiovascular risk profile in exercise-responder postmenopausal women: a pilot study. Menopause. 2010;17(5):1035-9.
- 18. Yoshizawa M, Maeda S, Miyaki A, Misono M, Choi Y, Shimojo N, et al. Additive beneficial effects of lactotripeptides and aerobic exercise on arterial compliance in postmenopausal women. Am J Physiol Heart Circ Physiol. 2009;297(5):H1899-903.
- 19. Deibert P, Konig D, Vitolins MZ, Landmann U, Frey I, Zahradnik HP, et al. Effect of a weight loss intervention on anthropometric measures and metabolic risk factors in pre- versus postmenopausal women. Nutr J. 2007;6:31.
- 20. Zaros PR, Pires CE, Bacci M, Jr., Moraes C, Zanesco A. Effect of 6-months of physical exercise on the nitrate/nitrite levels in hypertensive postmenopausal women. BMC Womens Health. 2009;9:17.
- 21. Seals DR, Tanaka H, Clevenger CM, Monahan KD, Reiling MJ, Hiatt WR, et al. Blood pressure reductions with exercise and sodium restriction in postmenopausal women with elevated systolic pressure: role of arterial stiffness. J Am Coll Cardiol. 2001 ;38(2):506-13.
- 22. Staffileno BA, Braun LT, Rosenson RS. The accumulative effects of physical activity in hypertensive post-menopausal women. J Cardiovasc Risk. 2001;8(5):283-90.
- 23. Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(16):1640-5.
- 24. Block G, Norkus E, Hudes M, Mandel S, Helzlsouer K. Which plasma antioxidants are most related to fruit and vegetable consumption? Am J Epidemiol. 2001;154(12):1113-8.
- 25. Karvonen MJ, Kentala E, Mustala O. The effects of training on heart rate; a longitudinal study. Ann Med Exp Biol Fenn. 1957;35(3):307-15.
- 26. Eng J. Sample size estimation: how many individuals should be studied? Radiology. 2003; 227(2):309-13.
- 27. MacDonald JR. Potential causes, mechanisms, and implications of post exercise hypotension. J Hum Hypertens. 2002;16(4):225-36.
- 28. Jessup JV, Lowenthal DT, Pollock ML, Turner T. The effects of endurance exercise training on ambulatory blood pressure in normotensive older adults. Geriatr Nephrol Urol. 1998;8(2):103-9.
- 29. Moreira WD, Fuchs FD, Ribeiro JP, Appel LJ. The effects of two aerobic training intensities on ambulatory blood pressure in hypertensive patients: results of a randomized trial. J Clin Epidemiol. 1999;52(7):637-42.
- 30. Pinto A, Di Raimondo D, Tuttolomondo A, Fernandez P, Arnao V, Licata G. Twenty-four hour ambulatory blood pressure monitoring to evaluate effects on blood pressure of physical activity in hypertensive patients. Clin J Sport Med. 2006;16(3):238-43.
- 31. Figueroa A, Park SY, Seo DY, Sanchez-Gonzalez MA, Baek YH. Combined resistance and endurance exercise training improves arterial stiffness, blood pressure, and muscle strength in postmenopausal women. Menopause. 2011;18(9):980-4.
- 32. Cardoso CG Jr., Rosas FC, Oneda B, Labes E, Tinucci T, Abrahao SB, et al. Aerobic training abolishes ambulatory blood pressure increase induced by estrogen therapy: a double blind randomized clinical trial. Maturitas. 2011; 69(2):189-94.
- 33. Mujica V, Urzua A, Leiva E, Diaz N, Moore-Carrasco R, Vasquez M, et al. Intervention with education and exercise reverses the metabolic syndrome in adults. J Am Soc Hypertens. 2010; 4(3):148-53.
- 34. Jou HJ, Hsu IP, Huang HT, Liu IL, Chien PL, Li IC, et al. A hospital-based therapeutic lifestyle program for women with metabolic syndrome. Taiwan J Obstet Gynecol. 2010;49(4):432-7.
- 35. American College of Sports Medicine Position Stand. The recommended quantity and quality of exercise for developing and maintaining cardiorespiratory and muscular fitness, and flexibility in healthy adults. Med Sci Sports Exerc. 1998; 30(6):975-91.
- 36. Sociedade Brasileira de Cardiologia. Sociedade Brasileira de Hipertensão. Sociedade Brasileira de Nefrologia. Diretrizes brasileiras de hipertensão. Arq Bras Cardiol.2010;95(1 supl.):1-51.
- 37. Kenney MJ, Seals DR. Postexercise hypotension. Key features, mechanisms, and clinical significance. Hypertension. 1993;22(5):653-64.
- 38. Harvey PJ, Morris BL, Kubo T, Picton PE, Su WS, Notarius CF, et al. Hemodynamic after-effects of acute dynamic exercise in sedentary normotensive postmenopausal women. J Hypertens. 2005;23(2):285-92.
- 39. Rodriguez D, Silva V, Prestes J, Rica RL, Serra AJ, Bocalini DS, et al. Hypotensive response after water-walking and land-walking exercise sessions in healthy trained and untrained women. Int J Gen Med. 2011;4:549-54.
- 40. Senitko AN, Charkoudian N, Halliwill JR. Influence of endurance exercise training status and gender on postexercise hypotension. J Appl Physiol. 2002;92(6):2368-74.
Publication Dates
-
Publication in this collection
18 Oct 2012 -
Date of issue
Nov 2012
History
-
Received
16 Nov 2011 -
Accepted
28 May 2012 -
Reviewed
16 Nov 2011