Athletes; cardiomyopathy, dilated; exercise; running; athletic performance
CASE REPORT
IInstituto do Coração da Faculdade de Medicina da Universidade de São Paulo, São Paulo, SP - Brazil
IIHospital Israelita Albert Einstein, São Paulo, SP - Brazil
IIIEscola de Educação Física e Esporte da Universidade de São Paulo, São Paulo, SP - Brazil
Mailing address
Keywords: Athletes; cardiomyopathy, dilated; exercise; running; athletic performance.
Case report
This case report was approved by the CAPPesq-HC and the patient authorized its publication.
A 55-year-old amateur runner was referred to the Sports Cardiology Ambulatory for cardiovascular screening and aerobic performance improvement. In the last 10 years he had trained moderate (between 74-90% of maximal heart rate) and vigorous (above 90% of maximal heart rate) aerobic running from 10 to 30 km/day, 6 days/week. Indeed, he was engaged in running competitions once a month and ran 10 km in 42 minutes. The athlete had no symptoms of cardiovascular disorders, but presented family history of premature sudden death (mother and father).
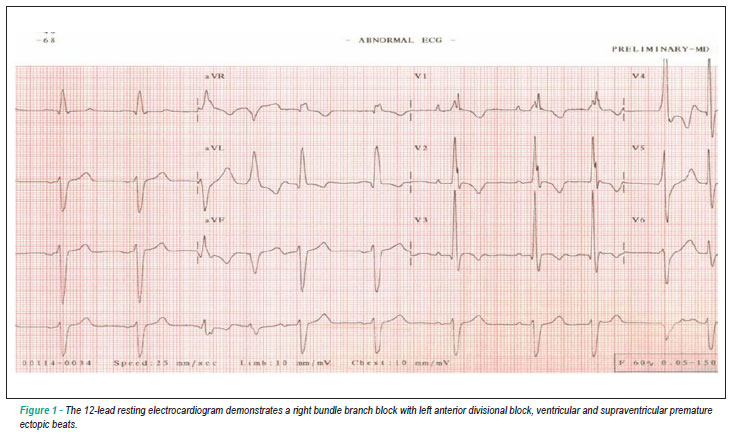
The 12-lead resting ECG revealed sinus rhythm, right bundle branch block with left anterior divisional block associated with ventricular and supraventricular premature ectopic beats (Fig. 1). Echocardiography showed moderated ventricular dilatation with increased right and left ventricular internal dimensions (50 mm and 60 mm, respectively), and reduced left ventricular ejection fraction (LVEF, 30%). The cardiopulmonary exercise test on treadmill (ramp protocol) showed a peak oxygen uptake (peak VO2) of 42 ml.kg-1.min-1 (125% of peak VO2 predicted for his age)1, maximal heart rate 147bpm (90% of maximal predicted heart rate), maximal oxygen pulse 14.8 ml/beat, anaerobic threshold at 59% peak VO2, respiratory compensation point at 85% peak VO2 and 7.8 mph with 9% inclination at the peak of exercise.
Both ECG alterations and the fact that the athlete was born in Minas Gerais, an endemic region for Chagas' disease in Brazil, led us to request a blood serologic test. The blood test confirmed Chagas' disease as the etiology for the diagnosis of the myocardiopathy. A further examination revealed a high degree of ventricular ectopic beats (242 beats per hour) and non-sustained polymorphic ventricular tachycardia during the 24-hour Holter monitoring. A clinical flowchart is shown in figure 2. Autonomic studies by microneurography2 showed sympathetic outflow comparable to the one found in age-paired healthy individuals (20 bursts/min). Similarly, forearm blood flow and forearm vascular conductance responses to handgrip exercise evaluated by plethysmography were not different from the responses observed in our laboratory in healthy individuals (1.72 - 2.57 ml.min-1.100 ml tissue-1 and 1.96 - 2.68 Units, respectively). Noncardiac manifestations of Chagas disease were not present in this patient.
Based on the clinical evaluation, the athlete was disqualified for competition in accordance to international recommendations3. Pharmacological therapy was started (ACE-inhibitor - 40 mg per day, beta-blocker - 12.5 mg per day and diuretic - 25 mg per day) and moderate aerobic exercise training (walking/running with heart rate kept between anaerobic threshold and respiratory compensation point, 4 days/week, 10 km/day) was recommended. After 6 months of follow-up, the athlete reported a syncope episode not related to exercise and a electrophysiological study was performed. This examination revealed sustained and non-tolerated monomorphic ventricular tachycardia. To prevent sudden death, an implantable cardioverter defibrillator (ICD) was implanted. During this follow up, the peak VO2 reduced to 31 ml.kg-1.min-1 (98% of peak VO2 predicted for his age)1, but he remained asymptomatic and no shocks were reported during exercise training sessions. In the last follow-up, the peak VO2 was significantly reduced to 19.9 ml.kg-1.min-1, (63% of the predicted for his age), which was associated with inadequate pacemaker function related to reduced ventricular sensitivity and supraventricular arrhythmia. The mode of stimulation of pacemaker was changed to VVI mode and the demand frequency reduced by 40ppm, which resulted in the immediate improvement of his functional capacity.
Discussion
Dilated cardiomyopathy is characterized by left ventricular dilatation and impaired systolic function. The present patient presented two risk factors for the development of dilated cardiomyopathy, family history (father and mother who died of sudden death before 40 years old) and infection by Chagas disease. Chagas disease is a parasitic disease transmitted to humans through the feces of infected blood-sucking insects - a protozoan called Trypanosoma cruzi. The World Health Organization4 indicates that 18 million persons are chronically infected with Trypanosoma cruzi (T cruzi) and 200.000 new cases occur each year. Cardiac involvement is the most frequent and serious manifestation of chronic Chagas' disease, typically producing atrial and ventricular arrhythmias, cardiac failure and thromboembolic phenomena. The clinical course of Chagas' heart disease is varied and the identification of patients who are at risk of dying remains a challenge.
Although dilated cardiomyopathy represents only 2% of all causes of arrhythmic sudden death in young/adults engaged in sports activities5, its diagnosis in athletes has important clinical implications. Athletes with dilated cardiomyopathy can be completely asymptomatic, which significantly increases the risk of sudden death during intense exercise. This case provides some very interesting findings. Despite the presence of dilated cardiomyopathy, our athlete had a remarkable functional capacity, which strongly suggests that his exercise capacity was greatly dependent on peripheral adaptation. This finding is consistent with previous studies that showed that exercise training improves exercise tolerance in patients with moderate or severe left ventricular dysfunction6,7, improving oxygen extraction and utilization, muscle oxidative enzymes8 and changes in oxidative muscle fibers composition9.
Another remarkable finding was the normal neurovascular control in the reported case. Despite the left ventricular dysfunction, the muscle sympathetic nerve activity and the response of muscle blood flow during handgrip exercise were within the normal range. Previous studies have consistently shown that both the levels of sympathetic outflow and muscle blood flow are directly associated with the severity of left ventricular dysfunction. Why was this association not seen in the present patient? The answer to this question is out of the scope of the present case report, but it is legitimate to attribute the normal neurovascular control to the high level of exercise training in our patient. In fact, previous investigations clearly demonstrated that exercise training dramatically reduced muscle sympathetic nerve activity and significantly improved muscle blood flow in patients with left ventricular dysfunction7. It is possible that the attenuation in vasoconstriction, and hence, the preserved response of muscle blood flow during exercise contributed to the high level of peak VO2 in our patient.
It is also important to stress the necessity of accompanying the parameters of ICD in patients with dilated cardiomyopathy performing regular exercise training. We observed that a simple inappropriate adjustment of the pacemaker parameters was sufficient to result in clinical and functional capacity worsening. In this case, the cardiopulmonary test was fundamental to dictate the best adjustment of the parameters. The interval time of clinical monitoring of dilated cardiomyopathy patients is essential and should be considered individually. The evaluation of our patient was done every 3 months.
In fact, few data are available about the actual safety in sports participation for patients with ICD. As this population expands, clinicians are being asked about what sports activities are allowed for these patients. Although the theoretical risks include an increased frequency of arrhythmias with exercise, an increase in defibrillation threshold leading to device therapy failure, inappropriate shocks secondary to sinus tachycardia due to damage in device and lead systems, Lampert et al10 demonstrated that only 10% of physicians recommended avoidance of all activities more strenuous than golf or bowling. Our athlete, similarly, did not have any complications during his training, never received inappropriate shocks, or presented damage to the device or lead systems.
This case report strengthens that high exercise performance and the absence of symptoms does not exclude the presence of cardiomyopathy. Moreover, it affirms that accompanying patients with ICD is essential, mainly those performing regular exercise training.
Potential Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Sources of Funding
There were no external funding sources for this study.
Study Association
This study is not associated with any post-graduation program.
References
- 1. Morris CK, Myers J, Froelicher VF, Kawaguchi T, Ueshima K, Hideg A. Nomogram based on metabolic equivalents and age for assessing aerobic exercise capacity in men. J Am Coll Cardiol. 1993; 22 (1): 175-82.
- 2. Negrão CE, Rondon MUPB, Tinucci T, Alves MJN, Roveda F, Braga AMW, et al. Abnormal neurovascular control during exercise is linked to heart failure severity, Am J Physiol. 2001; 280 (3): H1286-H1292.
- 3. Maron BJ, Mitchell JH. 36th Bethesda Conference: Recommendations for Determining Eligibility for Competition in Athletes with Cardiovascular Abnormalities. J Am Coll Cardiol. 2005; 5 (8): 1322-75.
-
4World Health Organization. The World Health Report 2002. Annexes and Tables. Geneva: World Health Organization, 2002:192.
- 5. Bille K, Figueiras D, Schamasch P, Kappenberger L, Brenner JI, Meijboom FJ, et al. Sudden cardiac death in athletes: The Lausanne Recommendations. Eur J of Cardiovasc Prev Rehabil. 2006, 13 (6): 859-75.
- 6. Duscha BD, Schulze PC, Robbins JL, Forman DE. Implications of chronic heart failure on peripheral vasculature and skeletal muscle before and after exercise training. Heart Fail Rev. 2008; 13 (1): 21-37.
- 7. Roveda F, Middlekauff HR, Rondon MU, Reis SF, Souza M, Nastari L, et al. The effects of exercise training on sympathetic neural activation in advanced heart failure: a randomized controlled trial. J Am Coll Cardiol. 2003; 42 (5): 854-60.
- 8. Haykowsky MJ, Ezckowitz JA, Armstrong PW. Therapeutic exercise for individuals with heart failure: special attention to older women with heart failure. J Card Fail. 2004; 10 (2): 165-73.
- 9. Rusko H, Rahkila P, Karvinen E. Anaerobic threshold, skeletal muscle enzymes and fiber composition in young female cross-country skiers. Acta Physiol Scand. 1980; 108 (3): 263-8.
- 10. Lampert R, Cannom D, Olshansky B. Safety of sports participation in patients with implantable cardioverter defibrillators: a survey of heart rhythm society members. J Cardiovasc Electrophysiol. 2006; 17 (1): 11-5.
Long distance runner with dilated cardiomyopathy and excellent performance
Publication Dates
-
Publication in this collection
04 Feb 2011 -
Date of issue
Jan 2011
History
-
Accepted
01 Sept 2009 -
Reviewed
24 Aug 2009 -
Received
20 Mar 2009