BACKGROUND: Skin tags are dermatological lesions commonly found in the general population and have been associated with diabetes mellitus, obesity, insulin resistance and atherosclerosis. Early detection of patients with insulin resistance may play an important preventive role. OBJECTIVE: To evaluate the association between skin tags in the neck or axillary regions and insulin resistance. METHODS: A cross-sectional study involving adult patients receiving care at a university teaching hospital. Cases were defined as patients with > 5 skin tags in the neck region and/or axillae. Insulin resistance was estimated using the HOMA-IR index. Results were adjusted for the other known covariates of risk for insulin resistance using a multiple logistic regression model. RESULTS: Ninety-eight cases and 103 controls were evaluated. There was no difference between the groups with respect to age or gender. Skin tags were directly associated with HOMA-IR values (odds ratio = 1.4), hypertriglyceridemia and body mass index, irrespective of adjustment for diabetes mellitus, age, skin phototype, gender, family history of diabetes mellitus or hip/waist ratio. Qualitatively elevated HOMA-IR levels (>3.8) were also significantly associated (odds ratio = 7.5). CONCLUSIONS: The presence of multiple skin tags was strongly associated with insulin resistance irrespective of other risk factors.
FUNDAMENTOS: Acrocórdons são lesões dermatológicas comuns na população e estão associados ao diabetes mellitus, à obesidade, à resistência insulínica e à aterosclerose. A identificação precoce de pacientes com resistência insulínica pode ter papel preventivo primário. OBJETIVO: Avaliar a associação entre presença de acrocórdons cervicais ou axilares e resistência insulínica. MÉTODOS: Estudo transversal com pacientes dermatológicos adultos atendidos em hospital universitário. Casos foram definidos como portadores de mais de cinco acrocórdons cervicais e/ou axilares. A resistência insulínica foi estimada pelo índice HOMA-IR. Resultados foram ajustados pelas demais covariáveis de risco para resistência insulínica conhecidos, a partir de regressão logística múltipla. RESULTADOS: Avaliaram-se 98 casos e 103 controles, que não diferiram entre si quanto à idade ou ao gênero. Acrocórdons se associaram diretamente aos valores de HOMA-IR (Odds Ratio = 1,4), hipertrigliceridemia e índice de massa corpórea, independentemente do ajuste por diabetes mellitus, idade, fototipo, gênero, história de diabetes mellitus familiar e relação cintura/quadril. Níveis qualitativamente elevados de HOMA-IR (> 3,8) também evidenciaram associação significativa (Índice de probabilidade = 7,5). CONCLUSÕES: Presença de múltiplos acrocórdons se associou à resistência insulínica, independentemente dos demais fatores de risco.
Diabetes Mellitus; Estudos transversais; Obesidade; Resistência à insulina
INVESTIGATION
IDermatologist, Masters student in Pathology, Botucatu School of Medicine, Paulista State University (UNESP), Botucatu, SP, Brazil
IINutritionist, Botucatu (SP), Brazil
IIIMedical Resident, Department of Dermatology and Radiotherapy, Botucatu School of Medicine, Paulista State University (UNESP), Botucatu, SP, Brazil
IVDermatologist, Department of Dermatology and Radiotherapy, Botucatu School of Medicine, Paulista State University (UNESP), Botucatu, SP, Brazil
VAssistant Professor, PhD, Department of Dermatology and Radiotherapy, Botucatu School of Medicine, Paulista State University (UNESP), Botucatu, SP, Brazil
Mailing Address
ABSTRACT
BACKGROUND: Skin tags are dermatological lesions commonly found in the general population and have been associated with diabetes mellitus, obesity, insulin resistance and atherosclerosis. Early detection of patients with insulin resistance may play an important preventive role.
OBJECTIVE: To evaluate the association between skin tags in the neck or axillary regions and insulin resistance.
METHODS: A cross-sectional study involving adult patients receiving care at a university teaching hospital. Cases were defined as patients with > 5 skin tags in the neck region and/or axillae. Insulin resistance was estimated using the HOMA-IR index. Results were adjusted for the other known covariates of risk for insulin resistance using a multiple logistic regression model.
RESULTS: Ninety-eight cases and 103 controls were evaluated. There was no difference between the groups with respect to age or gender. Skin tags were directly associated with HOMA-IR values (odds ratio = 1.4), hypertriglyceridemia and body mass index, irrespective of adjustment for diabetes mellitus, age, skin phototype, gender, family history of diabetes mellitus or hip/waist ratio. Qualitatively elevated HOMA-IR levels (>3.8) were also significantly associated (odds ratio = 7.5).
CONCLUSIONS: The presence of multiple skin tags was strongly associated with insulin resistance irrespective of other risk factors.
Keywords: Cross-sectional studies; Diabetes Mellitus; Insulin resistance; Obesity;
INTRODUCTION
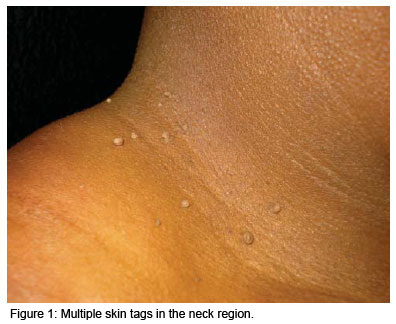
Skin tags, also known as acrochordons, are the most common fibroepithelial skin tumor. They consist of acquired benign polyps that grow in the natural folds of the skin such as the neck, axillae, inguinal, thigh, perineal and inframammary regions, in the eyelids and in the intergluteal folds.1,2 They are pedunculated, soft papules that protrude from the skin's surface (Figure 1), may present singly or as multiple lesions and may vary in size from 2 to 10 mm. They tend to grow progressively in size and do not involute spontaneously. They may be normochromic or hyperchromic and, although asymptomatic, constitute the subject of frequent complaints in dermatology clinics for esthetic reasons or due to traumatism resulting from contact with clothing or jewelry.1-3 Depending on the geographical region of the country, statistics in 2006 showed that 0.9 to 1.2% of all diagnoses in dermatology consultations in Brazil concerned skin tags.4 These lesions are extremely common in the adult population over 40 years of age (46%), and increase in incidence in the elderly, reaching 59% at 70 years of age.3 There is a familial component; however, the genetic segregation pattern and ethnic characteristics have yet to be defined. There is no difference in incidence between males and females. Skin tags have been associated with pregnancy, acromegaly, symptomatic intestinal polyps, dyslipidemia, obesity, diabetes mellitus (DM), atherosclerosis and various syndromes including polycystic ovary syndrome, Birt-Hogg-Dubé Syndrome and Cowden syndrome. HPV (human papillomavirus) DNA (desoxyribonucleic acid) has been found in 88% of acrochordon lesions.5-9 Variations in estrogen levels and trophic hormones such as IGF-1 (insulin-like growth factor-1), insulin, TGF (transforming growth factor- ) and epidermal growth factor (EGF) are involved in its genesis and development.8-10
Peripheral insulin resistance (IR) is a systemic metabolic abnormality that exerts a significant effect on the overall mortality of the general population by increasing propensity to cardiovascular events and certain malignant neoplasias. IR is the central element in the metabolic syndrome (syndrome X), which affects 20-30% of the population in industrialized countries and is associated with risk factors for atherosclerosis such as type II DM, systemic arterial hypertension, abdominal obesity and dyslipidemia. Furthermore, IR, together with smoking, alcoholism and traffic accidents, represents one of the most significant modifiable factors in promoting health and reducing mortality in the general population.11-13
Bearing in mind that IR develops prior to the appearance of associated diseases, at which time they are still impossible to detect, the early identification and treatment of these patients may play an important role in primary prevention, encouraging lifestyle changes and permitting specific treatments to be implemented.
Various skin conditions are associated with IR including pseudoacanthosis nigricans, hirsutism, acne, hidradenitis suppurativa, oiliness, alopecia, papulosis of the fingers and skin tags.13-15
IR is detectable using an oral glucose tolerance test, glucose curve or indexes such as the homeostatic model assessment of insulin resistance (HOMA-IR) and Quick indexes, which compare basal insulin production and fasting glucose. In addition, IR may also be suspected from phenotypical characteristics such as body mass index (BMI) and waist/hip ratio. The HOMA-IR index is widely used in clinical tests and is based on the product of fasting glucose and basal insulin.16,17
Investigation of the association between multiple skin tags and IR has yet to be studied in the Brazilian population and was the objective of the present trial.
METHODS
A cross-sectional study was conducted involving 201 adult patients attending the dermatology outpatient clinic of the University Teaching Hospital, Botucatu School of Medicine (UNESP) between February 2008 and February 2009. All patients were informed with respect to the nature of the study and signed an informed consent form prior to enrollment.
Cases were defined as patients with > 5 skin tags in the neck region or axillae, while the other dermatology patients, who had no lesions at the time of examination and no prior history of having had skin tags removed, were invited to form a control group. Patients were excluded from the study if they reported acromegaly, pheochromocytoma, hemochromatosis, Cushing's syndrome or glucagonoma.
All the patients were interviewed and examined individually and various anthropometric measurements were taken by the same investigator in all cases. The laboratory tests (fasting glucose and basal insulin) were all performed in the same laboratory.
The dependent variable in this study was the presence of skin tags in the neck region or axillae, and the principal independent variable was the HOMA-IR index.
The association between the groups was initially evaluated using a bivariate model and then adjusted for the following covariates: age, gender, skin phototype, waist/hip ratio, body mass index, presence of pseudoacanthosis nigricans, DM, familial DM, hypercholesterolemia, hypertriglyceridemia, glycemia, basal insulin and HOMA-IR, using a conditional multiple logistic regression model. Patients were considered to have DM, hypercholesterolemia and hypertriglyceridemia if they reported having been diagnosed with these conditions or presented previous or current fasting tests performed at the institution's own laboratory, with results indicating glucose levels > 126 mg/dl and total cholesterol and triglyceride levels > 200 mg/dl, the normal reference values at this laboratory.
The frequencies of the categorical variables were compared using the chi-square test (for trend, if appropriate) and Fisher's exact test. Continuous variables were described as means or medians and compared using Student's t-test, the Mann-Whitney test or the Kruskal-Wallis test. The normality of distribution was calculated using the Shapiro-Wilk test. Construction of the multivariate logistic model was based on the sequential inclusion algorithm (stepwise forward) of covariates that could affect probability, with significance established as < 0.3 at each step. Calculation of the association between the variables was based on the odds ratios (OR) and their respective 95% confidence intervals (95%CI).
Calculation of sample size was based on a preliminary test conducted with 50 cases and 50 controls, confirming a power of 0.9 and alpha level of 0.01 for the bivariate comparison of the proportions of abnormal HOMA-IR levels. This number was then increased to include a further five cases for each covariate adjustment in the final logistic model.
Data were analyzed and tables constructed using the MS Excel 2003 and SPSS 17 software programs.18 Two-tailed significance level was defined as 0.05.
RESULTS
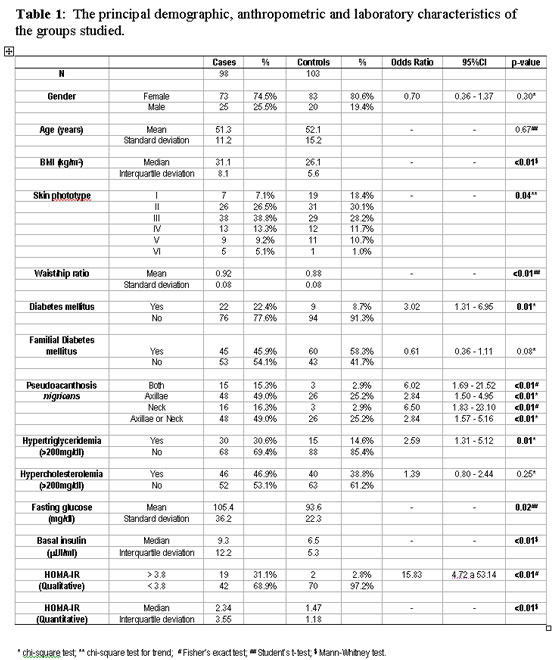
A total of 98 cases and 103 controls were interviewed. No patient refused to participate in the study; nevertheless, only 133 patients (66.2%) provided samples for the laboratory tests. There were no statistically significant differences between the patients who failed to undergo laboratory testing and those who underwent testing with respect to the group to which they were allocated (case or controls), gender, age or BMI; therefore, this factor had no effect on the internal validity of the study. Moreover, the 61 cases and 72 controls who underwent laboratory testing fulfilled all the previously established requisites. Table 1 shows the frequency of the variables and the bivariate analysis between cases and controls.
Skin tags were present in the neck region in 85. 7% of cases and in the axillae in 62.2%, while in 28.6% of patients these lesions were located at other sites. In 53.1% of cases, lesions were found both in the neck region and in the axillae.
There was a predominance of women in both the study and control groups, and the age of these patients ranged from 30 to 70 years. There was a prevalence of lighter skin phototypes in the control group compared to the study group, indicating a need for later adjustment in the multivariate analysis.
The presence of skin tags was primarily and directly associated with BMI, waist/hip ratio, DM, hypertriglyceridemia, the presence of pseudoacanthosis nigricans, glucose and insulin levels and HOMA-IR (quantitative and qualitative). The most common form of pseudoacanthosis nigricans identified in both groups was the axillary form; nevertheless, when the neck region was affected, this was more strongly associated with the presence of skin tags.
Multivariate analysis corroborated the finding of higher levels of HOMA-IR in the case group, an association that persisted irrespective of adjustment for the covariates (Table 2). The association between skin tags and BMI, hypertriglyceridemia and a family history of DM remained significant.
When only the qualitative HOMA-IR value (> 3.8) was taken into consideration, an increase was found in 31.1% of cases and in 2.8% of controls, emphasizing the positive association between these variables. The odds ratio adjusted for age, skin phototype, gender, DM, BMI, waist/hip ratio, familial DM and hypertriglyceridemia was 7.5 (95%CI: 1.38-40.9; p < 0.02).
Comparative analysis of the patients with skin tags in the neck region, in the axillae or in both regions showed no statistically significant differences with respect to HOMA-IR values (p>0.3).
DISCUSSION
An independent association was found between the presence of more than five skin tags and an increase of 1.4 units in the HOMA-IR index in dermatology patients. The significance of the association found in this study between BMI and hypertriglyceridemia reinforces the concept that skin tags may constitute a marker for IR.
Male gender and age are other known risk factors that were not confirmed in this sample, while the finding that a family history of DM was more common among controls was contradictory to reports from previous studies. These issues need to be investigated further in future studies and the possibility of a memory bias should be taken into consideration.
The metabolic syndrome is a systemic abnormality that results in various trophic alterations in the organism. In a hospital-based study involving 118 patients with skin tags, IR or DM was identified in 40.6% of cases.19 In the present study, conducted in a normal outpatient population, the general prevalence of elevated HOMA-IR levels was low, albeit with a tendency towards higher values among cases.
In agreement with the findings of the present study, a greater association with insulinemia than with fasting glucose was also reported by Norris et al.,20 reinforcing the argument that skin tags, together with pseudoacanthosis nigricans, may represent a marker for the identification of IR prior to the manifestation of diseases resulting from the hypermetabolic syndrome, since only 15% of patients with IR have no skin manifestations.9
Insulin is a hormone that promotes tissue growth and stimulates glucose uptake in the tissues at an intensity that varies from one individual to another. When IR is present, the cells are less responsive to the effect of this hormone. To compensate, the pancreas begins to produce greater quantities of insulin.1,9,21 This hyperinsulinism promotes an increase in IGF-1 and a reduction in insulin-like growth factor-binding protein 3 (IGFBP-3), one of the ligands to retinoid X receptor ? (RXR?), responsible for antiproliferative gene transcription. Both the hyperinsulinism and the increase in IGF-1 directly induce epithelial and fibroblastic growth by activating receptors, possibly explaining the prevalence of skin tags and pseudoacanthosis nigricans in these groups.1
Barghava and Mathur reported an association between multiple skin tags, pseudoacanthosis nigricans, seborrheic keratoses, obesity and glucose intolerance, suggesting that it may represent a syndrome that results from tissue growth factor secretion.22,23
The high prevalence of skin tags in acromegaly emphasizes the importance of growth factors in the genesis and development of these lesions, since these patients express higher levels of insulin, growth hormone (GH) and IGF-1. 24
More intense EGF receptor staining was also found in the epithelium of skin tags in patients with dysplastic nevus syndrome and it has been speculated that this tissue expression may also be induced by other circulating tissue growth factors in addition to insulin and IGF-1. Nevertheless, levels of growth factors such as GH have not yet been investigated in healthy patients with skin tags or in patients with dysplastic nevus syndrome.25
The color of the skin tags has not been associated with any particular clinical characteristic and probably reflects aspects related to constitutional pigmentation.1,9 In one study carried out in India22 and in another conducted in Israel 26, the majority of skin tags were reported as being hyperchromic, a feature not reported in studies carried out in the West.
In agreement with the findings of the present study, other authors have also reported an association between skin tags and cardiovascular risk factors such as dyslipidemia and obesity, indicating a greater risk of atherosclerotic disease.22,27 Other alterations related to IR include hyperuricemia, increased levels of plasminogen activator inhibitor-1, increased IGF-1 and a greater quantity of small LDL particles, factors that are also associated with coronary atherosclerosis. 12,28 Various dermatological findings have been related to atherosclerosis; therefore, skin tags should also be investigated, since a confirmed association may collaborate towards stratifying risks in the general population.29
Other studies that have evaluated patients with skin tags have reported altered glucose metabolism in 28-81% of patients, depending on the population evaluated, which is in agreement with the 41% found in the current sample.7,19,22,27
The total number of skin tags in an individual has been correlated by some authors with fasting glucose levels and BMI; however, these correlations remain under debate.1,7,9,20,26,27
The topography of the lesions is not predictive of abnormal carbohydrate metabolism, as shown in the present study; nevertheless, women had more inframammary lesions than men, no doubt as a result of the prominence of the female breasts.1,7,9,20,26,27
In another study9, multiple skin tags were found to be predictive of IR, with a sensitivity that was greater than when the presence of pseudoacanthosis nigricans is used. The design of the present study did not permit this comparison to be made with respect to IR, since the presence of more than five skin tags constituted the main criterion for the selection of the patients (dependent variable); nevertheless, skin tags were shown to be markers of IR irrespective of the presence of pseudoacanthosis nigricans.
Adult patients with skin tags should be alerted to the risk of developing IR, hypertriglyceridemia, overweight and possibly DM and associated cardiovascular complications such as acute myocardial infarction, cerebrovascular disease, peripheral arterial disease, erectile dysfunction, cognitive decline, fatty liver and renovascular disease. Moreover, IR has been associated with the development of malignant neoplasias such as adenocarcinoma of the bowel, breast, endometrium, kidney, esophagus and prostate, which may be explained by the increased levels of tissue growth factors in these patients.11,30 Nonetheless, no studies have yet compared the presence of skin tags and these specific outcomes.
The use of medication, the presence of chronic diseases, physical activity, diet and nutritional status are factors that may directly affect the determination of IR according to the HOMA-IR index, and these cannot be adequately controlled in cross-sectional studies. On the other hand, the magnitude of the association between IR and skin tags found in this study and corroborated in the literature, which persisted even following adjustment for the confounding variables, and the identification in the case group of other markers of IR such as BMI, DM and abdominal obesity, make it unlikely that this association would have occurred or would have been strongly affected by these biases.
Another point that should be emphasized is that the reference values for HOMA-IR do not constitute a consensus in the literature, but vary from 2.7 to 8.2 in accordance with ethnic characteristics, age and BMI. The present data were tested for different cut-off points and were found to be significant at all levels. In the laboratory of this institution, a limit of 3.8 is adopted, which was also used for this study. On the other hand, the identification of an association between skin tags and quantitative HOMA-IR levels, adjusted for age and BMI, minimize the bias resulting from the adoption of specific cut-off points.
There is a scarcity of population-based studies characterizing skin tags, their clinical and laboratory features, natural history, prognostic factors and their possible ethnic and cultural idiosyncrasies. Their association with IR, in addition to corroborating previous data on the physiopathogenesis of the disease, should encourage other investigators to conduct further studies with larger sample sizes to evaluate other variables with the objective of reaching a more precise estimation of risk in the various segments of the population. Prospective studies would also allow general and specific mortality indexes to be measured. Likewise, it is important to investigate the significance of skin tags in the different areas of the body, to assess their differences in number and color, and to evaluate the effect of therapies for IR (pharmacological or otherwise) in their genesis.
CONCLUSION
In dermatological outpatients, the presence of multiple skin tags was associated with insulin resistance, overweight and hypertriglyceridemia, irrespective of the presence of other known risk factors.
REFERENCES
References
- 1. Rasi A, Soltani-Arabshahi R, Shahbazi N. Skin tag as a cutaneous marker for impaired carbohydrate metabolism: a case-control study. Int J Dermatol. 2007;46:1155-9.
- 2. Pariser RJ. Benign neoplasms of the skin. Med Clin North Am. 1998;82:1285-307.
- 3. Banik R, Lubach D. Skin tags: localization and frequencies according to sex and age. Dermatologica. 1987;174:180-3.
- 4. Sociedade Brasileira de Dermatologia. Perfil nosológico das consultas dermatológicas no Brasil. An Bras Dermatol. 2006;81:549-58.
- 5. Dianzani C, Calvieri S, Pierangeli A, Imperi M, Bucci M, Degener AM. The detection of human papillomavirus DNA in skin tags. Br J Dermatol. 1998;138:649-51.
- 6. Erdo¤an BS, Aktan S, Rota S, Ergin S, Evliyao¤lu D. Skin tags and atherosclerotic risk factors. J Dermatol. 2005;32:371-5.
- 7. Margolis J, Margolis LS. Skin tags - a frequent sign of diabetes mellitus. N Engl J Med. 1976;294:1184.
- 8. Mathur SK, Bhargava P. Insulin resistance and skin tags. Dermatology. 1997;195:184.
- 9. Sudy E, Urbina F, Maliqueo M, Sir T. Screening of glucose/insulin metabolic alterations in men with multiple skin tags on the neck. J Dtsch Dermatol Ges. 2008;6:852-5.
- 10. Piette AM, Meduri B, Fritsch J, Fermanian J, Piette JC, Chapman A. Do skin tags constitute a marker for colonic polyps? A prospective study of 100 asymptomatic patients and metaanalysis of the literature. Gastroenterology. 1988;95:1127-9.
- 11. Carvalheira JBC, Saad MJA. Doenças Associadas à Resistência à Insulina / Hiperinsulinemia, não Incluídas na Síndrome Metabólica. Arq Bras Endocrinol Metab. 2006;50:360-7.
- 12. Bonora E, Formentini G, Calcaterra F, Lombardi S, Marini F, Zenari L et al. HOMA-estimated insulin resistance is an independent predictor of ardiovascular disease in type 2 diabetic subjects: prospective data from the Verona Diabetes Complications Study. Diabetes Care. 2002;25:1135-41.
- 13. Garcia Hidalgo L. Dermatological complications of obesity. Am J Clin Dermatol. 2002;3:497-506.
- 14. Kede MPV, Figueira AL, Porto JA. Manifestações cutâneas no diabetes mellitus. An Bras Dermatol. 1993;68:21-4.
- 15. Levine N. Brown patches, skin tags on axilla. Are this patient's velvety plaques related to his obesity and diabetes? Geriatrics. 1996;51:27.
- 16. Geloneze B, Vasquez AC, Stabe CF, Pareja JC, Rosado LE, Queiroz EC et al. HOMA1-IR and HOMA2-IR indexes in identifying insulin resistance and metabolic syndrome - Brazilian Metabolic Syndrome Study (BRAMS) Arq Bras Endocrinol Metab. 2009;53:281-7.
- 17. McAuley KA, Williams SM, Mann JI, Walker RJ, Lewis-Barned NJ, Temple LA et al. Diagnosing insulin resistance in the general population. Diabetes Care. 200;24:460-4.
- 18 SPSS.com [homepage]. Statistical Package for Social Science (SPSS). Release Version 17.0.1. Chicago (IL): SPSS Incorporation; 2008. [cited 2009 Jul 10]. Available from: http://www.spss.com
- 19. Agarwal JK, Nigam PK. Acrochordon: a cutaneous sign of carbohydrate intolerance. Australas J Dermatol. 1987;28:132-3.
- 20. Norris PG, McFadden J, Gale E, Griffiths WA. Skin tags are more closely related to fasting insulin than fasting glucose levels. Acta Derm Venereol. 1988;68:367-8.
- 21. Hermanns-Lê T, Scheen A, Piérard GE. Acanthosis nigricans associated with insulin resistance: pathophysiology and management. Am J Clin Dermatol. 2004;5:199-203.
- 22. Bhargava P, Mathur D. Acrochordon, diabetes and associations. Indian J Dermatol Venereol Leprol. 1996;62:226-8.
- 23. Ellis DL, Kafka SP, Chow JC, Nanney LB, Inman WH, McCadden ME et al. Melanoma growth factors, acanthosis nigricans, the sign of Leser-Trelat and multiple acrochordons. N Engl J Med. 1987;317:1582-7.
- 24. Ben-Shlomo A, Melmed S. Skin manifestations in acromegaly. Clin Dermatol. 2006;24:256-9.
- 25. Ellis DL, Nanney LB, King LE Jr. Increased epidermal growth factor receptors in seborrheic keratoses and acrochordons of patients with the dysplastic nevus syndrome. J Am Acad Dermatol. 1990;23:1070-7.
- 26. Kahana M, Grossman E, Feinstein A, Ronnen M, Cohen M, Millet MS. Skin tags: a cutaneous marker for diabetes mellitus. Acta Derm Venereol. 1987;67:175-7.
- 27. Demir S, Demir Y. Acrochordon and impaired carbohydrate metabolism. Acta Diabetol. 2002;39:57-9.
- 28. Crook MA. Skin tags and the atherogenic lipid profile. J Clin Pathol. 2000;53:873-4.
- 29. Miot HA, Medeiros LM, Siqueira CRS, Cardoso LC, Gumieiro JH, Pandini Filho MA et al. Associação entre doença arterial coronariana e as pregas lobular diagonal e anterotragal em homens. An Bras Dermatol. 2006;81:29-33.
- 30. Hsing AW, Gao YT, Chua S JR, Deng J, Stanczyk FZ. Insulin resistance and prostate cancer risk. J Natl Cancer Inst. 2003;95:66-71.

 Association between skin tags and insulin resistance
Association between skin tags and insulin resistance