BACKGROUND: Melanoma incidence and mortality rates have increased over the past 30 years in the Caucasian population. In Brazil, data on non-capital cities are scarce, making epidemiological stu dies necessary. OBJECTIVES: To evaluate the incidence of and classify cutaneous melanomas in Blumenau from 1980 to 2009. METHOD: Data from 1002 histopathological examinations of individuals from Blumenau were collected, considering sex, age, primary site of involvement, histological type, level of invasion (Clark's level) and tumor thickness (Breslow's depth). The gross and adjusted coefficients of annual incidences were calculated based on the number of melanoma cases and the population estimated by the Brazilian Institute of Geography and Statistics (IBGE) between 1980 and 2009. RESULTS: The incidence rates of melanoma reached 22.4 cases per 100,000 inhabitants/year; 31.5 in women and 30.4 in men at the adjusted rate. The incidence rates standardized by decade, age and sex were 141 male and 103 female cases per 100,000/inhabitants aged 65 to 69 years. Superficial spreading melanoma occurred in 53% of the cases, followed by nodular melanoma (37%), and the primary site of involvement was the trunk (47%). 62.5% of the cases were diagnosed early, with Breslow < 1mm. CONCLUSION: The incidence of malignant melanoma has increased fivefold from 1980 to 2009 and early diagnosis has increased 151% as a result of primary prevention
FUNDAMENTOS: A incidência do melanoma e a mortalidade pela doença aumentaram nos últimos 30 anos na população caucasiana. No Brasil, dados em municípios não-capitais são escassos, necessitando de estudos epidemiológicos. OBJETIVOS: Avaliar a incidência e classificar melanomas cutâneos em Blumenau de 1980 a 2009. MÉTODO: Foram coletadas informações de 1.002 exames histopatológicos de indivíduos de Blumenau, considerando sexo, idade, localização primária, tipo histológico, nível de invasão (Clark) e espessura tumoral (Breslow). Os coeficientes de incidência anuais brutos e ajustados foram calculados utilizandose o número de melanomas e a população estimada pelo Instituto Brasileiro de Geografia e Estatística entre 1980 e 2009. RESULTADOS: As taxas de incidência do melanoma atingiram 22,4 casos/100.000 habitantes/ano, 31,5 nas mulheres e 30,4 nos homens na taxa ajustada. As taxas de incidência padronizadas por década, faixa etária e sexo atingiram 141 casos em homens e 103 no sexo feminino por 100.000 habitantes/ano entre 65 a 69 anos. O melanoma disseminativo superficial aconteceu em 53% dos casos, seguido do melanoma nodular com 37%, e a principal localização foi no tronco (47%). Os diagnósticos precoces atingiram 62,5% com Breslow < 1 mm. CONCLUSÃO: A incidência do melanoma maligno aumentou em cinco vezes entre 1980 e 2009 e o diagnóstico precoce aumentou 151% como resultado da prevenção primária.
Detecção precoce de câncer; Diagnóstico precoce; Epidemiologia; Melanoma; Morbidade
INVESTIGATION
Cutaneous melanoma - a 30-year-long epidemiological study conducted in a city in southern Brazil, from 1980-2009*
Nilton Naser
Ph.D. in Dermatology, Universidade Federal do Rio de Janeiro (Federal University of Rio de Janeiro - UFRJ) - Professor of dermatology, Fundação Universidade Regional de Blumenau (Regional University Foundation of Blumenau - FURB) - Blumenau (SC), Brazil
Mailing address Mailing address: Nilton Nasser Rua Curt Hering, 20 CEP 89010-030 Blumenau - SC, Brazil Phone: +55 47 3322 1246 E-mail: ninasser.bnu@terra.com.br
ABSTRACT
BACKGROUND: Melanoma incidence and mortality rates have increased over the past 30 years in the Caucasian population. In Brazil, data on non-capital cities are scarce, making epidemiological stu dies necessary.
OBJECTIVES: To evaluate the incidence of and classify cutaneous melanomas in Blumenau from 1980 to 2009. METHOD: Data from 1002 histopathological examinations of individuals from Blumenau were collected, considering sex, age, primary site of involvement, histological type, level of invasion (Clark's level) and tumor thickness (Breslow's depth). The gross and adjusted coefficients of annual incidences were calculated based on the number of melanoma cases and the population estimated by the Brazilian Institute of Geography and Statistics (IBGE) between 1980 and 2009.
RESULTS: The incidence rates of melanoma reached 22.4 cases per 100,000 inhabitants/year; 31.5 in women and 30.4 in men at the adjusted rate. The incidence rates standardized by decade, age and sex were 141 male and 103 female cases per 100,000/inhabitants aged 65 to 69 years. Superficial spreading melanoma occurred in 53% of the cases, followed by nodular melanoma (37%), and the primary site of involvement was the trunk (47%). 62.5% of the cases were diagnosed early, with Breslow < 1mm.
CONCLUSION: The incidence of malignant melanoma has increased fivefold from 1980 to 2009 and early diagnosis has increased 151% as a result of primary prevention
Keywords: Early detection of cancer; Early diagnosis; Epidemiology; Melanoma; Morbidity
INTRODUCTION
Due to its high incidence and mortality, melanoma is considered the skin cancer of greatest medical relevance. The American Cancer Society (ACS) estimates 68,720 new cases of melanoma in the United States in 2009 with 8,650 deaths, mostly male deaths, constituting a serious public health issue. 1.2 In the white population the incidence rate of melanoma increased from 7.5 cases per 100,000 population in 1973 to 21.9 cases per 100,000 population in 2002 (nearly a 300% increase).2.4
Melanoma mortality rates have stabilized in the United States, Australia and European countries. 3.4
The higher incidence of melanoma can be attributed to an increase in risk factors such as characteristics of the host's skin (white skin, presence of dysplastic nevi, susceptibility to ultraviolet radiation and episodes of sunburn), geographic latitude, immunosuppression and family history of the disease. 2-7
In Brazil, according to estimates from the National Cancer Institute (INCA) for 2010, we will have 113,850 new cases of nonmelanoma skin cancer (basal cell and squamous cell carcinoma) and 5,930 cases of melanoma, totaling 24.5% of new cases of cancer.8
The annual incidence rates of melanoma per 100,000 population estimated for the capital cities of southern Brazil in 2008-2009 by INCA and which serve as a reference for the rates found in Blumenau were 8.3 (male) and 7.39 (female) in Curitiba-PR, 6.84 (male) and 7.94 (female) in Florianópolis and 9.25 (male) and 10.12 (female) in Porto Alegre. 9
Blumenau, a city in southern Brazil, is located to the northeast of Santa Catarina, latitude 26 º 55 10" south, longitude 49 º 03 58", 21 meters of altitude above sea level. In 2000, the study population of Blumenau represented 4.89% of the population of Santa Catarina and 0.15% of Brazil's population.10
Due to the colonization of the city by German immigrants, just over half of the population is of German descent. Another large section of the population is of Italian descent, since the cities in the vicinity of Blumenau were mostly colonized by Italian immigrants. Portuguese descendants are also present, albeit in a more modest number, and many are of mixed origin. In the 2000 census, the ethnic makeup of the city was 247,527 (94.55%) white, 9,171 (3.5%) pardos (mixed race), 3,042 (1.16%) African descendants, 340 (0.13%) indigenous and 252 (0.10%) yellow. 10
The Caucasian population of Blumenau, comprised mostly of German and Italian descendants with skin phototypes I and II, according to Fitzpatrick classification scale, is subject to intense sun radiation in the summer, with an UV-index between 11.5 and 13.0, according to the National Institute for Space Research and very high according to the Environonmental Protection Agency/Operational Satellites (EPA / Nooa)-United States of America. Therefore, this population faces major risk factors for melanoma.1, 11
In Brazil, data on the incidence rate of cutaneous melanoma are rare and underestimated in noncapital cities; thus, there is a need for specific epidemiological studies.
The objective of this 30-year-retrospective study is to describe the epidemiological characteristics of cutaneous melanoma in Blumenau, presenting statistical data that may serve as a reference for epidemiological studies and prevention of the disease in southern Brazil.
MATERIAL AND METHODS
We collected data on all cases of melanoma histopathologically diagnosed in the three pathology laboratories located in Blumenau, CIPAC (Laboratório de Citologia, Imunopatologia e Anatomia Patológica -Laboratory of Cytology, Immunopathology and Anatomic Pathology - between 1980 and 2009), BML (Laboratório Beatriz Moreira Leite, from 1980 to 1999) and Pathology Diagnóstico em Medicina from 2000 to 2009, a total of 1002 cases of cutaneous melanoma.
Data collected in protocol by the author included patient characteristics (age, sex, tumor location) and melanoma morphology (histological type, stage of invasion using Clark level and Breslow's depth). We only included cases from the city of Blumenau and excluded those of patients living in other municipalities for the accuracy of the calculations of morbidity rates.
Melanoma incidence coefficients in the city of Blumenau were calculated based on the annual population in 1980, 1991, 2000 and 2009 estimated by the Brazilian Institute of Geography and Statistics - IBGE. We also calculated the incidence coefficients adjusted to world standard population.10
We used the c2 test to assess the association between groups.
This research study was approved by the Ethics Committee for Research Involving Human Beings of Universidade Regional de Blumenau according to Protocol 122/07 16 July 2009.
RESULTS
Between 1980 and 2009 we found 10,981 cases of skin cancer in the histopathological exams studied. The frequency of basal cell carcinoma was 63.25% (n = 6884) and the incidence of squamous cell carcinoma was 28.25% (n = 3075). The number of melanoma cases found between 1980 and 2009 was 1,002, of which 44% (n = 441) affected men and 56% (n = 561), women. 12,13
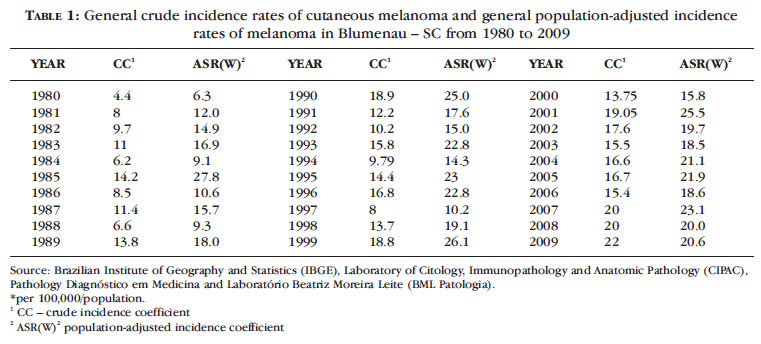
Table 1 shows the crude and world standard population-adjusted incidence rates of melanoma in the city of Blumenau between 1980 and 2009. Table 2 shows the crude and sex-adjusted incidence rates of cutaneous melanoma in the city of Blumenau between 1980 and 2009. 12,13
The general crude incidence rates were 18.8, 19.05 and 22.35 cases per 100,000 population/year of melanoma in 1999, 2001 and 2007, respectively. The general incidence rates adjusted to world standard population were 278, 25.5 and 23.1 in 1985, 2001 and 2007 (Table 1).
The sex-adjusted incidence rates were 30.4 / 100,000 male population in 1999, 23.3 in 1993 and 22 in 2009.
The crude incidence rates were 23.5 / 100,000 female population in 2001, 23 in 2002 and 31.5 in 2007. The adjusted rate was 24.6 in 2001 and 28.7 in 2007 (Table 2).
The age of highest incidence of the disease was over 50 years, with 64.9% of the cases (n = 649). Patients under 30 years old registered 4.4% of the cases (n = 44) (Table 3).
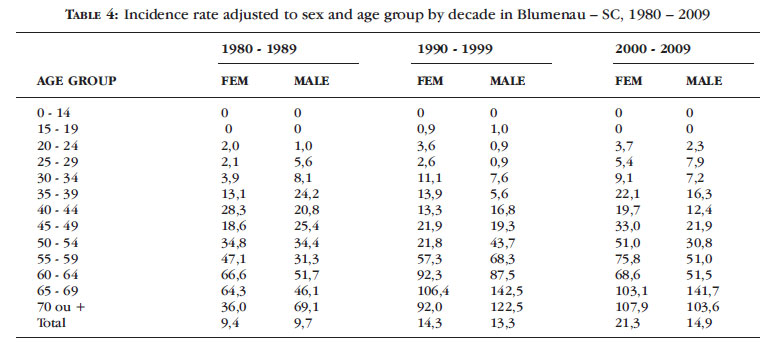
The incidence rate standardized for decade reached 141 cases per 100,000 population/year in the age group of 65-69 years (2000-2009) in males and 106.4 cases per 100,000 population/year in the same age group in females (1990 -1999). The age-adjusted rates in the three decades show a high incidence of melanoma in male and female patients over 55 years old (Table 4).
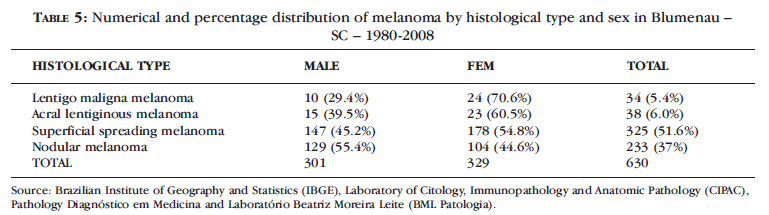
The most frequent histological type was superficial spreading melanoma (51.6%, n = 325) between 1980 and 2008, followed by nodular melanoma (37%, n = 233). Lentigo maligna melanoma reached 5.4% (n = 34) and acral lentiginous melanoma, 6.0% (n = 38) (Table 5).
Table 5 demonstrates that the distribution of histological type according to sex showed a predominance (54.8%, n = 178) of superficial spreading melanoma in women and (55.4%, n = 129) of nodular melanoma in men (55.4%, n = 129).
Table 6 shows the absolute and percentage distribution of cutaneous melanoma according to primary location and sex. 64.5% (n = 160) of the cases affected the trunk in men and 64% (n = 55) involved the upper limbs in women.
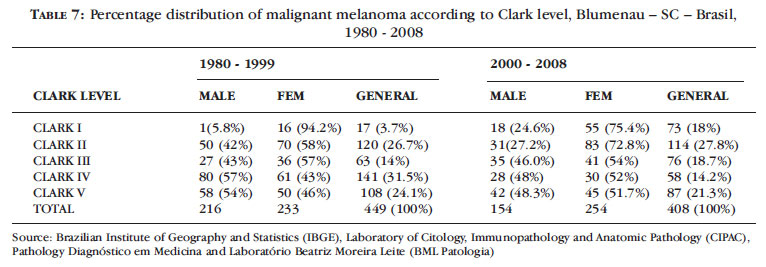
Table 7 shows the distribution of melanoma cases according to Clark level between 1980 and 1999 and between 2000 and 2008, evidentiating an increase from 30.4% (n = 137) to 45.8% (n = 187) in the diagnosis of Clark levels I and II between 2000 and 2008 in relation to the period 1980-1999.
When Clark level is evaluated over time, from 2000 to 2008, there is a significant increase in the number of cases diagnosed as Clark level I (c2 = 224.00, P = 0.000) and a decrease in those classified as Clark level IV.
Table 8 shows the percentage distribution of melanomas according to Breslow's depth between 1995 and 1999 and 2000 and 2008. Melanomas less than 1 mm thick reached 46.7% (n = 50) between 1995 and 1999 and increased to 62.55% (n = 212) between 2000 and 2008. There was a significant increase in the frequency of cases with Breslow's depth between 0-1mm (early diagnosis) from 2000 to 2008 and a decreased frequency of cases with Breslow's depth greater than 2mm c2 = 52.5, P <0.0001) .
Table 9 shows the relationship between early and late diagnoses from 1980 to 1990 (Clark Levels I and II), 1995-1999 and 2000-2008 (Breslow's depth). We noticed an increase of 87.5% in early diagnoses between 1995 and 1999 as compared with 1980 and 1990, and an increase of 151% in early diagnoses between 2000 and 2008, compared to 1980 and 1990.
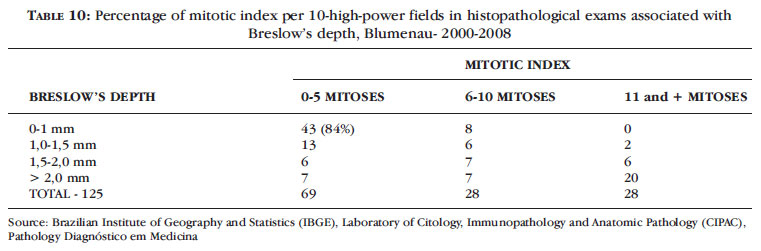
The mitotic index per 10 high-power fields in histopathological examinations of melanomas and in accordance with Breslow's depth is shown in Table 10. We noticed a mitotic index below 5 in 84% (n = 43) of melanomas with Breslow's depth less than 1 mm and 16% (n = 8) of melanomas less than 1mm with mitotic index up to 10.
DISCUSSION
This research study covers only histopathological examinations with a conclusive diagnosis of melanoma. Therefore, data are underestimated, resulting in lower-than-expected rates (population survey), which are high in relation to the incidence rates found in Brazil.
Our study shows that the morbidity of cutaneous melanoma in Blumenau increased from 4.4 to 22.4 cases per 100,000 population, reaching 21.6 in men in 1990, 20.0 in 1999 and 22.35 in 2007; the populationadjusted incidence reached 27.8, 25.5 and 23.1 in 1985, 2001 and 2007, respectively.
The highest notified incidence worldwide was in Queensland, Australia, with 56 new cases per 100,000 population/year in men and 43 in women. 4.5
Mortality rates have stabilized in the U.S., Australia and European countries. 4,5,6,7
The population-adjusted rates were 19.6 cases of melanoma per 100,000 population/year based on diagnosed cases between 2000-2006 from 17 geographic areas of SEER (Surveillance, Epidemiology and End Results). 14
The incidence of cutaneous melanoma found in the region of Passo Fundo-RS was 5.67 cases per 100,000 population and the mortality rate was 2.16 / 100,000. 15
The morbidity coefficients of cutaneous melanoma in Blumenau are close to those expected for European descendants (Germans and Italians) and lightskinned Caucasians living in a geographic region with high ultraviolet radiation such as southern Brazil. 8,9,10,12,13
Sex
In Australia, the incidence of cutaneous melanoma has stabilized among young men and declined among young women; the incidence of the disease has increased 7.2% per year in men older than 75 years.14 In the UK, the incidence of melanoma is higher in women. 5.16
In Londrina (Parana), the prevalence was 54.46% in women. 17
In Germany, with a Caucasian population similar to the one found in Blumenau, the morbidity coefficient in Northrhine-Westphalia was 13.6 cases/100,000 male population and 18.5/100,000 female population. 18
In 2001 the Brazilian Melanoma Group found 57.4% of melanoma cases in men and 41.47% in women in a research study.19
In Blumenau, between 1980 and 2009, 56% of melanoma cases were diagnosed in women (Table 10). The crude incidence rate, higher in women, reached 31.5 casos/100,000 population in 2007. There were 20 cases/100,000 male population. The population-adjusted rate was 30.4 in 1999 (Table 2).
Age group
In the United States of America there is predominance of melanoma incidence in the age group above 55 years. 1
The incidence of melanoma in the United Kingdom and United States of America between 1973 and 2002 increased in all age groups in both men and women; there was an increase from 12.4 to 56.1 cases/100,000 male population aged between 55 and 64 years, and in the age group over 65 years the incidence increased from 18.8 to 104.4/100,000 population. 1,20
In this study there was a 66.3% incidence of melanoma in the age group over 50 years in from 1980-2008. In the age group below 20 years the incidence was 0.21% between 2000-2008. This is in agreement with the literature, which describes an incidence of 0.4% in this age group. 20
The incidence rates standardized for decade, age and sex in Blumenau showed high incidence coefficients in age groups above 50 years, with rates up to 141 cases per 100,000 male population/year and 103/100,000 female population (Table 5).
Primary location
Kraemer, 1994, in a study of 5.884 cases of melanoma, demonstrated that 55% were primarily located in areas unexposed to the sun and 45% were found in sun-exposed areas. 21
In Canada a 50 year-study showed that the most frequent location was the chest (unexposed area) in men and lower limbs in women. 22
In the United States this location was also the most frequent between 1960-2004. 1.20%
In Blumenau the most common primary location of cutaneous melanoma was the chest, with 47% of the cases (64.5% in men and 35.5% in women). Incidence in the lower limbs was more common in women (56.3%), than in men (44.7%) (Table 6).
Histology
In a study of 771 cases of melanoma in Texas and California, lentigo maligna melanoma was the most frequent (56%), followed by superficial spreading melanoma (29%). 23
In Londrina, Parana, Brazil, the most common histological types was nodular melanoma (41.09%), followed by superficial spreading melanoma (37.13%). 17
In the region of Passo Fundo, RS-Brazil, superficial spreading melanoma was the most frequent (61.6%) of all cases found. 15
In Blumenau superficial spreading melanoma was the most frequent (51.6%), especially in women (54.6%), whereas nodular melanoma was the most frequent in men (55.4%). Lentigo maligna melanoma appeared much more frequently in women (70.6% ) than in men (29.4%) (Table 5).
Level of invasion and mitotic index.
The thickness and level of invasion of primary cutaneous melanoma are the most important prognostic factors in patient survival, and reduction of tumor thickness on histopathological examination is equivalent to early diagnosis and improved survival rates. 24.25
Patients with primary cutaneous melanoma with Breslow's depth of less than 1mm are considered low risk and with excellent prognosis for survival, possibly leading to zero mortality. 24.25
The increase in the number of dermatologists in Blumenau - 04 in the 1970s and 18 in the 2000s, according to the Municipal Health Secretariat for the city of Blumenau, led to a improvement in early diagnosis with better survival of patients and consequent low mortality, despite the increased incidence of melanoma.
In Blumenau, between 1980 and 1990, there were 25% of diagnosed patients with Clark levels I and II, and between 1995-1999, this percentage increased to 46.7% (n = 50). Between 2000 and 2008, based on Breslow's depth, the percentage of early diagnosis was 62.55% (n = 212) for melanomas less than 1mm thick. A similar value was reported in the U.S., where 66% of all melanomas diagnosed between 1988 and 1999 were less than 1mm thick. 24
Therefore, this study shows that there was an increase in early diagnosis when we compare Breslow's depth from 2000 to 2009 with data from 1980 to 1990.
This would mean an estimated 48% reduction in mortality and consequent 151% increase of survival, considering that the survival of patients with malignant melanoma is inversely proportional to tumor thickness, measured by Breslow's depth and Clark level. (Table 9)
The mitotic index is an important prognostic factor, even in cases of thin cutaneous melanomas, especially those with a number of mitoses less than 6 per 10 high-power fields. 24.25
The final version of melanoma staging by the American Joint Committee on Cancer (AJCC) established in 2009 considers the mitotic index per mm2 one of the main prognostic factors for the survival of patients with melanoma.25
From 2000 to 2009, we found a mitotic index below 6 mitoses/10 high-power fields in 84% of melanomas with Breslow's depth less than 1 mm, indicating a good prognosis for these patients, with a better survival rate (Table 10).
The mitotic index found in our study cannot be compared with the mitotic index by mm2, recommended by AJCC, but it shows that in thin melanomas this index is lower, indicating better staging with a good prognosis. 1,24,25
Reduction in the thickness of cutaneous melanomas in histopathological diagnosis can be credited to prevention campaigns and the training of health professionals for early diagnosis and treatment, but this evidence can only be shown in longer studies (20 or more years) which monitor invasion levels and morbidity rates, such as the present work. 26
Race
The study population is constituted by Caucasians (94.5%), mostly descendants of German and Italian immigrants with skin phototypes I and II, according to Fitzpatrick classification scale. The percentage of annual incidence of melanoma is higher among the white population than in other races in the UK, United States, Australia and Germany. 1-4,8
The crude incidence rate of melanoma in all races is 25/100,000 male population and 15.8 / 100,000 female population, according to SEER. 14
This rate is 1.1 (men) and 1.3 (women) in the black race; the world average is 28.9 (men) and 18.7 (women) in the white race. 14
In the population of Blumenau, which is mostly white, the highest incidence rates of melanoma found, adjusted to world standard, were 30.4 among men (1999) and 28.7 among women (2007) (Table 4).
Sun radiation
It is well established that ultraviolet radiation B (UVB) is a risk factor for the appearance of cutaneous malignant melanoma, and sporadic and chronic sun exposure since childhood is of great importance in the etiology of this skin cancer. 3
Epidemiological studies have confirmed the hypothesis that most cases of melanoma are partly caused by excessive ultraviolet radiation exposure originating from sun radiation. 2, 27,28
Exposure to ultraviolet A radiation, found in tanning beds, has been associated to the appearance of cutaneous malignant melanoma in epidemiological studies. 29
The high incidence of cutaneous malignant melanoma in Blumenau has as important risk factors the high incidence of ultraviolet radiation in the region (UVB-Index between 11.5 and 13 in summer) and a Caucasian population (94.5%), descendants of Germans and Italians from northern Italy.
FINAL CONSIDERATIONS AND CONCLUSIONS
The results of this study may serve as a reference for most municipalities in the south of Brazil where there is intense sun radiation reaching light-skinned individuals, phototypes I and II, of European descent.
Increase in early diagnosis can be credited to education and prevention campaigns as already reported in other countries. 26,30
The treatment of thin melanomas at an early stage can lead to increased patient survival. 26,30
Increased early diagnosis and the consequent early treatment of melanomas in their initial stage are shown in this 30-year-retrospective epidemiological study.
This study (1980 to 2009) allows us to make the following observations:
1. The crude rate of melanoma increased from 4.4 to 22.4 / 100,000 population, with a peak of 31.5 in women. The adjusted incidence rate ranged from 6.3 to 26.1 with a peak of 30.4 in men.
2. Prevalence of melanoma incidence in women with 56% of cases (n = 561)
3. Increased incidence of melanoma in the age group above 50 years (64.9%);
4. Rare in the age group below 30 years (4.2% of cases, n = 42).
5. Prevalence of superficial spreading melanoma (51.6%, n = 315) and nodular melanoma with (37%, n = 233).
6. Superficial spreading melanoma was more common in men (55.4%, n = 176) and lentigo maligna melanoma in women (70.6%).
7. Increase of early diagnosis from 2000-2009 represented by 62.55% (n = 212) of diagnoses with Breslow's depth less than 1mm in relation to the period from 1995 to 1999.
8. Mitotic index below 6 /10 high-power fields was found in 84% of melanomas with Breslow's depth less than 1 mm.
9. Increase of 151% in early diagnosis from 2000-2009 compared to 1980-1990.
10. Increase in early diagnosis can be credited to education and prevention campaigns and improved diagnosis made by physicians and dermatologists in the city of Blumenau.
ACKNOWLEDGEMENTS
Laboratory of Cytology, Immunopathology and Anatomic Pathology (CIPAC), Pathology Diagnóstico em Medicina and Laboratório Beatriz Moreira Leite (BML Pathology)
Professors (PhD) Carlos Efrain Stein and Ernani T. de Santa Helena, Department of Statistics and Department of Medicine, Universidade Regional de Blumenau
Prof. Ubirani Barros Otero- National Cancer Institute (NCI) Dr. Maria Paula Curado
REFERENCES
1. Cancer.org [Internet]. American Cancer Society. Cancer facts and figures 2009. [cited 2009 Mar 26]. Available from: www.cancer.org/downloads/STT/CAFF2009PWSecured.pdf.
2. Markovic SN, Erickson LA, Rao RD, Weenig RH, Pockaj BA, Bardia A, et al. Malignant melanoma in the 21st century, part 1: epidemiology, risk factors, screening, prevention, and diagnosis. Mayo Clin Proc. 2007;82:364-80.
3. Leiter U, Garbe C. Epidemiology of melanoma and nonmelanoma skin cancer-the role of sunlight. Adv Exp Med Biol. 2008;624:89-103.
4. Kelly JW, Henderson MA, Thursfield VJ, Slavin J, Ainslie J, Giles GG.The management of primary cutaneous melanoma in Victoria in 1996 and 2000. Med J Aust. 2007;187:511-4.
5. Staples MP, Elwood M, Burton RC, Williams JL, Marks R, Giles GG. Non-melanom a skin cancer in Australia: the 2002 national survey and trends since 1985. Med J Aust. 2006;184:6-10.
6. Stewart BW, Kleihues P. World cancer report. Lyon (France): IARC Press; 2003.
7. Gandini S, Sera F, Cattaruzza MS, Pasquini P, Picconi O, Boyle P, et al. Metaanalysis of risk factors for cutaneous melanoma, II: sun exposure. Eur J Cancer. 2005;41:45-60.
8. Ministério da Saúde. Instituto Nacional de Câncer. Coordenação de Prevenção e Vigilância de Câncer. Estimativas 2010: Incidência de Câncer no Brasil. Rio de Janeiro: INCA; 2009. 98 p.
9. Ministério da Saúde. Instituto Nacional de Câncer. Taxa de incidência anual de neoplasias malignas por 100.000 habitantes, por localização, segundo Capital Sexo masculino e feminino no Brasil, 2008 e 2009. Rio de Janeiro: Inca; 2009.
10. BRASIL. IBGE. DPE. Departamento da População e Indicadores Sociais. Gerência de Estudos e Análises da Dinâmica Demográfica. Estimativas para as Unidades da Federação obtidas pela Metodologia AiBi, controlada pela projeção Brasil- Revisão 2000 (método dos componentes demográficos). Brasília; 2009.
11. International Agency for Research on Cancer (IARC). Solar and ultraviolet radiation. IARC Monogr Eval Carcinog Risks Hum. 1992;55:11.
12. Nasser N. Epidemiologia do melanoma maligno em Blumenau - SC. An Bras Dermatol. 1993;68:17-20.
13. Nasser N. Incidência de câncer de pele em Blumenau-SC (1980-1990). An Bras Dermatol. 1993;68:77-8.
14. Horner MJ, Ries LAG, Krapcho M, Neyman N, Aminou R, Howlader N, et al, editors. SEER Cancer Statistics Review, 1975-2006. Bethesda: National Cancer Institute; 2009.
15. Borges SZ, Bakos L, Cartell A, Wagner M, Agostini A, Lersch E. Distribution of clinical-pathological types of cutaneous melanomas and mortality rate in the region of Passo Fundo, RS, Brazil. Int J Dermatol. 2007;46:679-86.
16. Bishop JN, Bataille V, Gavin A, Lens M, Marsden J, Mathews T, et al. The prevention, diagnosis, referral and management of melanoma of the skin: concise guidelines. Clin Med. 2007;7:283-90.
17. Gon AS, Minelli L, Guembarovski AL. Melanoma Cutâneo Primário em Londrina. An Bras Dermatol. 2001;76:413-26.
18. Stang A, Ziegler S, Büchner U, Ziegler B, Jöckel KH, Ziegler V. Malignant melanoma and nonmelanoma skin cancers in Northrhine-Westphalia, Germany: a patient vs. diagnosis-based incidence approach. Int J Dermatol. 2007;46:564-70.
19. Enokihara MY, Waksman G, Chao LW, Belfort FA, Almeida FA. Statistical data of the Brazilian melanoma group: Analysis 2033 cases of the simplified report form. In: 5th International Conference on Melanoma, 2001, Veneza. Melanom Research. 2001;(Suppl 1):S1-80.
20. Lewis KG.Trends in pediatric melanoma mortality in the United States, 1968 through 2004. Dermatol Surg. 2008;34:152-9.
21. Kraemer KH, Lee MM, Andrews AD, Lambert WC. The role of sunlight and DNA repair in melanoma and nonmelanoma skin cancer. The xeroderma pigmentosum paradigm. Arch Dermatol. 1994;130:1018-21.
22. Pruthi DK, Guilfoyle R, Nugent Z, Wiseman MC, Demers AA. Canada Incidence and anatomic presentation of cutaneous malignant melanoma in central Canada during a 50-year period: 1956 to 2005. J Am Acad Dermatol. 2009;61:44-50.
23. Forman SB, Ferringer TC, Peckham SJ, Dalton SR, Sasaki GT, Libow LF, et al. Is superficial spreading melanoma still the most common form of malignant melanoma? J Am Acad Dermatol. 2008;58:1013-20.
24. Gimotty PA, Elder DE, Fraker DL, Botbyl J, Sellers K, Elenitsas R, et al. Identification of high-risk patients among those diagnosed with thin cutaneous melanomas. J Clin Oncol. 2007;25:1129-34.
25. Balch CM, Gershenwald JE, Soong SJ, Thompson JF, Atkins MB, Byrd DR, et al. Final Version of 2009 AJCC melanoma staging and Classification. J.Clin Oncol. 2009;27:6199-206.
26. Schneider JS, Moore DH 2nd, Mendelsohn ML. Screening program reduced melanoma mortality at the Lawrence Livermore National Laboratory, 1984 to 1996. J Am Acad Dermatol. 2008;58:741-9.
27. Lea CS, Scotto JA, Buffler PA, Fine J, Barnhill RL, Berwick M. Ambient UVB and melanoma risk in the United States: a case-control analysis. Ann Epidemiol. 2007;17:447-53.
28. Moan J, Porojnicu AC, Dahlback A. Ultraviolet radiation and malignant melanoma. Adv Exp Med Biol. 2008;624:104-16.
29. Ting W, Schultz K, Cac NN, Peterson M, Walling HW.Tanning bed exposure increases the risk of malignant melanoma. Int J Dermatol. 2007;46:1253-7.
30. Sneyd M, Cox B. The control of melanoma in New Zealand. N Z Med J 2006;119:U2169.
Received on 25.05.2010.
Approved by the Advisory Board and accepted for publication on 17.10.10.
Conflict of interest: None
Financial funding: None
References
- 1. Cancer.org [Internet]. American Cancer Society. Cancer facts and figures 2009. [cited 2009 Mar 26]. Available from: www.cancer.org/downloads/STT/CAFF2009PWSecured.pdf
- 2. Markovic SN, Erickson LA, Rao RD, Weenig RH, Pockaj BA, Bardia A, et al. Malignant melanoma in the 21st century, part 1: epidemiology, risk factors, screening, prevention, and diagnosis. Mayo Clin Proc. 2007;82:364-80.
- 3. Leiter U, Garbe C. Epidemiology of melanoma and nonmelanoma skin cancer-the role of sunlight. Adv Exp Med Biol. 2008;624:89-103.
- 4. Kelly JW, Henderson MA, Thursfield VJ, Slavin J, Ainslie J, Giles GG.The management of primary cutaneous melanoma in Victoria in 1996 and 2000. Med J Aust. 2007;187:511-4.
- 5. Staples MP, Elwood M, Burton RC, Williams JL, Marks R, Giles GG. Non-melanom a skin cancer in Australia: the 2002 national survey and trends since 1985. Med J Aust. 2006;184:6-10.
- 6. Stewart BW, Kleihues P. World cancer report. Lyon (France): IARC Press; 2003.
- 7. Gandini S, Sera F, Cattaruzza MS, Pasquini P, Picconi O, Boyle P, et al. Metaanalysis of risk factors for cutaneous melanoma, II: sun exposure. Eur J Cancer. 2005;41:45-60.
- 8. Ministério da Saúde. Instituto Nacional de Câncer. Coordenação de Prevenção e Vigilância de Câncer. Estimativas 2010: Incidência de Câncer no Brasil. Rio de Janeiro: INCA; 2009. 98 p.
- 9. Ministério da Saúde. Instituto Nacional de Câncer. Taxa de incidência anual de neoplasias malignas por 100.000 habitantes, por localização, segundo Capital Sexo masculino e feminino no Brasil, 2008 e 2009. Rio de Janeiro: Inca; 2009.
- 10 BRASIL. IBGE. DPE. Departamento da População e Indicadores Sociais. Gerência de Estudos e Análises da Dinâmica Demográfica. Estimativas para as Unidades da Federação obtidas pela Metodologia AiBi, controlada pela projeção Brasil- Revisão 2000 (método dos componentes demográficos). Brasília; 2009.
- 11. International Agency for Research on Cancer (IARC). Solar and ultraviolet radiation. IARC Monogr Eval Carcinog Risks Hum. 1992;55:11.
- 12. Nasser N. Epidemiologia do melanoma maligno em Blumenau - SC. An Bras Dermatol. 1993;68:17-20.
- 13. Nasser N. Incidência de câncer de pele em Blumenau-SC (1980-1990). An Bras Dermatol. 1993;68:77-8.
- 14. Horner MJ, Ries LAG, Krapcho M, Neyman N, Aminou R, Howlader N, et al, editors. SEER Cancer Statistics Review, 1975-2006. Bethesda: National Cancer Institute; 2009.
- 15. Borges SZ, Bakos L, Cartell A, Wagner M, Agostini A, Lersch E. Distribution of clinical-pathological types of cutaneous melanomas and mortality rate in the region of Passo Fundo, RS, Brazil. Int J Dermatol. 2007;46:679-86.
- 16. Bishop JN, Bataille V, Gavin A, Lens M, Marsden J, Mathews T, et al. The prevention, diagnosis, referral and management of melanoma of the skin: concise guidelines. Clin Med. 2007;7:283-90.
- 17. Gon AS, Minelli L, Guembarovski AL. Melanoma Cutâneo Primário em Londrina. An Bras Dermatol. 2001;76:413-26.
- 18. Stang A, Ziegler S, Büchner U, Ziegler B, Jöckel KH, Ziegler V. Malignant melanoma and nonmelanoma skin cancers in Northrhine-Westphalia, Germany: a patient vs. diagnosis-based incidence approach. Int J Dermatol. 2007;46:564-70.
- 19. Enokihara MY, Waksman G, Chao LW, Belfort FA, Almeida FA. Statistical data of the Brazilian melanoma group: Analysis 2033 cases of the simplified report form. In: 5th International Conference on Melanoma, 2001, Veneza.
- Melanom Research. 2001;(Suppl 1):S1-80.
- 20. Lewis KG.Trends in pediatric melanoma mortality in the United States, 1968 through 2004. Dermatol Surg. 2008;34:152-9.
- 21. Kraemer KH, Lee MM, Andrews AD, Lambert WC. The role of sunlight and DNA repair in melanoma and nonmelanoma skin cancer. The xeroderma pigmentosum paradigm. Arch Dermatol. 1994;130:1018-21.
- 22. Pruthi DK, Guilfoyle R, Nugent Z, Wiseman MC, Demers AA. Canada Incidence and anatomic presentation of cutaneous malignant melanoma in central Canada during a 50-year period: 1956 to 2005. J Am Acad Dermatol. 2009;61:44-50.
- 23. Forman SB, Ferringer TC, Peckham SJ, Dalton SR, Sasaki GT, Libow LF, et al. Is superficial spreading melanoma still the most common form of malignant melanoma? J Am Acad Dermatol. 2008;58:1013-20.
- 24. Gimotty PA, Elder DE, Fraker DL, Botbyl J, Sellers K, Elenitsas R, et al. Identification of high-risk patients among those diagnosed with thin cutaneous melanomas. J Clin Oncol. 2007;25:1129-34.
- 25. Balch CM, Gershenwald JE, Soong SJ, Thompson JF, Atkins MB, Byrd DR, et al. Final Version of 2009 AJCC melanoma staging and Classification. J.Clin Oncol. 2009;27:6199-206.
- 26. Schneider JS, Moore DH 2nd, Mendelsohn ML. Screening program reduced melanoma mortality at the Lawrence Livermore National Laboratory, 1984 to 1996. J Am Acad Dermatol. 2008;58:741-9.
- 27. Lea CS, Scotto JA, Buffler PA, Fine J, Barnhill RL, Berwick M. Ambient UVB and melanoma risk in the United States: a case-control analysis. Ann Epidemiol. 2007;17:447-53.
- 28. Moan J, Porojnicu AC, Dahlback A. Ultraviolet radiation and malignant melanoma. Adv Exp Med Biol. 2008;624:104-16.
- 29. Ting W, Schultz K, Cac NN, Peterson M, Walling HW.Tanning bed exposure increases the risk of malignant melanoma. Int J Dermatol. 2007;46:1253-7.
- 30. Sneyd M, Cox B. The control of melanoma in New Zealand. N Z Med J 2006;119:U2169.

 Cutaneous melanoma: a 30-year-long epidemiological study conducted in a city in southern Brazil, from 1980-2009
Cutaneous melanoma: a 30-year-long epidemiological study conducted in a city in southern Brazil, from 1980-2009