Abstract
With the development of new cancer therapies, systemic toxicity profile and effects on survival achieved an important improvement. However, a constellation of toxicities has emerged, even more remarkably, cutaneous adverse events. This report, developed by a board of Brazilian experts in oncodermatology, aims to establish a guideline for the dermatological care of oncologic patients. When possible, evidence-based recommendations were made, but in many cases, when strong evidence was not available, a consensus was reached, based on some data supporting therapies combined with personal experiences.
KEYWORDS
Antineoplastic agents; Antineoplastic agents, immunological; Dermatology; Drug-related side effects and adverse reactions; Medical oncology; Molecular targeted therapy
Introduction
With the advance of cancer treatment, systemic toxicities and survival faced an important improvement. However, its use is often related to dermatologic adverse events (DAE), which have a high frequency, are often symptomatic, may be disfiguring, and might cause an important impact on patient's quality of life (QoL). Another important issue relies on the fact that those skin toxicities might lead to dose reductions or even discontinuation of cancer therapy, with impact on the disease outcome. For those reasons, it is important for dermatologists to know the most common types of reactions in order to be able to help patients and oncologists on the prevention and management of those toxicities.11 Bensadoun RJ, Humbert P, Krutman J, Luger T, Triller R, Rougier A, et al. Daily baseline skin care in the prevention, treatment, and supportive care of skin toxicity in oncology patients: recommendations from a multinational expert panel. Cancer Manag Res. 2013;5:401-8.,22 Balagula Y, Rosen ST, Lacouture ME. The emergence of supportive oncodermatology: the study of dermatologic adverse events to cancer therapies. J Am Acad Dermatol. 2011;65:624-35.
A multidisciplinary team with a good interaction between the different specialists (e.g.: oncologists, dermatologists, nurses) is fundamental for the best supportive care for cancer patients and their families. Different societies and associations are dedicated to the research, support and education in all aspects of cancer treatment, such as the Multinational Association of Supportive Care in Cancer (MASCC), the American Society of Clinical Oncology (ASCO), the Oncology Nurse Society (ONS), and the National Comprehensive Cancer Network (NCCN).
The aim of this paper was to establish a guideline to help professionals on the dermatological care of oncologic patients. When possible, evidence-based recommendations were made, but when strong evidence was not available, a consensus was reached based on some data supporting therapies combined with personal experiences. Levels of evidence are defined bellow and are reported for each treatment in tables 1-33 Shekelle PG, Woolf SH, Eccles M, Grimshaw J. Developing clinical guidelines. West J Med. 1999;170:348-51..
Categories of evidence based on types of studies 33 Shekelle PG, Woolf SH, Eccles M, Grimshaw J. Developing clinical guidelines. West J Med. 1999;170:348-51.:
IA - Evidence from meta-analysis of Randomized Controlled Trials (RCT);
IB - Evidence from at least one Randomized Controlled Trial (RCT);
IIA - Evidence from at least one controlled study without randomization;
IIB - Evidence from at least one other type of experimental study;
III - Evidence from non-experimental descriptive studies, such as comparative studies, correlation studies and case-control studies;
IV - Evidence from expert committee reports, opinions or clinical experience of respected authorities, or both.
Categories of agents
Conventional chemotherapeutic drugs
Conventional cytotoxic chemotherapy still plays an important role in cancer treatment. It works primarily through the inhibition of cell division. It is associated with many adverse events (AE), especially in some systems that share with the tumor the property of rapid cell proliferation and therefore a high rate of cell division, such as the hematopoietic and gastrointestinal systems, and the skin.22 Balagula Y, Rosen ST, Lacouture ME. The emergence of supportive oncodermatology: the study of dermatologic adverse events to cancer therapies. J Am Acad Dermatol. 2011;65:624-35.,44 Reyes-Habito CM, Roh EK. Cutaneous reactions to chemotherapeutic drugs and targeted therapies for cancer: Part I. Conventional chemotherapeutic drugs. J Am Acad Dermatol. 2014;71:203. Examples of common effects are emesis, cytopenias, alopecia, mucositis and nail changes. They are associated with dose, type of drug, time of infusion and are in most cases reversible with the end of chemotherapy cycles.
Belonging to this class of drugs are agents such as antimetabolites (e.g. capecitabine, fludarabine, cladribine, gemcitabine, 5-fluoracil), alkylating agents (e.g. cyclophosphamide, platins), topoisomerase inhibitors (e.g. irinotecan, topotecan, etoposide), anthracyclines (e.g. doxorubicin, daunorubicin), bleomycin, antimicrotubule agents (e.g. taxanes and vinca alkaloids). The related cutaneous adverse events to this class, most frequent causing agents and management (with level of evidence) are summarized in table 1.
Targeted therapies and immunomodulatory agents ("checkpoint" inhibitors)
In the last decade an enormous development on oncologic treatments have occurred, with the emergence of numerous targeted agents and immune checkpoint related agents. With those new mechanisms of action a totally new scope of adverse reactions has arisen, and professionals may not be familiar with the spectrum of dermatological toxicities.55 Reyes-Habito CM, Roh EK. Cutaneous reactions to chemotherapeutic drugs and targeted therapy for cancer: Part II. Targeted therapy. J Am Acad Dermatol. 2014;71:217.
6 Macdonald JB, Macdonald B, Golitz LE, LoRusso P, Sekulic A. Cutaneous adverse effects of targeted therapies: Part I: Inhibitors of the cellular membrane. J Am Acad Dermatol. 2015;72:203-18.
7 Macdonald JB, Macdonald B, Golitz LE, LoRusso P, Sekulic A. Cutaneous adverse effects of targeted therapies: Part II: Inhibitors of intracellular molecular signaling pathways. J Am Acad Dermatol. 2015;72:221-36.-88 Tischer B, Huber R, Kraemer M, Lacouture ME. Dermatologic events from EGFR inhibitors: the issue of the missing patient voice. Support Care Cancer. 2017;25:651-60. In one hand, those therapies were crucial for the improvement of survival. On the other hand, they created a new challenge: with the continuous and prolonged use of these drugs, patients and professionals have to deal with the chronic aspects of the toxicities, not anymore related with a specific number of chemotherapy cycles, but lasting for months to years, with an important impact on quality of life.
Targeted therapies inhibit specific molecules involved in tumor development and growth, having a more specific action than conventional chemotherapy, with greater efficacy and less toxicity. Many of those molecules are mutated or overexpressed on tumors, but are also present in normal tissues such as the skin. This justifies the common dermatological side effects related to this class. They might be monoclonal antibodies, large molecules that target extracellular components; or small molecule inhibitors, that can enter cells, block receptor signaling, and interfere with downstream intracellular molecules (they will be referred as Tyrosine Kinase Inhibitors - TKI). 99 Gerber DE. Targeted therapies: a new generation of cancer treatments. Am Fam Physician. 2008;77:311-19. The main agents on this class are Epidermal Growth Factor Receptor Inhibitors - EGFRi (e.g. cetuximab, panitumumab, gefitinib, erlotinib, lapatinib); antiangiogenic agents, inhibitors of Vascular Endothelial Growth Factor Receptor - VEGFRi (e.g. bevacizumab, sorafenib); inhibitors of the Mitogen-Activated Protein Kinase (MAPK) pathway, such as RAFi (e.g. vemurafenib, dabrafenib), MEKi (e.g. cobimetinib, trametinib); mTOR inhibitors (e.g. everolimus); multikinase inhibitors (e.g. vandetanib, pazopanib, sunitinib); and Hedgehog pathway inhibitors (vismodegib). Related cutaneous adverse events to the agents on this class, their management and levels of evidence are summarized in table 2.
More recently, the comprehension of the regulatory processes involved in the restriction of the immune response to cancer, led to the development of a new promising group of agents, the "immune checkpoint" targeted agents, also known as immunotherapy. They have the goal of releasing the immune system against tumor cells, blocking inhibitory receptors expressed on T-cells such as Programmed Death 1 (PD-1) or Cytotoxic T Lymphocyte-associated Antigen 4 (CTLA-4). This releasing/activation of the immune system also affects normal cells, explaining the most common adverse events related to this class, also known as Immune-Related Adverse Events (irAE). On this group we find the CTLA-4 inhibitor (ipilimumab), PD-1 and PD-1 ligand inhibitors (nivolumab, pembrolizumab, atezolizumab).77 Macdonald JB, Macdonald B, Golitz LE, LoRusso P, Sekulic A. Cutaneous adverse effects of targeted therapies: Part II: Inhibitors of intracellular molecular signaling pathways. J Am Acad Dermatol. 2015;72:221-36.,1010 Friedman CF, Proverbs-Singh TA, Postow MA. Treatment of the immune-related adverse effects of immune checkpoint inhibitors: a review. JAMA Oncol. 2016;2:1346-53.
11 Puzanov I, Diab A, Abdallah K, Bingham CO, Brogdon C, Dadu R, et al. Society for Immunotherapy of Cancer Toxicity Management Working Group Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer. 2017;5:95.
12 Rapoport BL, van Eeden R, Sibaud V, Epstein JB, Klastersky J, Aapro M, et al. Supportive care for patients undergoing immunotherapy. Support Care Cancer. 2017;25:3017-30.-1313 Lacouture ME, Wolchok JD, Yosipovitch G, Kähler KC, Busam KJ, Hauschild A. Ipilimumab in patients with cancer and the management of dermatologic adverse events. J Am Acad Dermatol. 2014;71:161-9. Related cutaneous adverse events to the agents on this class, their management and levels of evidence are summarized on table 3.
Grading the dermatological adverse events
The National Cancer Institute's Common Terminology Criteria for Adverse Events (CTCAE) is a standardized tool used in oncology trials to document and grade toxic effects of oncologic therapies. It is available online with open access at https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm.
It divides AE into systems and has a specific chapter for skin and subcutaneous tissue disorders. It helps establish a common terminology and language between different experts (oncologists, dermatologists, nurses, etc.) and to standardize treatments or dose modifications based on the severity of specific manifestations.
Impact of dermatological adverse events on quality of life
Quality of life is an important issue when dealing with oncologic patients. Different studies have already shown that grading severity of AE are different from the physician and the patient's perspectives. 1414 Rosen AC, Case EC, Dusza SW, Balagula Y, Gordon J, West DP, et al. Impact of dermatologic adverse events on quality of life in 283 cancer patients: a questionnaire study in a dermatology referral clinic. Am J Clin Dermatol. 2013;14:327-33. Therefore, using patient's self-reporting instruments for DAE are also important. Different instruments are available such as Skindex-16©, Skindex-29©, Dermatology Life Quality Index (DLQI), DIELH-24 or even questionnaires for specific agents such as the Functional Assessment of Cancer Therapy-Epidermal Growth Factor Receptor Inhibitors-18 (FACT-EGFRI-18). 1515 Chan A, Cameron MC, Garden B, Boers-Doets CB, Schindler K, Epstein JB, et al. A systematic review of patient-reported outcome instruments of dermatologic adverse events associated with targeted cancer therapies. Support Care Cancer. 2015;23:2231-44.
When comparing different classes of drugs, the number of DAEs and its impact on QoL seemed to be greater on patients on targeted versus non-targeted therapies (difference = 8.9, p = 0.02). 1515 Chan A, Cameron MC, Garden B, Boers-Doets CB, Schindler K, Epstein JB, et al. A systematic review of patient-reported outcome instruments of dermatologic adverse events associated with targeted cancer therapies. Support Care Cancer. 2015;23:2231-44.
Cutaneous toxicity as a predictive marker for clinical outcome
A recent review tried to address the association of cutaneous toxicities and clinical outcome. For papulopustular eruption induced by EGFRi, this association has already been established (four trials, all with p < 0.05). For hand-foot skin reaction associated with sorafenib, it was associated with reduced risk of death on a systematic review of 12 cohort studies (p < 0.00001; hazard ratio = 0.45). However, authors concluded that for other toxicities such as vitiligo and immunotherapy for melanoma (data based mostly on retrospective analyses), the analyses are still observational and exploratory and need further investigation from larger prospective studies. 1616 Rzepecki AK, Cheng H, McLellan BN. Cutaneous toxicity as a predictive biomarker for clinical outcome in patients receiving anticancer therapy. J Am Acad Dermatol. 2018;79:545-55.
Daily baseline skin care
Xerosis is a common dermatological condition in patients on cancer treatment, with incidences ranging from 1 to 84% and can collaborate to a decrease in QoL and to the occurrence of other DAE, such as infections, sensitization to allergens and pruritus. It is even more frequent with the use of targeted therapies (for xerosis - RR = 2.99, 95% IC 2.0-4.3, p < 0.001; for pruritus RR = 2.56, 95% IC 1.51-4.35, p < 0.001).1717 Valentine J, Belum VR, Duran J, Ciccolini K, Schindler K, Wu S, et al. Incidence and risk of xerosis with targeted anticancer therapies. J Am Acad Dermatol. 2015;72:656-67.,1818 Santoni M, Conti A, Andrikou K, Bittoni A, Lanese A, Pistelli M, et al. Risk of pruritus in cancer patients treated with biological therapies: a systematic review and meta-analysis of clinical trials. Crit Rev Oncol Hematol. 2015;96:206-19. The skin also becomes more sensitive to the ultraviolet radiation and more prone to skin pigmentation. Therefore, it is fundamental to educate patients on preventive measures, even prior to the start of any therapy, that help maintaining skin barrier function, possibly decreasing the occurrence and severity of DAE.
Some important measures to all cancer patients are: avoidance of alcohol- based lotions and irritating products, limited shower time, use of gentle cleansers (pH-neutral soaps or syndets), regular use of emollients and sun protective measures (e.g.: broad spectrum sunscreen - SPF30 or higher with a UVA-PF, protective clothes). 11 Bensadoun RJ, Humbert P, Krutman J, Luger T, Triller R, Rougier A, et al. Daily baseline skin care in the prevention, treatment, and supportive care of skin toxicity in oncology patients: recommendations from a multinational expert panel. Cancer Manag Res. 2013;5:401-8.
The use of deodorants is controversial, but some data show no evidence of harm, and therefore, patients might use those products in order to maintain their regular routine and help on their well-being. 1919 Chan RJ, Webster J, Chung B, Marquart L, Ahmed M, Garantziotis S. Prevention and treatment of acute radiation-induced skin reactions: a systematic review and meta-analysis of randomized controlled trials. BMC Cancer. 2014;14:53. The use of make-up or camouflage may also help on the self-esteem. Preference for noncomedogenic products is recommended.
Dermatological adverse events
As dermatologists usually start their evaluation on skin exam, the different DAE will be grouped in types of skin reactions and areas of involvement. In tables 1-3, readers can find the DAE grouped by class of agents and the level of evidence of the recommendations for each treatment used on this guideline.
Pigmentary changes
Hyperpigmentation can occur at different sites and in different patterns (Fig. 1). They can start days to months after initiation and most of the times fade months after discontinuation of therapy. It can occur: (a) On photo distributed areas, preceded or not by photosensitivity signs; (b) As a serpentine supravenous hyperpigmentation after peripheral chemotherapy infusion (e.g. fluorouracil, docetaxel), that might be preceded or not by erythema and inflammation; (c) In a diffuse pattern, sometimes reticulated (doxorubicin, hydroxyurea, methotrexate); (d) Localized in areas of pressure, flexural areas or under occlusive dressings (ifosfamide, thiotepa); (e) As acral pigmentation (along the crease lines or macular, over the palms and soles).44 Reyes-Habito CM, Roh EK. Cutaneous reactions to chemotherapeutic drugs and targeted therapies for cancer: Part I. Conventional chemotherapeutic drugs. J Am Acad Dermatol. 2014;71:203.,2020 Sibaud V, Leboeuf NR, Roche H, Belum VR, Gladieff L, Deslandres M, et al. Dermatological adverse events with taxane chemotherapy. Eur J Dermatol. 2016;26:427-43.
Different hyperpigmentation patterns: (A) serpentine supravenous hyperpigmentation after peripheral chemotherapy infusion (fluorouracil); (B) nail plate pigmentation (daunorubicin); (C) acral lentiginoses (doxorubicin).
Flagellate dermatitis and pigmentation is related to bleomycin treatment (20%-30% of patients) and occur as a pruritic erythematous linear streaks, followed by pigmentation (Fig. 2). Busulfan treatment can cause an "Addison-like" pigmentation, with a tan appearance. 44 Reyes-Habito CM, Roh EK. Cutaneous reactions to chemotherapeutic drugs and targeted therapies for cancer: Part I. Conventional chemotherapeutic drugs. J Am Acad Dermatol. 2014;71:203.
Flagellate dermatitis associated to bleomycin treatment: (A) pruritic erythematous linear streaks, (B) followed by linear pigmentation.
Patients should be oriented to adhere to sun-protective measures and bleaching agents might also be used to accelerate clearing.
Targeted therapies can also cause pigmentary changes such as a yellow pigmentation on sunitinib treated patients (VEGFRi). Hypopigmentation (diffuse or localized) is associated with c-kit inhibitors such as imatinib, especially in patients with darker skin, as c-kit also regulates melanocyte function. They are usually reversible with dose reduction or discontinuation. 55 Reyes-Habito CM, Roh EK. Cutaneous reactions to chemotherapeutic drugs and targeted therapy for cancer: Part II. Targeted therapy. J Am Acad Dermatol. 2014;71:217.
More recently, immunotherapy has been linked to vitiligo-like lesions, mainly but not only in melanoma treated patients. Associated hair depigmentation can also be observed. No definitive treatment exists and it usually persists after the completion of immunotherapy. Reports of white hair repigmentation are also available.1010 Friedman CF, Proverbs-Singh TA, Postow MA. Treatment of the immune-related adverse effects of immune checkpoint inhibitors: a review. JAMA Oncol. 2016;2:1346-53.
11 Puzanov I, Diab A, Abdallah K, Bingham CO, Brogdon C, Dadu R, et al. Society for Immunotherapy of Cancer Toxicity Management Working Group Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer. 2017;5:95.-1212 Rapoport BL, van Eeden R, Sibaud V, Epstein JB, Klastersky J, Aapro M, et al. Supportive care for patients undergoing immunotherapy. Support Care Cancer. 2017;25:3017-30.,2121 Yin ES, Totonchy MB, Leventhal JS. Nivolumab-associated vitiligo-like depigmentation in a patient with acute myeloid leukemia: a novel finding. JAAD Case Rep. 2017;3:90-2.
Nail changes
Nail alterations during cancer treatment are frequent, usually well tolerated and disappear under cessation of treatment. However, in some circumstances they might be symptomatic and interfere with patient's daily activities. 2222 Robert C, Sibaud V, Mateus C, Verschoore M, Charles C, Lanoy E, et al. Nail toxicities induced by systemic anticancer treatments. Lancet Oncol. 2015;16:e181-9.
Conventional chemotherapeutic agents are usually related to alterations such as melanonychia (diffuse, transverse or longitudinal), leukonychia, onycholysis, Beau's lines, onychomadesis and onychorrhexis.
Taxanes are associated with painful subungual hemorrhage, followed by onycholysis and sometimes subungual abscess formation that are quite symptomatic, and might lead to dose reductions or treatment discontinuation (Fig. 3). The use of frozen gloves and socks during infusion might reduce the severity of nail alterations, but they are usually not well tolerated (nail toxicity, one study, 45 patients, side-to-side, 11% vs. 51%, p = 0.0001).22 Balagula Y, Rosen ST, Lacouture ME. The emergence of supportive oncodermatology: the study of dermatologic adverse events to cancer therapies. J Am Acad Dermatol. 2011;65:624-35.,44 Reyes-Habito CM, Roh EK. Cutaneous reactions to chemotherapeutic drugs and targeted therapies for cancer: Part I. Conventional chemotherapeutic drugs. J Am Acad Dermatol. 2014;71:203.,2020 Sibaud V, Leboeuf NR, Roche H, Belum VR, Gladieff L, Deslandres M, et al. Dermatological adverse events with taxane chemotherapy. Eur J Dermatol. 2016;26:427-43. Drainage of hemorrhage and abscesses might be needed for symptoms relief, as well as culture guided antibiotic treatments.
PATEO syndrome (PeriArticular Thenar Erythema and Onycholysis): docetaxel treated patient presenting with (A) erythematous lesions with a distinct distribution to the dorsal aspects of the hands and (B) associated nail changes - subungual hemorrhage and onycholysis.
Both conventional and targeted treatments can cause nail plate alterations such as brittle nails, nail cracking and onychoschizia. Those effects can be handled with preventive measures such as reduction of contact to water, use of cotton gloves beneath plastic or rubber gloves for any wet work, avoidance of any damaging procedure (e.g.: aggressive manicuring), and hydration with thick emollients. Oral biotin and lacquers such as hydroxypropyl chitosan and poly-ureaurethane might be used, although no controlled study is yet available. 2222 Robert C, Sibaud V, Mateus C, Verschoore M, Charles C, Lanoy E, et al. Nail toxicities induced by systemic anticancer treatments. Lancet Oncol. 2015;16:e181-9.
For onycholysis, avoiding traumas (e.g.: keeping nails short) and humidity is important. If signs of pseudomonas colonization (green discoloration), a topical antibiotic might be used (we suggest ophthalmologic solutions such as tobramycin or ciprofloxacin eye drops twice daily).
VEGFRi are associated with asymptomatic splinter subungual hemorrhages (black, red or brown longitudinal lines) in 25-70% of patients, with fingernails being mostly affected.55 Reyes-Habito CM, Roh EK. Cutaneous reactions to chemotherapeutic drugs and targeted therapy for cancer: Part II. Targeted therapy. J Am Acad Dermatol. 2014;71:217.,77 Macdonald JB, Macdonald B, Golitz LE, LoRusso P, Sekulic A. Cutaneous adverse effects of targeted therapies: Part II: Inhibitors of intracellular molecular signaling pathways. J Am Acad Dermatol. 2015;72:221-36.
EGFRi are related to frequent nail toxicities, occurring in around 17.2% of patients on a previous meta-analysis. 2323 Garden BC, Wu S, Lacouture ME. The risk of nail changes with epidermal growth factor receptor inhibitors: a systematic review of the literature and meta-analysis. J Am Acad Dermatol. 2012;67:400-8. Periungueal fissures, paronychia and pyogenic granuloma-like lesions start to develop two or more months after initiation of therapy, on both finger and toe nails (Fig. 4). They are initially sterile, but secondary infection may occur. Preventive measures include the ones listed above and also the use of comfortable shoes, sometimes with cushioning inserts for the affected nails, adequate nail cutting and the use of antiseptic solutions such as Burrow's solution soaks, white vinegar soaks (1:1), bleach soaks (1/4 cup bleach: approx. 10 l water), chlorhexidine or povidone iodine66 Macdonald JB, Macdonald B, Golitz LE, LoRusso P, Sekulic A. Cutaneous adverse effects of targeted therapies: Part I: Inhibitors of the cellular membrane. J Am Acad Dermatol. 2015;72:203-18.,2424 Burtness B, Anadkat M, Basti S, Hughes M, Lacouture ME, McClure JS, et al. NCCN Task Force Report: management of dermatologic and other toxicities associated with EGFR inhibition in patients with cancer. J Natl Compr Canc Netw. 2009;7(Suppl. 1):S5-21.
25 Melosky B, Leighl NB, Rothenstein J, Sangha R, Stewart D, Papp K. Management of egfr tki-induced dermatologic adverse events. Curr Oncol. 2015;22:123-32.-2626 Lacouture ME, Anadkat M, Jatoi A, Garawin T, Bohac C, Mitchell E. Dermatologic toxicity occurring during anti-EGFR monoclonal inhibitor therapy in patients with metastatic colorectal cancer: a systematic review. Clin Colorectal Cancer. 2018;17:85-6. or a solution of sodium hypoclorite 2.5%, sodium chloride 1.0%, deionized water qsp 100 mL (5 drops/1 l). The use of oral tetracyclines is controversial as they were related to a decrease on the incidence of paronychia in some trials and showed no benefits on others. 2727 Dsouza PC, Kumar S. Role of systemic antibiotics in preventing epidermal growth factor receptor: tyrosine kinase inhibitors-induced skin toxicities. Asia Pac J Oncol Nurs. 2017;4:323-29. For fissuring, protective coverings such as hydrocolloid, biological glue or even cyanoacrylate glue to relieve pain and promote healing, barrier creams (petroleum jelly, zinc oxide cream) and thick emollients might be used. For granulomas, if secondary infection is suspected, culture is indicated and proper antibiotics should be prescribed. For non infected lesions, destructive measures such as trichloroacetic acid (70-90%) or cryotherapy might be used. Potent topical steroids, occlusive or not, are also indicated. 2828 Wnorowski AM, de Souza A, Chachoua A, Cohen DE. The management of EGFR inhibitor adverse events: a case series and treatment paradigm. Int J Dermatol. 2012;51:223-32. Sometimes surgical treatment might be necessary. Recently, the use of topical betaxolol was described for treating a pyogenic granuloma-like lesion induced by EGFRi. 2929 Yen CF, Hsu CK, Lu CW. Topical betaxolol for treating relapsing paronychia with pyogenic granuloma-like lesions induced by epidermal growth factor receptor inhibitors. J Am Acad Dermatol. 2018;78:e143-44.
EGFR inhibitors related adverse events: (A and B) inflammatory papulopustular rash with associated xerosis (*); (C) trychomegaly and hypertrichosis; (D) periungual fissures and (E) pyogenic granuloma-like lesions. (A, B, D and E on cetuximab treated patients; C on panitumumab treated patient).
Acral reactions
Hand-foot syndrome (HFS), also known as palmoplantar erythrodysestesia, is a DAE related to conventional chemotherapeutic drugs, common with agents such as capecitabine, doxorubicin, cytarabine, 5-FU. It is preceded by prodrome symptoms such as tingling or pain at the extremities, followed by a symmetric, sharply demarcated erythema and edema of the palms and soles (Fig. 5). Vesicles and bullae might also be present. Pain usually limits daily activities and might be a cause of dose reduction or discontinuation. Incidence is higher with prolonged infusions or oral agents.22 Balagula Y, Rosen ST, Lacouture ME. The emergence of supportive oncodermatology: the study of dermatologic adverse events to cancer therapies. J Am Acad Dermatol. 2011;65:624-35.,44 Reyes-Habito CM, Roh EK. Cutaneous reactions to chemotherapeutic drugs and targeted therapies for cancer: Part I. Conventional chemotherapeutic drugs. J Am Acad Dermatol. 2014;71:203.
Toxic erythema of chemotherapy (TEC): combination of different lesions caused by direct toxicity of chemotherapy agents with (A) lesions on flexural areas (intertriginous eruption associated with chemotherapy) and (B) on palms and soles (Hand-foot syndrome - HFS).
PATEO syndrome (PeriArticular Thenar Erythema and Onycholysis) is a variant of HFS, specific to the taxanes (docetaxel) (Fig. 3). It is characterized by scaly erythematous lesions with a distinct distribution to the dorsal aspects of the hands (overlying the joints) and thenar eminences. More rarely, it can also affect the dorsum of the feet. Although common, nails are not universally affected. It is usually bilateral, not necessarily symmetric. It may start on the first chemotherapy cycle or develop progressively. Burning sensation and pain are the most reported symptoms. 2020 Sibaud V, Leboeuf NR, Roche H, Belum VR, Gladieff L, Deslandres M, et al. Dermatological adverse events with taxane chemotherapy. Eur J Dermatol. 2016;26:427-43.
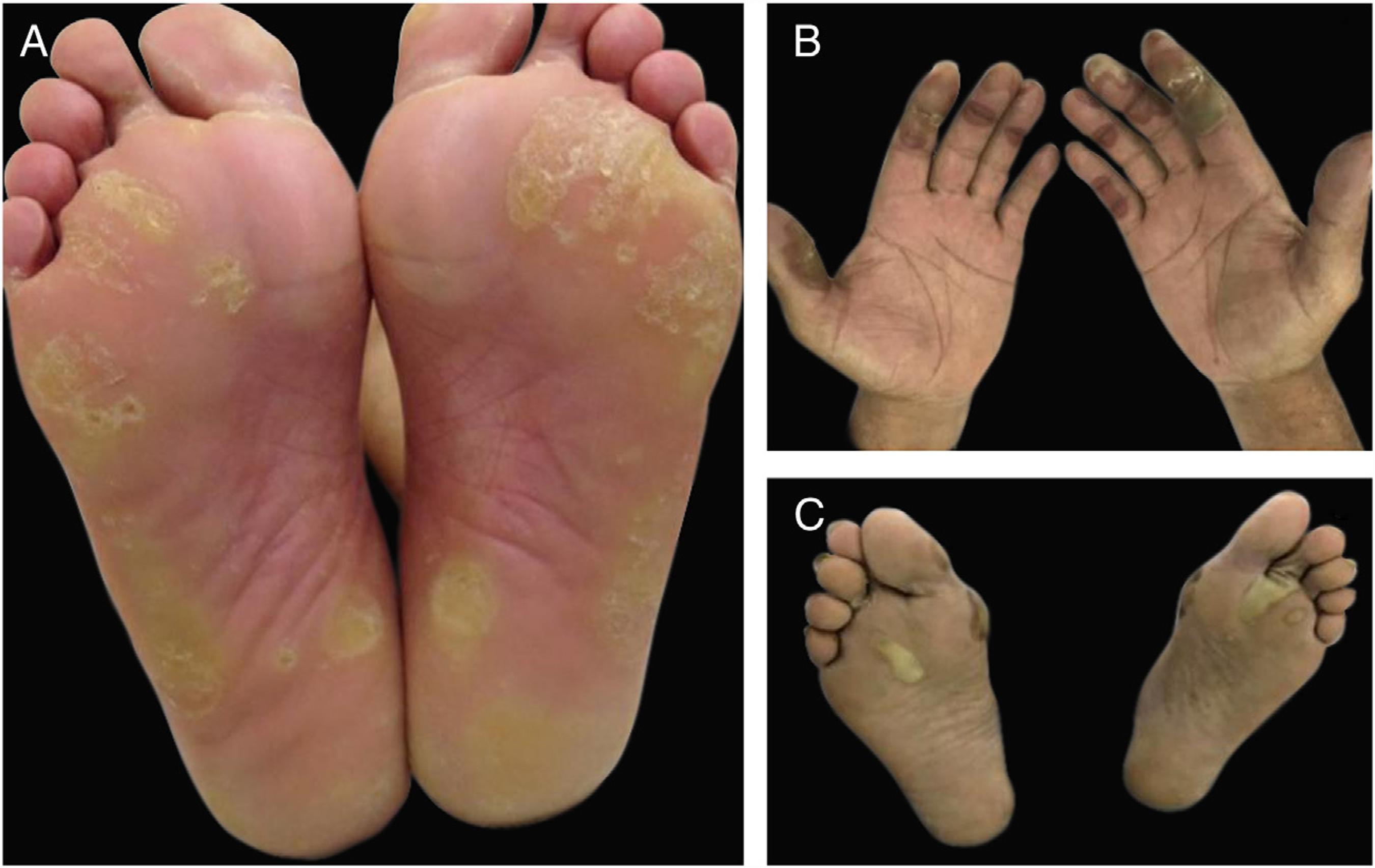
Hand-foot skin reaction (HFSR) is another variant of acral toxicity related to targeted therapies, with both monoclonal antibodies and small molecules tyrosine kinase inhibitors especially with those targeting VEGFR (bevacizumab, sorafenib, sunitinib) and BRAF (vemurafenib) (Fig. 6). Despite the similar palmoplantar distribution, dose dependency and associated pain, HFSR develop on friction or trauma-prone areas (pressure areas), such as the heel and lateral aspects of the soles and web spaces. Lesions start 2-4 weeks after beginning of therapy and are characterized by hyperkeratosis, resembling skin calluses, occasionally with superficial blistering and erythematous halos.22 Balagula Y, Rosen ST, Lacouture ME. The emergence of supportive oncodermatology: the study of dermatologic adverse events to cancer therapies. J Am Acad Dermatol. 2011;65:624-35.,55 Reyes-Habito CM, Roh EK. Cutaneous reactions to chemotherapeutic drugs and targeted therapy for cancer: Part II. Targeted therapy. J Am Acad Dermatol. 2014;71:217.,66 Macdonald JB, Macdonald B, Golitz LE, LoRusso P, Sekulic A. Cutaneous adverse effects of targeted therapies: Part I: Inhibitors of the cellular membrane. J Am Acad Dermatol. 2015;72:203-18.,3030 Lacouture ME, Wu S, Robert C, Atkins MB, Kong HH, Guitart J, et al. Evolving strategies for the management of hand-foot skin reaction associated with the multitargeted kinase inhibitors sorafenib and sunitinib. Oncologist. 2008;13:1001-11.,3131 Anderson R, Jatoi A, Robert C, Wood LS, Keating KN, Lacouture ME. Search for evidence-based approaches for the prevention and palliation of hand-foot skin reaction (HFSR) caused by the multikinase inhibitors (MKIs). Oncologist. 2009;14:291-302. Depending on the drug, lesions might improve or not over time. 3232 Flaherty KT, Brose MS. Sorafenib-related hand-foot skin reaction improves not worsens, with continued treatment. Clin Cancer Res. 2009;15:7749.
Hand-foot skin reaction (HFSR) associated with antiangiogenic agents (VEGFRi): (A) hyperkeratotic lesions (sorafenib) and (B) bullous lesions (axitinib) on areas of pressure and friction.
Prevention
HFS and PATEO: on a prior systematic review, the only two evidenced based measures for prevention (to decrease incidence and/or severity) were the use of a nonsteroidal anti-inflammatory drug (NSAIDs - celocoxib) (for any grade: Odds Ratio - OR = 0.47, 95% IC 0.29-0.78, p = 0.003; for Grade 2-3: OR = 0.39, 95% IC 0.20-0.73, p = 0.003) and dose reduction. One study showed benefit with the use of regional cooling during chemotherapy infusion (incidence 36% × 7.1%, p = 0.0097). However, as previously discussed, frozen gloves and socks are usually not well tolerated by patients; regarding to NSAIDs, the risks and benefits of their use must be weighted; regarding dose reductions, it has direct impact on disease outcome.3333 Macedo LT, Lima JP, dos Santos LV, Sasse AD. Prevention strategies for chemotherapy-induced hand-foot syndrome: a systematic review and meta-analysis of prospective randomised trials. Support Care Cancer. 2014;22:1585-93.,3434 Huang XZ, Chen Y, Chen WJ, Zhang X, Wu CC, Wang ZN, et al. Clinical evidence of prevention strategies for capecitabine-induced hand-foot syndrome. Int J Cancer. 2018;142:2567-77. The use of emollients seemed promisor, but with no statistical significant difference. Pyridoxine was not effective for the prevention.3333 Macedo LT, Lima JP, dos Santos LV, Sasse AD. Prevention strategies for chemotherapy-induced hand-foot syndrome: a systematic review and meta-analysis of prospective randomised trials. Support Care Cancer. 2014;22:1585-93.
34 Huang XZ, Chen Y, Chen WJ, Zhang X, Wu CC, Wang ZN, et al. Clinical evidence of prevention strategies for capecitabine-induced hand-foot syndrome. Int J Cancer. 2018;142:2567-77.-3535 Yap YS, Kwok LL, Syn N, Chay WY, Chia JWK, Tham CK, et al. Predictors of hand-foot syndrome and pyridoxine for prevention of capecitabine-induced hand-foot syndrome: a randomized clinical trial. JAMA Oncol. 2017;3:1538-45. A small RCT showed benefits with the use of an antioxidant-containing ointment when compared to placebo on pegylated liposomal doxorubicin treated patients. 3636 Jung S, Sehouli J, Chekerov R, Kluschke F, Patzelt A, Fuss H, et al. Prevention of palmoplantar erythrodysesthesia in patients treated with pegylated liposomal doxorubicin (Caelyx®). Support Care Cancer. 2017;25:3545-49. For HFSR one RCT showed a decreased incidence in any grade and >Grade 2 reaction with the use of urea 10% cream compared to "best supportive care" (for any grade: OR = 0.457, 95% CI 0.34-0.60, p < 0.001, for ≥Grade 2: OR = 0.635, 95% CI 0.46-0.86, p = 0.004). Results might be questioned, though, because "best supportive care" was indeed no preventive care.3737 Ren Z, Zhu K, Kang H, Lu M, Qu Z, Lu L, et al. Randomized controlled trial of the prophylactic effect of urea-based cream on sorafenib-associated hand-foot skin reactions in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2015;33:894-900.,3838 Negri FV, Porta C. Urea-based cream to prevent sorafenib-induced hand-and-foot skin reaction: which evidence?. J Clin Oncol. 2015;33:3219-20. Another small prospective trial showed a decrease in occurrence of HFSR on sorafenib treated patients with the ingestion of a Japanese food (Bonito broth) when compared to no ingestion (Hazard Ratio, HR = 0.097, 95% CI 0.011-0.846, p = 0.035 on multivariable analysis). This food is shown to increase peripheral blood flow in humans. 3939 Kamimura K, Shinagawa-Kobayashi Y, Goto R, Ogawa K, Yokoo T, Sakamaki A, et al. Effective prevention of sorafenib-induced hand-foot syndrome by dried-bonito broth. Cancer Manag Res. 2018;10:805-13. Another isolated report showed success with the use of topical calcipotriol in one case. 4040 Demirkan S, Gündüz Ö, Devrim T. Sorafenib-asssociated hand-foot syndrome treated with topical calcipotriol. JAAD Case Rep. 2017;3:354-57.
Despite the absence of a strong evidence, we do recommend the following preventive measures22 Balagula Y, Rosen ST, Lacouture ME. The emergence of supportive oncodermatology: the study of dermatologic adverse events to cancer therapies. J Am Acad Dermatol. 2011;65:624-35.,55 Reyes-Habito CM, Roh EK. Cutaneous reactions to chemotherapeutic drugs and targeted therapy for cancer: Part II. Targeted therapy. J Am Acad Dermatol. 2014;71:217.
6 Macdonald JB, Macdonald B, Golitz LE, LoRusso P, Sekulic A. Cutaneous adverse effects of targeted therapies: Part I: Inhibitors of the cellular membrane. J Am Acad Dermatol. 2015;72:203-18.-77 Macdonald JB, Macdonald B, Golitz LE, LoRusso P, Sekulic A. Cutaneous adverse effects of targeted therapies: Part II: Inhibitors of intracellular molecular signaling pathways. J Am Acad Dermatol. 2015;72:221-36.,3030 Lacouture ME, Wu S, Robert C, Atkins MB, Kong HH, Guitart J, et al. Evolving strategies for the management of hand-foot skin reaction associated with the multitargeted kinase inhibitors sorafenib and sunitinib. Oncologist. 2008;13:1001-11.,3131 Anderson R, Jatoi A, Robert C, Wood LS, Keating KN, Lacouture ME. Search for evidence-based approaches for the prevention and palliation of hand-foot skin reaction (HFSR) caused by the multikinase inhibitors (MKIs). Oncologist. 2009;14:291-302.,4141 von Moos R, Thuerlimann BJ, Aapro M, Rayson D, Harrold K, Sehouli J, et al. Pegylated liposomal doxorubicin-associated hand-foot syndrome: recommendations of an international panel of experts. Eur J Cancer. 2008;44:781-90.:
Educate patients of early signs and symptoms;
Use thick cotton gloves and/or socks;
Apply emollient creams (urea based emollients in hyperkeratotic type) to hands and feet regularly;
Avoid irritants such as alcohol, harsh cleansing agents and tight clothing and shoes;
Avoid extremes of temperature, pressure and friction (e.g.: repetitive activities, stressful manual work, etc.).
For HFSR also include a pretreatment evaluation with a podiatrist with callosity chopping and the use of orthopedic shoe inserts when needed.
Treatment
Also low evidence exists on treatment measures. Dose reductions are effective but interferes with disease related outcomes. One small prospective non-comparative trial showed improvement in QoL and decrease on HFS symptoms with the use of a topical non-occlusive polymer for 8 weeks. 4242 Fabbrocini G, Cristaudo A, Ionescu MA, Panariello L, Robert G, Pellicano M, et al. Topical non-occlusive polymers in hand-foot syndrome. G Ital Dermatol Venereol. 2018;153:165-71. For HFSR, one small randomized phase II trial showed benefit with the treatment of Grade 1 toxicity with a hydrocolloid dressing containing ceramide with a low-friction external surface when compared to urea 10% cream (Grade 2-3, 29% vs. 69%, p = 0.03). 4343 Shinohara N, Nonomura N, Eto M, Kimura G, Minami H, Tokunaga S, et al. A randomized multicenter phase II trial on the efficacy of a hydrocolloid dressing containing ceramide with a low-friction external surface for hand-foot skin reaction caused by sorafenib in patients with renal cell carcinoma. Ann Oncol. 2014;25:472-76. Recently, a systematic review showed benefits of different Chinese herbs on the treatment of acral toxicities. However, most of the studies were not blinded and with a lower quality.4444 Deng B, Sun W. Herbal medicine for hand-foot syndrome induced by fluoropyrimidines: a systematic review and meta-analysis. Phytother Res. 2018;32:1211-28.,4545 Tian A, Zhou A, Bi X, Hu S, Jiang Z, Zhang W, et al. Efficacy of topical compound danxiong granules for treatment of dermatologic toxicities induced by targeted anticancer therapy: a randomized, double-blind placebo-controlled trial. Evid Based Complement Alternat Med. 2017;2017:3970601. One case report and a small case series suggest benefits on the use of topical Henna for capecitabine HFS.4646 Yucel I, Guzin G. Topical henna for capecitabine induced hand-foot syndrome. Invest New Drugs. 2008;26:189-92.,4747 Ilyas S, Wasif K, Saif MW. Topical henna ameliorated capecitabine-induced hand-foot syndrome. Cutan Ocul Toxicol. 2014;33:253-55. Besides the already mentioned preventive measures, recommendations of this board for the treatment of those toxicities include22 Balagula Y, Rosen ST, Lacouture ME. The emergence of supportive oncodermatology: the study of dermatologic adverse events to cancer therapies. J Am Acad Dermatol. 2011;65:624-35.,55 Reyes-Habito CM, Roh EK. Cutaneous reactions to chemotherapeutic drugs and targeted therapy for cancer: Part II. Targeted therapy. J Am Acad Dermatol. 2014;71:217.
6 Macdonald JB, Macdonald B, Golitz LE, LoRusso P, Sekulic A. Cutaneous adverse effects of targeted therapies: Part I: Inhibitors of the cellular membrane. J Am Acad Dermatol. 2015;72:203-18.-77 Macdonald JB, Macdonald B, Golitz LE, LoRusso P, Sekulic A. Cutaneous adverse effects of targeted therapies: Part II: Inhibitors of intracellular molecular signaling pathways. J Am Acad Dermatol. 2015;72:221-36.,3030 Lacouture ME, Wu S, Robert C, Atkins MB, Kong HH, Guitart J, et al. Evolving strategies for the management of hand-foot skin reaction associated with the multitargeted kinase inhibitors sorafenib and sunitinib. Oncologist. 2008;13:1001-11.,3131 Anderson R, Jatoi A, Robert C, Wood LS, Keating KN, Lacouture ME. Search for evidence-based approaches for the prevention and palliation of hand-foot skin reaction (HFSR) caused by the multikinase inhibitors (MKIs). Oncologist. 2009;14:291-302.,4141 von Moos R, Thuerlimann BJ, Aapro M, Rayson D, Harrold K, Sehouli J, et al. Pegylated liposomal doxorubicin-associated hand-foot syndrome: recommendations of an international panel of experts. Eur J Cancer. 2008;44:781-90.:
Continuing the use of preventive measures;
Maintaining the use of emollients, and on the case of HFSR, include the use of keratolytic agents (e.g.: urea 10-40%, salicylic acid, etc.);
Add a potent topical steroid;
For relief of symptoms, cool compresses or emergence of hands and feet on cool water, topical anesthetics and NSAIDs might be used;
Dose reduction or treatment interruption is sometimes necessary until symptoms decrease.
Skin rashes
One of the problems of better defining the DAE in many oncology trials is that frequently investigators report the different types of cutaneous eruptions as a skin "rash". Yet, as we will see, there are different types of cutaneous eruptions, associated with different classes of drugs and with different treatment options.
Acute hypersensitivity reactions: those Type I immunoglobulin E-mediated reactions may occur within minutes to hours of infusion. They manifest as regular hypersensitivity reactions to conventional drugs, as pruritus, flushing, urticaria and even anaphylaxis. The difference relies though on the way we deal with it. With conventional drugs, patients are usually oriented to avoid re-exposure. When dealing with oncologic treatments, the chemotherapeutic agent is fundamental for disease related survival. Therefore, we usually maintain the drug for the next cycles and manage the reaction with a slower infusion, a premedication with corticosteroids and antihistamines before every infusion and a closer monitoring.44 Reyes-Habito CM, Roh EK. Cutaneous reactions to chemotherapeutic drugs and targeted therapies for cancer: Part I. Conventional chemotherapeutic drugs. J Am Acad Dermatol. 2014;71:203.,66 Macdonald JB, Macdonald B, Golitz LE, LoRusso P, Sekulic A. Cutaneous adverse effects of targeted therapies: Part I: Inhibitors of the cellular membrane. J Am Acad Dermatol. 2015;72:203-18.,77 Macdonald JB, Macdonald B, Golitz LE, LoRusso P, Sekulic A. Cutaneous adverse effects of targeted therapies: Part II: Inhibitors of intracellular molecular signaling pathways. J Am Acad Dermatol. 2015;72:221-36.
Exanthema: many treatments may be related to a non-specific maculopapular rash or morbiliform eruption that starts gradually, sometimes weeks after the start of drug, with mild symptoms such as pruritus. Those DAE can be handled with anti-histamines and topical corticosteroids when limited, or with short courses of oral corticosteroids when more disseminated. All class of agents might cause those kind of reactions, such as kinase inhibitors (e.g.: BRAFi - vemurafenib/kit and BCR-ABL inhibitors - imatinib, dasatinib), "checkpoint" inhibitors (ipilimumab, nivolumab), and conventional chemotherapeutic agents (bleomycin, carboplatin, etoposide, etc.).44 Reyes-Habito CM, Roh EK. Cutaneous reactions to chemotherapeutic drugs and targeted therapies for cancer: Part I. Conventional chemotherapeutic drugs. J Am Acad Dermatol. 2014;71:203.,66 Macdonald JB, Macdonald B, Golitz LE, LoRusso P, Sekulic A. Cutaneous adverse effects of targeted therapies: Part I: Inhibitors of the cellular membrane. J Am Acad Dermatol. 2015;72:203-18.,77 Macdonald JB, Macdonald B, Golitz LE, LoRusso P, Sekulic A. Cutaneous adverse effects of targeted therapies: Part II: Inhibitors of intracellular molecular signaling pathways. J Am Acad Dermatol. 2015;72:221-36. Only in rare occasions severe reactions such as toxic epidermal necrolysis (TEN), Steven-Johnson syndrome (SSJ) or drug rash with eosinophilia and systemic symptoms (DRESS) might occur, but it must be remembered that a maculopapular rash may represent the first manifestation of those life-threatening conditions. Special attention should be given when using a targeted therapy after the use of an immunotherapy (e.g.: melanoma patients treated with immunotherapy and then switched to a BRAFi) as it may be associated with a higher risk of severe skin toxicity (> Grade 3 and SSJ/TEN). Some authors suggest at least a 4 week interval between treatments with those agents.77 Macdonald JB, Macdonald B, Golitz LE, LoRusso P, Sekulic A. Cutaneous adverse effects of targeted therapies: Part II: Inhibitors of intracellular molecular signaling pathways. J Am Acad Dermatol. 2015;72:221-36.,4848 Harding JJ, Pulitzer M, Chapman PB. Vemurafenib sensitivity skin reaction after ipilimumab. N Engl J Med. 2012;366:866-68.
49 Johnson DB, Wallender EK, Cohen DN, Likhari SS, Zwerner JP, Powers JG, et al. Severe cutaneous and neurologic toxicity in melanoma patients during vemurafenib administration following anti-PD-1 therapy. Cancer Immunol Res. 2013;1:373-77.-5050 Ludlow SP, Pasikhova Y. Cumulative dermatologic toxicity with ipilimumab and vemurafenib responsive to corticosteroids. Melanoma Res. 2013;23:496-97.
When a patient is on immunotherapy, skin adverse events to conventional drugs (e.g.: antibiotics) might be more intense, so it is always important to exclude other causative agents, before relating the rash to the "checkpoint" inhibitor.
Toxic erythema of chemotherapy (TEC): this term is suggested by some authors to unify different manifestations such as HFS, intertriginous eruption of chemotherapy or other used histopathologic terms such as "eccrine squamous syringometaplasia", all related to a direct toxicity of the chemotherapeutic agent and not due to an allergic reaction. This type of DAE is characterized by overlapping features of bilateral painful erythema, edema, and even bullous lesions located on hands and feet (see hand-foot syndrome), and sometimes also affecting intertriginous areas such as axilla and groins (less frequently ears, knees and elbows) (Fig. 5). It is important to distinguish, because those manifestations are usually self-limited, often resolving with desquamation and post-inflammatory pigmentation and not demanding aggressive measures. In addition, lesions develop 2-3 weeks after the chemotherapy cycle, usually when patient's defenses are lower (e.g.: neutropenia), being many times misdiagnosed as infections or graft-versus-host disease (GVHD). Treatment relies on topical corticosteroids and emollients, and educating the patient about the nature of the manifestation. It often recrudesces on the subsequent cycles. It might be milder with dose reductions.2020 Sibaud V, Leboeuf NR, Roche H, Belum VR, Gladieff L, Deslandres M, et al. Dermatological adverse events with taxane chemotherapy. Eur J Dermatol. 2016;26:427-43.,5151 Bolognia JL, Cooper DL, Glusac EJ. Toxic erythema of chemotherapy: a useful clinical term. J Am Acad Dermatol. 2008;59:524-9.
Papulopustular rash: also known as "acne-like rash" or "folliculitis", this is the most common dermatological toxicity of EGFRi treatment (Fig. 4). It usually appears 1-2 weeks after initiation of therapy, starts with erythema, followed by the eruption of papules and pustules (sterile) on the face, scalp, upper chest and back, with a lack of comedones. Sometimes lesions extend to the limbs. Skin is usually dry, itchy and sensible (patients refer sensation of burning, stinging, tenderness). This AE has a high impact on patient's QoL and on social aspects of daily living, being a cause of dose reduction or even treatment discontinuation. The rash tends to improve around the 8th week, but usually persists, as a milder eruption, with periods of improvement and worsening. Other features that are present, especially later on treatment, are the already discussed paronychia, periungual fissures and granulomas, xerosis, pruritus and the yet to be discussed hair alterations such as trichomegaly, hypertrichosis and non-scarring alopecia.66 Macdonald JB, Macdonald B, Golitz LE, LoRusso P, Sekulic A. Cutaneous adverse effects of targeted therapies: Part I: Inhibitors of the cellular membrane. J Am Acad Dermatol. 2015;72:203-18.,2323 Garden BC, Wu S, Lacouture ME. The risk of nail changes with epidermal growth factor receptor inhibitors: a systematic review of the literature and meta-analysis. J Am Acad Dermatol. 2012;67:400-8.
24 Burtness B, Anadkat M, Basti S, Hughes M, Lacouture ME, McClure JS, et al. NCCN Task Force Report: management of dermatologic and other toxicities associated with EGFR inhibition in patients with cancer. J Natl Compr Canc Netw. 2009;7(Suppl. 1):S5-21.-2525 Melosky B, Leighl NB, Rothenstein J, Sangha R, Stewart D, Papp K. Management of egfr tki-induced dermatologic adverse events. Curr Oncol. 2015;22:123-32.,5252 Chanprapaph K, Vachiramon V, Rattanakaemakorn P. Epidermal growth factor receptor inhibitors: a review of cutaneous adverse events and management. Dermatol Res Pract. 2014;2014:734249.
Prevention
General daily baseline measures have already been discussed and are of great importance on this group of agents. They include the regular use of emollients, photo protective measures (sun exposure might worsen the eruption), avoidance of irritating agents, limited shower time and use of gentle cleansers. The use of systemic antibiotics (mostly tetracycline agents) on the first 6-8 weeks of treatment has been evaluated in some trials with discordant results. The available data suggest some benefit of the preventive treatment in decreasing the incidence and mainly the intensity of the papulopustular rash when compared to no treatment or even to the reactive treatment (started once the rash is already present).2626 Lacouture ME, Anadkat M, Jatoi A, Garawin T, Bohac C, Mitchell E. Dermatologic toxicity occurring during anti-EGFR monoclonal inhibitor therapy in patients with metastatic colorectal cancer: a systematic review. Clin Colorectal Cancer. 2018;17:85-6.,5353 Lacouture ME, Keefe DM, Sonis S, Jatoi A, Gernhardt D, Wang T, et al. A phase II study (ARCHER 1042) to evaluate prophylactic treatment of dacomitinib-induced dermatologic and gastrointestinal adverse events in advanced non-small-cell lung cancer. Ann Oncol. 2016;27:1712-18.,5454 Kripp M, Prasnikar N, Vehling-Kaiser U, Quidde J, Al-Batran SE, Stein A, et al. AIO LQ-0110: a randomized phase II trial comparing oral doxycycline versus local administration of erythromycin as preemptive treatment strategies of panitumumab-mediated skin toxicity in patients with metastatic colorectal cancer. Oncotarget. 2017;8:105061-71. This is also the opinion of this expert panel. We suggest the use of oral tetracyclines (e.g. doxycycline 100 mg bid.) during the first 6 to 8 weeks of treatment with EGFRi. On one randomized phase II trial, topical erythromycin was inferior to oral doxycycline for the prevention of EGFR skin toxicity. 5454 Kripp M, Prasnikar N, Vehling-Kaiser U, Quidde J, Al-Batran SE, Stein A, et al. AIO LQ-0110: a randomized phase II trial comparing oral doxycycline versus local administration of erythromycin as preemptive treatment strategies of panitumumab-mediated skin toxicity in patients with metastatic colorectal cancer. Oncotarget. 2017;8:105061-71. Another trial showed some benefit with the preventive use of low potency topical steroids, but we recommend their use only as a reactive treatment.
Treatment
Despite the acne-like appearance, topical agents used to treat acne and acneiform eruptions such as benzoyl peroxide, retinoic acids and salicylic acid containing products are contra-indicated. If the rash occurs, preventive measures should be maintained and to that, topical steroids can be added. If more pronounced and tetracycline was not started for prevention, it can now be initiated.66 Macdonald JB, Macdonald B, Golitz LE, LoRusso P, Sekulic A. Cutaneous adverse effects of targeted therapies: Part I: Inhibitors of the cellular membrane. J Am Acad Dermatol. 2015;72:203-18.,2323 Garden BC, Wu S, Lacouture ME. The risk of nail changes with epidermal growth factor receptor inhibitors: a systematic review of the literature and meta-analysis. J Am Acad Dermatol. 2012;67:400-8.,2424 Burtness B, Anadkat M, Basti S, Hughes M, Lacouture ME, McClure JS, et al. NCCN Task Force Report: management of dermatologic and other toxicities associated with EGFR inhibition in patients with cancer. J Natl Compr Canc Netw. 2009;7(Suppl. 1):S5-21. Sometimes dose reductions might be necessary. If excessive crusting or secretion, cultures are indicated to exclude secondary bacterial infection. In those cases, culture guided antimicrobial treatment is indicated. Recently, a single RCT showed benefit with the use of a Chinese herbal topical compound (compared to placebo) for the treatment of the DAE related to targeted therapy, including the papulopustular rash. 4545 Tian A, Zhou A, Bi X, Hu S, Jiang Z, Zhang W, et al. Efficacy of topical compound danxiong granules for treatment of dermatologic toxicities induced by targeted anticancer therapy: a randomized, double-blind placebo-controlled trial. Evid Based Complement Alternat Med. 2017;2017:3970601. In addition, there are some reports and some personal experience on the use of low dose systemic isotretinoin for refractory cases. 5555 Requena C, Llombart B, Sanmartín O. Acneiform eruptions induced by epidermal growth factor receptor inhibitors: treatment with oral isotretinoin. Cutis. 2012;90:77-80.
Acneiform eruption: conventional chemotherapeutic regimens frequently contain high doses of systemic corticosteroids, such as dexamethasone or prednisone. For that reason, steroid related acneiform eruption might occur and should be treated similar to the non-oncologic patients.
Photosensitivity reactions: oncologic treatments have been reported to cause both phototoxic or photoallergic reactions. Many conventional agents such as 5-FU and taxanes (mainly to UVB) have reported inflammatory rashes on photo-exposed areas.44 Reyes-Habito CM, Roh EK. Cutaneous reactions to chemotherapeutic drugs and targeted therapies for cancer: Part I. Conventional chemotherapeutic drugs. J Am Acad Dermatol. 2014;71:203.,2020 Sibaud V, Leboeuf NR, Roche H, Belum VR, Gladieff L, Deslandres M, et al. Dermatological adverse events with taxane chemotherapy. Eur J Dermatol. 2016;26:427-43. With EGFRi, not only photosensitive eruptions can occur, but also the other DAE can be exacerbated by photo exposure. 66 Macdonald JB, Macdonald B, Golitz LE, LoRusso P, Sekulic A. Cutaneous adverse effects of targeted therapies: Part I: Inhibitors of the cellular membrane. J Am Acad Dermatol. 2015;72:203-18. One of the most photosensitizing agent is vemurafenib (BRAFi) with a proved sensitivity for UVA radiation, which is present on fluorescence lamps and passes through windows.55 Reyes-Habito CM, Roh EK. Cutaneous reactions to chemotherapeutic drugs and targeted therapy for cancer: Part II. Targeted therapy. J Am Acad Dermatol. 2014;71:217.,77 Macdonald JB, Macdonald B, Golitz LE, LoRusso P, Sekulic A. Cutaneous adverse effects of targeted therapies: Part II: Inhibitors of intracellular molecular signaling pathways. J Am Acad Dermatol. 2015;72:221-36.,5656 Woods JA, Ferguson JS, Kalra S, Degabriele A, Gardner J, Logan P, et al. The phototoxicity of vemurafenib: an investigation of clinical monochromator phototesting and in vitro phototoxicity testing. J Photochem Photobiol B. 2015;151:233-8.,5757 Zimmer L, Vaubel J, Livingstone E, Schadendorf D. Side effects of systemic oncological therapies in dermatology. J Dtsch Dermatol Ges. 2012;10:475-86. Vandetanib (TKI) is also associated to a UVA sensitivity. Preventive sun protective measures are fundamental. If reaction occurs, it might be handled with topical steroids or short courses of oral steroids.
Keratosis pilaris-like eruption: this adverse event has been linked to the use of BRAFi and is characterized by diffuse follicular keratotic papules in a generalized distribution, resembling keratosis pilaris. Topical keratolytic agents might be used. 77 Macdonald JB, Macdonald B, Golitz LE, LoRusso P, Sekulic A. Cutaneous adverse effects of targeted therapies: Part II: Inhibitors of intracellular molecular signaling pathways. J Am Acad Dermatol. 2015;72:221-36. Vemurafenib could also cause folliculocentric morbilliform rash (Fig. 7).
BRAF inhibitor related adverse events: multiple keratoachantomas (A) and low grade squamous cell carcinomas (B) after withdrawal of MEK inhibitor and maintenance of BRAF inhibitor; (C) associated keratosis pilaris-like eruption on the lower limbs.
Scalp and hair abnormalities
Some general recommended measures for hair and scalp daily care include the use of a gentle shampoo, avoiding hot water, hair dyes and hair foaming.
Conventional chemotherapy-induced alopecia (CIA): is one of the most distressing events in cancer patients treated with conventional agents. It is caused mainly by an anagen effluvium and is usually (although not always) completely reversible 2-6 months after treatment is discontinued.22 Balagula Y, Rosen ST, Lacouture ME. The emergence of supportive oncodermatology: the study of dermatologic adverse events to cancer therapies. J Am Acad Dermatol. 2011;65:624-35.,2020 Sibaud V, Leboeuf NR, Roche H, Belum VR, Gladieff L, Deslandres M, et al. Dermatological adverse events with taxane chemotherapy. Eur J Dermatol. 2016;26:427-43. It affects more often scalp hair, but eyebrows, eyelashes, and other body areas might also be affected. Hair loss will be influenced not only by the drug, but also by the route of administration, dosing and schedule (e.g.: high dose, intravenous, intermittent regimens are more prone to cause complete alopecia). Examples of agents with a high risk include cyclophosphamide, doxorubicin, irinotecan and taxanes (docetaxel and paclitaxel).
Prevention
A systematic review of preventive measures found no benefit with the use of topical minoxidil, or scalp compression. Benefit was found with the use of a scalp cooling device (RR = 0.38, CI 95% 0.32-0.45, p < 0.001).5858 Shin H, Jo SJ, Kim DH, Kwon O, Myung SK. Efficacy of interventions for prevention of chemotherapy-induced alopecia: a systematic review and meta-analysis. Int J Cancer. 2015;136:E442-54.
59 Vasconcelos I, Wiesske A, Schoenegg W. Scalp cooling successfully prevents alopecia in breast cancer patients undergoing anthracycline/taxane-based chemotherapy. Breast. 2018;40:1-3.
60 Shah VV, Wikramanayake TC, DelCanto GM, van den Hurk C, Wu S, Lacouture ME, et al. Scalp hypothermia as a preventative measure for chemotherapy-induced alopecia: a review of controlled clinical trials. J Eur Acad Dermatol Venereol. 2018;32:720-34.-6161 Nangia J, Wang T, Osborne C, Niravath P, Otte K, Papish S, et al. Effect of a scalp cooling device on alopecia in women undergoing chemotherapy for breast cancer: the SCALP randomized clinical trial. JAMA. 2017;317:596-605. Scalp cooling systems include static devices (cool caps) and dynamic scalp cooling systems. Patients should be warned that treatment efficacy is variable (around 50-80% depending on agent). Available data suggest that this technology is most effective for taxane-based chemotherapy regimens compared with anthracycline-based chemotherapy regimens. 6262 Kruse M, Abraham J. Management of chemotherapy-induced alopecia with scalp cooling. J Oncol Pract. 2018;14:149-54. Besides, the use of the cooling devices are not always well tolerated as they may cause symptoms like headache and scalp pain. Devices are also not reimbursable by insurance companies and might have a high cost. Recent studies have shown that the incidence of scalp metastasis is not increased in breast cancer patients with localized disease treated with scalp cooling.6363 Rugo HS, Klein P, Melin SA, Hurvitz SA, Melisko ME, Moore A, et al. Association between use of a scalp cooling device and alopecia after chemotherapy for breast cancer. JAMA. 2017;317:606-14.,6464 Rugo HS, Voigt J. Scalp hypothermia for preventing alopecia during chemotherapy. A systematic review and meta-analysis of randomized controlled trials. Clin Breast Cancer. 2018;18:19-28. However, there are two reported cases of disease recurrence in patients with hematological malignancies (mycosis fungoides and acute myeloid leukemia).6565 Witman G, Cadman E, Chen M. Misuse of scalp hypothermia. Cancer Treat Rep. 1981;65:507-8.,6666 Forsberg SA. Scalp cooling therapy and cytotoxic treatment. Lancet. 2001;357:1134. Because of that, scalp cooling should be avoided in hematological malignancies. 6767 Rubio-Gonzalez B, Juhász M, Fortman J, Mesinkovska NA. Pathogenesis and treatment options for chemotherapy-induced alopecia: a systematic review. Int J Dermatol. 2018;57:1417-24.
Treatment
Minoxidil was not effective in preventing CIA, but a small trial showed that minoxidil 2% was associated with a faster regrowth of hair (time to regrowth of 86 vs. 136 days on the placebo group). 6868 Yeager CE, Olsen EA. Treatment of chemotherapy-induced alopecia. Dermatol Ther. 2011;24:432-42. Other agents such as topical calcitriol are under investigation, but still with no definitive results.22 Balagula Y, Rosen ST, Lacouture ME. The emergence of supportive oncodermatology: the study of dermatologic adverse events to cancer therapies. J Am Acad Dermatol. 2011;65:624-35.,6969 Freites-Martinez A, Shapiro J, Goldfarb S, Nangia J, Jimenez JJ, et al. CME. Part 1: Hair disorders in cancer patients. J Am Acad Dermatol. 2019;80:1179-96. Based on the studies and on authors daily practice, we recommend the use of topical minoxidil 5% once daily after the end of the chemotherapy cycles. For eyebrows and eyelashes, topical bimatoprost might be used (one controlled study for eyelash showed benefit at 12 months). 7070 Glaser DA, Hossain P, Perkins W, Griffiths T, Ahluwalia G, Weng E, et al. Long-term safety and efficacy of bimatoprost solution 0.03% application to the eyelid margin for the treatment of idiopathic and chemotherapy-induced eyelash hypotrichosis: a randomized controlled trial. Br J Dermatol. 2015;172:1384-94. Biotin and other oral supplements might be added. Camouflage and support for patients are also important strategies.
Reversibility: CIA is completely reversible in most of the cases. When incomplete/suboptimal hair regrowth occurs after 6 months of discontinuing therapy, it is considered a persistent CIA (pCIA). It has a usually diffuse, non-scaring pattern and occur more often with pre bone marrow transplantation high dose regimens (usually busulfan and cyclophosphamide) or with taxanes.2020 Sibaud V, Leboeuf NR, Roche H, Belum VR, Gladieff L, Deslandres M, et al. Dermatological adverse events with taxane chemotherapy. Eur J Dermatol. 2016;26:427-43.,7171 Freites-Martinez A, Shapiro J, van den Hurk C, Goldfarb S, Jimenez J, Rossi AM, et al. CME Part 2: Hair disorders in cancer survivors. Persistent chemotherapy-induced alopecia, persistent radiotherapy-induced alopecia, and hair growth disorders related to endocrine therapy or cancer surgery. J Am Acad Dermatol. 2018;S0190:9622.
Another frequent pattern of persistent alopecia on cancer patients is described as endocrine therapy-induced alopecia. For many hormone receptor-positive breast cancer survivor's selective estrogen receptor modulators (e.g., tamoxifen, toremifene), aromatase inhibitors (e.g., anastrozole, letrozole, exemestane) and gonadotropin-releasing hormone agonist (e.g., leuprolide) are usually administered for 5-10 years to reduce the risk of recurrence.55 Reyes-Habito CM, Roh EK. Cutaneous reactions to chemotherapeutic drugs and targeted therapy for cancer: Part II. Targeted therapy. J Am Acad Dermatol. 2014;71:217.
6 Macdonald JB, Macdonald B, Golitz LE, LoRusso P, Sekulic A. Cutaneous adverse effects of targeted therapies: Part I: Inhibitors of the cellular membrane. J Am Acad Dermatol. 2015;72:203-18.
7 Macdonald JB, Macdonald B, Golitz LE, LoRusso P, Sekulic A. Cutaneous adverse effects of targeted therapies: Part II: Inhibitors of intracellular molecular signaling pathways. J Am Acad Dermatol. 2015;72:221-36.
8 Tischer B, Huber R, Kraemer M, Lacouture ME. Dermatologic events from EGFR inhibitors: the issue of the missing patient voice. Support Care Cancer. 2017;25:651-60.
9 Gerber DE. Targeted therapies: a new generation of cancer treatments. Am Fam Physician. 2008;77:311-19.-1010 Friedman CF, Proverbs-Singh TA, Postow MA. Treatment of the immune-related adverse effects of immune checkpoint inhibitors: a review. JAMA Oncol. 2016;2:1346-53. This estrogen deprivation might lead to androgenetic pattern alopecia. Topical minoxidil can be used. As for the use of systemic therapies for androgenic alopecia (e.g., spironolactone, finasteride), there is a putative risk of hormonal stimulation of endocrine receptor-positive tumors, so the use of these agents must be discussed with the oncologist and used with caution.6767 Rubio-Gonzalez B, Juhász M, Fortman J, Mesinkovska NA. Pathogenesis and treatment options for chemotherapy-induced alopecia: a systematic review. Int J Dermatol. 2018;57:1417-24.,7171 Freites-Martinez A, Shapiro J, van den Hurk C, Goldfarb S, Jimenez J, Rossi AM, et al. CME Part 2: Hair disorders in cancer survivors. Persistent chemotherapy-induced alopecia, persistent radiotherapy-induced alopecia, and hair growth disorders related to endocrine therapy or cancer surgery. J Am Acad Dermatol. 2018;S0190:9622.
Targeted therapies induced hair abnormalities: changes in hair quality, texture and growth pattern might be seen around the 2nd or 3rd month of treatment.
Scalp: scalp hair grows slower and with a fragile quality. A seborrheic dermatitis-like rash may develop (especially with VEGFRi and BRAFi). Also the papulopustular rash affecting the face and trunk might involve the scalp (especially with EGFRi). Alopecia is usually mild and with an androgenetic pattern, but cases of inflammatory non-scarring alopecia have been described, as cases of erlotinib-induced cicatricial alopecia.55 Reyes-Habito CM, Roh EK. Cutaneous reactions to chemotherapeutic drugs and targeted therapy for cancer: Part II. Targeted therapy. J Am Acad Dermatol. 2014;71:217.,66 Macdonald JB, Macdonald B, Golitz LE, LoRusso P, Sekulic A. Cutaneous adverse effects of targeted therapies: Part I: Inhibitors of the cellular membrane. J Am Acad Dermatol. 2015;72:203-18.
For those inflammatory changes, we suggest the use of anti-dandruff shampoos and topical corticosteroids (lotion or shampoo). If bacterial infection is suspected, cultured guided antibiotics are indicated.
Face, eyelashes and eyebrows: trichomegaly (longer, thicker and often curled eyelashes) and hypertrichosis are frequent (Fig. 4). Inward eyelashes may result in keratitis, therefore eyelash clipping is advised and patients with ocular symptoms should be referred to an ophthalmologist. For hypertrichosis, topical, cosmetic interventions can be used (e.g. waxing or bleaching). When available laser and photoepilation treatments are most effective and are not contra-indicated. 6969 Freites-Martinez A, Shapiro J, Goldfarb S, Nangia J, Jimenez JJ, et al. CME. Part 1: Hair disorders in cancer patients. J Am Acad Dermatol. 2019;80:1179-96. Creams for epilation should be avoided due to their sensitizing potential in those subjects that already have a skin barrier dysfunction.
Immunotherapy induced hair abnormalities: cases of alopecia with a clinical and histologic pattern consistent with alopecia areata (AA) have been reported. Patients should be treated similarly to non-oncologic AA cases. Also reports of hair depigmentation and repigmentation have been described. Even though we advise patients to avoid hair dyeing, it is not contra-indicated, and if there is an important cosmetic concern, dyes might be used. 6969 Freites-Martinez A, Shapiro J, Goldfarb S, Nangia J, Jimenez JJ, et al. CME. Part 1: Hair disorders in cancer patients. J Am Acad Dermatol. 2019;80:1179-96.
Changes in melanocytic nevi
BRAF inhibitors: might be associated to the appearance of eruptive melanocytic nevi (EMN), change of preexisting nevi (both the increase and acquisition of dermatoscopic structures as well as the regression of nevi that have the BRAF mutation) and the appearance of new melanomas.7272 Göppner D, Müller J, Krüger S, Franke I, Gollnick H, Quist SR. High incidence of naevi-associated BRAF wild-type melanoma and dysplastic naevi under treatment with the class I BRAF inhibitor vemurafenib. Acta Derm Venereol. 2014;94:517-20.,7373 Perier-Muzet M, Thomas L, Poulalhon N, Debarbieux S, Bringuier PP, Duru G, et al. Melanoma patients under vemurafenib: prospective follow-up of melanocytic lesions by digital dermoscopy. J Invest Dermatol. 2014;134:1351-8. Therefore, close dermatoscopic follow-up is recommended. The involution of BRAF inhibitor-induced EMN following the concomitant addition of MEK inhibitor has been described. 7474 Chen FW, Tseng D, Reddy S, Daud AI, Swetter SM. Involution of eruptive melanocytic nevi on combination BRAF and MEK inhibitor therapy. JAMA Dermatol. 2014;150:1209-12.
Immunotherapy: recently, regression of multiple melanocytic nevi after immunotherapy for melanoma has been described.7575 Libon F, Arrese JE, Rorive A, Nikkels AF. Ipilimumab induces simultaneous regression of melanocytic naevi and melanoma metastases. Clin Exp Dermatol. 2013;38:276-9.,7676 Burillo-Martinez S, Morales-Raya C, Prieto-Barrios M, Rodriguez-Peralto JL, Ortiz-Romero PL. Pembrolizumab-induced extensive panniculitis and nevus regression: two novel cutaneous manifestations of the post-immunotherapy granulomatous reactions spectrum. JAMA Dermatol. 2017;153:721-2.
Other particular toxicities
Epidermal neoplasms related to BRAF inhibitors: keratoacanthomas, squamous cell carcinomas and verrucal keratosis are a common DAE of BRAFi, they might also occur with some antiangiogenic agents (Fig. 7). This is probably due to the paradoxical stimulation of the MAPK pathway in BRAF wild-type cells. When MEK inhibitors are used in association, this AE is much less frequent. Lesions can be treated with surgical excision (when only a few lesions are present), destructive treatments (cryotherapy, curettage, etc.), topical treatments (keratolytics, 5-FU, imiquimod) or photodynamic therapy. 77 Macdonald JB, Macdonald B, Golitz LE, LoRusso P, Sekulic A. Cutaneous adverse effects of targeted therapies: Part II: Inhibitors of intracellular molecular signaling pathways. J Am Acad Dermatol. 2015;72:221-36.
Stomatitis related to mTOR inhibitors (rapamycin, everolimus, sirolimus): stomatitis is the most common AE and might be severe leading to dose adjustments. Different from conventional chemotherapy mucositis (broad ulceration with pseudo membrane formations), mTOR related stomatitis manifests as discrete aphthae on non-keratinized epithelium. They can be handled with antiseptic washes, topical steroids and anesthetics. 77 Macdonald JB, Macdonald B, Golitz LE, LoRusso P, Sekulic A. Cutaneous adverse effects of targeted therapies: Part II: Inhibitors of intracellular molecular signaling pathways. J Am Acad Dermatol. 2015;72:221-36.
Other eruptions related to immunotherapy: a non-specific maculopapular rash, as reported above, represents the most prevalent type of Immune-Related Adverse Events (irAE) to this class of agents. However, peculiar types of reactions have been described and include:
Lichenoid reactions: those eruptions can develop on both skin and/or mucosae (oral and genital). Oral involvement might also include xerostomia and taste change;
Psoriasis and rosacea: this agents might induce exacerbation or new onset of psoriasis;
Auto-immune bullous diseases: the development of auto-immune blistering disorders, especially bullous pemphigoid, have been reported.
Sarcoidosis: also reports of sarcoidosis (new onset or reactivation) are being recently published.
Vitiligo: immunotherapy has been linked to vitiligo-like lesions, mainly but not only in melanoma treated patients. 6464 Rugo HS, Voigt J. Scalp hypothermia for preventing alopecia during chemotherapy. A systematic review and meta-analysis of randomized controlled trials. Clin Breast Cancer. 2018;18:19-28.
A point of discussion is about whether or not the use of corticosteroids may antagonize the efficacy of immunotherapy. Current clinical data is limited and controversial. In some retrospective analyses, it was not associated with inferior responses to the oncologic treatment. A recent systematic review concluded that it "may not necessarily lead to poorer clinical outcomes". 7777 Garant A, Guilbault C, Ekmekjian T, Greenwald Z, Murgoi P, Vuong T. Concomitant use of corticosteroids and immune checkpoint inhibitors in patients with hematologic or solid neoplasms: a systematic review. Crit Rev Oncol Hematol. 2017;120:86-92. On the other hand, retrospective analysis on lung cancer patients using corticosteroids at baseline or for irAE suggested a possible deleterious effect.7878 Arbour KC, Mezquita L, Long N, Rizvi H, Auclin E, Ni A, et al. Impact of baseline steroids on efficacy of programmed cell death-1 and programmed death-ligand 1 blockade in patients with non-small-cell lung cancer. J Clin Oncol. 2018;36:2872-8.,7979 Scott SC, Pennell NA. Early use of systemic corticosteroids in patients with advanced NSCLC treated with nivolumab. J Thorac Oncol. 2018;13:1771-5. Most of the current guidelines and expert panels do not contra-indicate its use.77 Macdonald JB, Macdonald B, Golitz LE, LoRusso P, Sekulic A. Cutaneous adverse effects of targeted therapies: Part II: Inhibitors of intracellular molecular signaling pathways. J Am Acad Dermatol. 2015;72:221-36.,1010 Friedman CF, Proverbs-Singh TA, Postow MA. Treatment of the immune-related adverse effects of immune checkpoint inhibitors: a review. JAMA Oncol. 2016;2:1346-53.
11 Puzanov I, Diab A, Abdallah K, Bingham CO, Brogdon C, Dadu R, et al. Society for Immunotherapy of Cancer Toxicity Management Working Group Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer. 2017;5:95.
12 Rapoport BL, van Eeden R, Sibaud V, Epstein JB, Klastersky J, Aapro M, et al. Supportive care for patients undergoing immunotherapy. Support Care Cancer. 2017;25:3017-30.-1313 Lacouture ME, Wolchok JD, Yosipovitch G, Kähler KC, Busam KJ, Hauschild A. Ipilimumab in patients with cancer and the management of dermatologic adverse events. J Am Acad Dermatol. 2014;71:161-9. As there are no definitive conclusions, we suggest that corticosteroids should be used with caution and always discussing its use with the rest of the team, especially the oncologists. For steroid-refractory cases, other immunomodulatory agents such as mycophenolate, infliximab, methotrexate and others, and also rituximab for blistering diseases might be necessary and a few reports with their use have been published.
Final considerations
Dermatological adverse events are one of the most frequently observed toxicities from cancer treatments. Even though they rarely appear as life-threatening manifestations, they can lead to dose reductions or even discontinuation of oncologic therapy, interfering with disease outcome. In addition, they have a great impact in patient's quality of life. Being able to recognize and manage those skin-related toxicities gives dermatologists an important role on the multidisciplinary team, fundamental for the best supportive care of cancer patients.
Larger prospective randomized trials focusing on the management of the dermatological adverse events are still needed, but with the increasing development and recognition of the field of oncodermatology, this reality is each day closer.
-
☆
How to cite this article: Cury-Martins J, Eris APM, Abdalla CMZ, Silva GB, Moura VPT, Sanches JA. Management of dermatologic adverse events from cancer therapies: recommendations of an expert panel. An Bras Dermatol. 2020;95:221-37.
-
☆☆
Study conducted at the Faculdade de Medicina, Universidade de São Paulo, São Paulo, SP, Brazil.
-
Financial supportGalderma.
References
-
1Bensadoun RJ, Humbert P, Krutman J, Luger T, Triller R, Rougier A, et al. Daily baseline skin care in the prevention, treatment, and supportive care of skin toxicity in oncology patients: recommendations from a multinational expert panel. Cancer Manag Res. 2013;5:401-8.
-
2Balagula Y, Rosen ST, Lacouture ME. The emergence of supportive oncodermatology: the study of dermatologic adverse events to cancer therapies. J Am Acad Dermatol. 2011;65:624-35.
-
3Shekelle PG, Woolf SH, Eccles M, Grimshaw J. Developing clinical guidelines. West J Med. 1999;170:348-51.
-
4Reyes-Habito CM, Roh EK. Cutaneous reactions to chemotherapeutic drugs and targeted therapies for cancer: Part I. Conventional chemotherapeutic drugs. J Am Acad Dermatol. 2014;71:203.
-
5Reyes-Habito CM, Roh EK. Cutaneous reactions to chemotherapeutic drugs and targeted therapy for cancer: Part II. Targeted therapy. J Am Acad Dermatol. 2014;71:217.
-
6Macdonald JB, Macdonald B, Golitz LE, LoRusso P, Sekulic A. Cutaneous adverse effects of targeted therapies: Part I: Inhibitors of the cellular membrane. J Am Acad Dermatol. 2015;72:203-18.
-
7Macdonald JB, Macdonald B, Golitz LE, LoRusso P, Sekulic A. Cutaneous adverse effects of targeted therapies: Part II: Inhibitors of intracellular molecular signaling pathways. J Am Acad Dermatol. 2015;72:221-36.
-
8Tischer B, Huber R, Kraemer M, Lacouture ME. Dermatologic events from EGFR inhibitors: the issue of the missing patient voice. Support Care Cancer. 2017;25:651-60.
-
9Gerber DE. Targeted therapies: a new generation of cancer treatments. Am Fam Physician. 2008;77:311-19.
-
10Friedman CF, Proverbs-Singh TA, Postow MA. Treatment of the immune-related adverse effects of immune checkpoint inhibitors: a review. JAMA Oncol. 2016;2:1346-53.
-
11Puzanov I, Diab A, Abdallah K, Bingham CO, Brogdon C, Dadu R, et al. Society for Immunotherapy of Cancer Toxicity Management Working Group Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer. 2017;5:95.
-
12Rapoport BL, van Eeden R, Sibaud V, Epstein JB, Klastersky J, Aapro M, et al. Supportive care for patients undergoing immunotherapy. Support Care Cancer. 2017;25:3017-30.
-
13Lacouture ME, Wolchok JD, Yosipovitch G, Kähler KC, Busam KJ, Hauschild A. Ipilimumab in patients with cancer and the management of dermatologic adverse events. J Am Acad Dermatol. 2014;71:161-9.
-
14Rosen AC, Case EC, Dusza SW, Balagula Y, Gordon J, West DP, et al. Impact of dermatologic adverse events on quality of life in 283 cancer patients: a questionnaire study in a dermatology referral clinic. Am J Clin Dermatol. 2013;14:327-33.
-
15Chan A, Cameron MC, Garden B, Boers-Doets CB, Schindler K, Epstein JB, et al. A systematic review of patient-reported outcome instruments of dermatologic adverse events associated with targeted cancer therapies. Support Care Cancer. 2015;23:2231-44.
-
16Rzepecki AK, Cheng H, McLellan BN. Cutaneous toxicity as a predictive biomarker for clinical outcome in patients receiving anticancer therapy. J Am Acad Dermatol. 2018;79:545-55.
-
17Valentine J, Belum VR, Duran J, Ciccolini K, Schindler K, Wu S, et al. Incidence and risk of xerosis with targeted anticancer therapies. J Am Acad Dermatol. 2015;72:656-67.
-
18Santoni M, Conti A, Andrikou K, Bittoni A, Lanese A, Pistelli M, et al. Risk of pruritus in cancer patients treated with biological therapies: a systematic review and meta-analysis of clinical trials. Crit Rev Oncol Hematol. 2015;96:206-19.
-
19Chan RJ, Webster J, Chung B, Marquart L, Ahmed M, Garantziotis S. Prevention and treatment of acute radiation-induced skin reactions: a systematic review and meta-analysis of randomized controlled trials. BMC Cancer. 2014;14:53.
-
20Sibaud V, Leboeuf NR, Roche H, Belum VR, Gladieff L, Deslandres M, et al. Dermatological adverse events with taxane chemotherapy. Eur J Dermatol. 2016;26:427-43.
-
21Yin ES, Totonchy MB, Leventhal JS. Nivolumab-associated vitiligo-like depigmentation in a patient with acute myeloid leukemia: a novel finding. JAAD Case Rep. 2017;3:90-2.
-
22Robert C, Sibaud V, Mateus C, Verschoore M, Charles C, Lanoy E, et al. Nail toxicities induced by systemic anticancer treatments. Lancet Oncol. 2015;16:e181-9.
-
23Garden BC, Wu S, Lacouture ME. The risk of nail changes with epidermal growth factor receptor inhibitors: a systematic review of the literature and meta-analysis. J Am Acad Dermatol. 2012;67:400-8.
-
24Burtness B, Anadkat M, Basti S, Hughes M, Lacouture ME, McClure JS, et al. NCCN Task Force Report: management of dermatologic and other toxicities associated with EGFR inhibition in patients with cancer. J Natl Compr Canc Netw. 2009;7(Suppl. 1):S5-21.
-
25Melosky B, Leighl NB, Rothenstein J, Sangha R, Stewart D, Papp K. Management of egfr tki-induced dermatologic adverse events. Curr Oncol. 2015;22:123-32.
-
26Lacouture ME, Anadkat M, Jatoi A, Garawin T, Bohac C, Mitchell E. Dermatologic toxicity occurring during anti-EGFR monoclonal inhibitor therapy in patients with metastatic colorectal cancer: a systematic review. Clin Colorectal Cancer. 2018;17:85-6.
-
27Dsouza PC, Kumar S. Role of systemic antibiotics in preventing epidermal growth factor receptor: tyrosine kinase inhibitors-induced skin toxicities. Asia Pac J Oncol Nurs. 2017;4:323-29.
-
28Wnorowski AM, de Souza A, Chachoua A, Cohen DE. The management of EGFR inhibitor adverse events: a case series and treatment paradigm. Int J Dermatol. 2012;51:223-32.
-
29Yen CF, Hsu CK, Lu CW. Topical betaxolol for treating relapsing paronychia with pyogenic granuloma-like lesions induced by epidermal growth factor receptor inhibitors. J Am Acad Dermatol. 2018;78:e143-44.
-
30Lacouture ME, Wu S, Robert C, Atkins MB, Kong HH, Guitart J, et al. Evolving strategies for the management of hand-foot skin reaction associated with the multitargeted kinase inhibitors sorafenib and sunitinib. Oncologist. 2008;13:1001-11.
-
31Anderson R, Jatoi A, Robert C, Wood LS, Keating KN, Lacouture ME. Search for evidence-based approaches for the prevention and palliation of hand-foot skin reaction (HFSR) caused by the multikinase inhibitors (MKIs). Oncologist. 2009;14:291-302.
-
32Flaherty KT, Brose MS. Sorafenib-related hand-foot skin reaction improves not worsens, with continued treatment. Clin Cancer Res. 2009;15:7749.
-
33Macedo LT, Lima JP, dos Santos LV, Sasse AD. Prevention strategies for chemotherapy-induced hand-foot syndrome: a systematic review and meta-analysis of prospective randomised trials. Support Care Cancer. 2014;22:1585-93.
-
34Huang XZ, Chen Y, Chen WJ, Zhang X, Wu CC, Wang ZN, et al. Clinical evidence of prevention strategies for capecitabine-induced hand-foot syndrome. Int J Cancer. 2018;142:2567-77.
-
35Yap YS, Kwok LL, Syn N, Chay WY, Chia JWK, Tham CK, et al. Predictors of hand-foot syndrome and pyridoxine for prevention of capecitabine-induced hand-foot syndrome: a randomized clinical trial. JAMA Oncol. 2017;3:1538-45.
-
36Jung S, Sehouli J, Chekerov R, Kluschke F, Patzelt A, Fuss H, et al. Prevention of palmoplantar erythrodysesthesia in patients treated with pegylated liposomal doxorubicin (Caelyx®). Support Care Cancer. 2017;25:3545-49.
-
37Ren Z, Zhu K, Kang H, Lu M, Qu Z, Lu L, et al. Randomized controlled trial of the prophylactic effect of urea-based cream on sorafenib-associated hand-foot skin reactions in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2015;33:894-900.
-
38Negri FV, Porta C. Urea-based cream to prevent sorafenib-induced hand-and-foot skin reaction: which evidence?. J Clin Oncol. 2015;33:3219-20.
-
39Kamimura K, Shinagawa-Kobayashi Y, Goto R, Ogawa K, Yokoo T, Sakamaki A, et al. Effective prevention of sorafenib-induced hand-foot syndrome by dried-bonito broth. Cancer Manag Res. 2018;10:805-13.
-
40Demirkan S, Gündüz Ö, Devrim T. Sorafenib-asssociated hand-foot syndrome treated with topical calcipotriol. JAAD Case Rep. 2017;3:354-57.
-
41von Moos R, Thuerlimann BJ, Aapro M, Rayson D, Harrold K, Sehouli J, et al. Pegylated liposomal doxorubicin-associated hand-foot syndrome: recommendations of an international panel of experts. Eur J Cancer. 2008;44:781-90.
-
42Fabbrocini G, Cristaudo A, Ionescu MA, Panariello L, Robert G, Pellicano M, et al. Topical non-occlusive polymers in hand-foot syndrome. G Ital Dermatol Venereol. 2018;153:165-71.
-
43Shinohara N, Nonomura N, Eto M, Kimura G, Minami H, Tokunaga S, et al. A randomized multicenter phase II trial on the efficacy of a hydrocolloid dressing containing ceramide with a low-friction external surface for hand-foot skin reaction caused by sorafenib in patients with renal cell carcinoma. Ann Oncol. 2014;25:472-76.
-
44Deng B, Sun W. Herbal medicine for hand-foot syndrome induced by fluoropyrimidines: a systematic review and meta-analysis. Phytother Res. 2018;32:1211-28.
-
45Tian A, Zhou A, Bi X, Hu S, Jiang Z, Zhang W, et al. Efficacy of topical compound danxiong granules for treatment of dermatologic toxicities induced by targeted anticancer therapy: a randomized, double-blind placebo-controlled trial. Evid Based Complement Alternat Med. 2017;2017:3970601.
-
46Yucel I, Guzin G. Topical henna for capecitabine induced hand-foot syndrome. Invest New Drugs. 2008;26:189-92.
-
47Ilyas S, Wasif K, Saif MW. Topical henna ameliorated capecitabine-induced hand-foot syndrome. Cutan Ocul Toxicol. 2014;33:253-55.
-
48Harding JJ, Pulitzer M, Chapman PB. Vemurafenib sensitivity skin reaction after ipilimumab. N Engl J Med. 2012;366:866-68.
-
49Johnson DB, Wallender EK, Cohen DN, Likhari SS, Zwerner JP, Powers JG, et al. Severe cutaneous and neurologic toxicity in melanoma patients during vemurafenib administration following anti-PD-1 therapy. Cancer Immunol Res. 2013;1:373-77.
-
50Ludlow SP, Pasikhova Y. Cumulative dermatologic toxicity with ipilimumab and vemurafenib responsive to corticosteroids. Melanoma Res. 2013;23:496-97.
-
51Bolognia JL, Cooper DL, Glusac EJ. Toxic erythema of chemotherapy: a useful clinical term. J Am Acad Dermatol. 2008;59:524-9.
-
52Chanprapaph K, Vachiramon V, Rattanakaemakorn P. Epidermal growth factor receptor inhibitors: a review of cutaneous adverse events and management. Dermatol Res Pract. 2014;2014:734249.
-
53Lacouture ME, Keefe DM, Sonis S, Jatoi A, Gernhardt D, Wang T, et al. A phase II study (ARCHER 1042) to evaluate prophylactic treatment of dacomitinib-induced dermatologic and gastrointestinal adverse events in advanced non-small-cell lung cancer. Ann Oncol. 2016;27:1712-18.
-
54Kripp M, Prasnikar N, Vehling-Kaiser U, Quidde J, Al-Batran SE, Stein A, et al. AIO LQ-0110: a randomized phase II trial comparing oral doxycycline versus local administration of erythromycin as preemptive treatment strategies of panitumumab-mediated skin toxicity in patients with metastatic colorectal cancer. Oncotarget. 2017;8:105061-71.
-
55Requena C, Llombart B, Sanmartín O. Acneiform eruptions induced by epidermal growth factor receptor inhibitors: treatment with oral isotretinoin. Cutis. 2012;90:77-80.
-
56Woods JA, Ferguson JS, Kalra S, Degabriele A, Gardner J, Logan P, et al. The phototoxicity of vemurafenib: an investigation of clinical monochromator phototesting and in vitro phototoxicity testing. J Photochem Photobiol B. 2015;151:233-8.
-
57Zimmer L, Vaubel J, Livingstone E, Schadendorf D. Side effects of systemic oncological therapies in dermatology. J Dtsch Dermatol Ges. 2012;10:475-86.
-
58Shin H, Jo SJ, Kim DH, Kwon O, Myung SK. Efficacy of interventions for prevention of chemotherapy-induced alopecia: a systematic review and meta-analysis. Int J Cancer. 2015;136:E442-54.
-
59Vasconcelos I, Wiesske A, Schoenegg W. Scalp cooling successfully prevents alopecia in breast cancer patients undergoing anthracycline/taxane-based chemotherapy. Breast. 2018;40:1-3.
-
60Shah VV, Wikramanayake TC, DelCanto GM, van den Hurk C, Wu S, Lacouture ME, et al. Scalp hypothermia as a preventative measure for chemotherapy-induced alopecia: a review of controlled clinical trials. J Eur Acad Dermatol Venereol. 2018;32:720-34.
-
61Nangia J, Wang T, Osborne C, Niravath P, Otte K, Papish S, et al. Effect of a scalp cooling device on alopecia in women undergoing chemotherapy for breast cancer: the SCALP randomized clinical trial. JAMA. 2017;317:596-605.
-
62Kruse M, Abraham J. Management of chemotherapy-induced alopecia with scalp cooling. J Oncol Pract. 2018;14:149-54.
-
63Rugo HS, Klein P, Melin SA, Hurvitz SA, Melisko ME, Moore A, et al. Association between use of a scalp cooling device and alopecia after chemotherapy for breast cancer. JAMA. 2017;317:606-14.
-
64Rugo HS, Voigt J. Scalp hypothermia for preventing alopecia during chemotherapy. A systematic review and meta-analysis of randomized controlled trials. Clin Breast Cancer. 2018;18:19-28.
-
65Witman G, Cadman E, Chen M. Misuse of scalp hypothermia. Cancer Treat Rep. 1981;65:507-8.
-
66Forsberg SA. Scalp cooling therapy and cytotoxic treatment. Lancet. 2001;357:1134.
-
67Rubio-Gonzalez B, Juhász M, Fortman J, Mesinkovska NA. Pathogenesis and treatment options for chemotherapy-induced alopecia: a systematic review. Int J Dermatol. 2018;57:1417-24.
-
68Yeager CE, Olsen EA. Treatment of chemotherapy-induced alopecia. Dermatol Ther. 2011;24:432-42.
-
69Freites-Martinez A, Shapiro J, Goldfarb S, Nangia J, Jimenez JJ, et al. CME. Part 1: Hair disorders in cancer patients. J Am Acad Dermatol. 2019;80:1179-96.
-
70Glaser DA, Hossain P, Perkins W, Griffiths T, Ahluwalia G, Weng E, et al. Long-term safety and efficacy of bimatoprost solution 0.03% application to the eyelid margin for the treatment of idiopathic and chemotherapy-induced eyelash hypotrichosis: a randomized controlled trial. Br J Dermatol. 2015;172:1384-94.
-
71Freites-Martinez A, Shapiro J, van den Hurk C, Goldfarb S, Jimenez J, Rossi AM, et al. CME Part 2: Hair disorders in cancer survivors. Persistent chemotherapy-induced alopecia, persistent radiotherapy-induced alopecia, and hair growth disorders related to endocrine therapy or cancer surgery. J Am Acad Dermatol. 2018;S0190:9622.
-
72Göppner D, Müller J, Krüger S, Franke I, Gollnick H, Quist SR. High incidence of naevi-associated BRAF wild-type melanoma and dysplastic naevi under treatment with the class I BRAF inhibitor vemurafenib. Acta Derm Venereol. 2014;94:517-20.
-
73Perier-Muzet M, Thomas L, Poulalhon N, Debarbieux S, Bringuier PP, Duru G, et al. Melanoma patients under vemurafenib: prospective follow-up of melanocytic lesions by digital dermoscopy. J Invest Dermatol. 2014;134:1351-8.
-
74Chen FW, Tseng D, Reddy S, Daud AI, Swetter SM. Involution of eruptive melanocytic nevi on combination BRAF and MEK inhibitor therapy. JAMA Dermatol. 2014;150:1209-12.
-
75Libon F, Arrese JE, Rorive A, Nikkels AF. Ipilimumab induces simultaneous regression of melanocytic naevi and melanoma metastases. Clin Exp Dermatol. 2013;38:276-9.
-
76Burillo-Martinez S, Morales-Raya C, Prieto-Barrios M, Rodriguez-Peralto JL, Ortiz-Romero PL. Pembrolizumab-induced extensive panniculitis and nevus regression: two novel cutaneous manifestations of the post-immunotherapy granulomatous reactions spectrum. JAMA Dermatol. 2017;153:721-2.
-
77Garant A, Guilbault C, Ekmekjian T, Greenwald Z, Murgoi P, Vuong T. Concomitant use of corticosteroids and immune checkpoint inhibitors in patients with hematologic or solid neoplasms: a systematic review. Crit Rev Oncol Hematol. 2017;120:86-92.
-
78Arbour KC, Mezquita L, Long N, Rizvi H, Auclin E, Ni A, et al. Impact of baseline steroids on efficacy of programmed cell death-1 and programmed death-ligand 1 blockade in patients with non-small-cell lung cancer. J Clin Oncol. 2018;36:2872-8.
-
79Scott SC, Pennell NA. Early use of systemic corticosteroids in patients with advanced NSCLC treated with nivolumab. J Thorac Oncol. 2018;13:1771-5.
Publication Dates
-
Publication in this collection
06 July 2020 -
Date of issue
May-Jun 2020
History
-
Received
13 Jan 2019 -
Accepted
12 Jan 2020 -
Published
15 Feb 2020