ABSTRACT
The purpose of this study was to evaluate the role of early diagnosis and surgical outcomes in determining the prognosis of BOSCC, which is frequently encountered in cattle and leads to significant economic losses. The immunohistochemical examinations of specimens revealed severe TNF-α expression, whereas the real-time PCR tests showed significant increases in IL-6 and TNF-α gene expressions. Among 56 cases that underwent surgery, 31 local excision, 19 enucleation, and 6 exenteration procedures were performed, and the cases were followed up for 2 years. Various wound site complications were observed in only 16.1% of the surgical cases. No relapse was observed in 76.8% of the cases, tumor relapse was observed between the 16th and 23rd months in 12.6%, and the rates of cattle that were removed from the herd due to postoperative complications within the first 3 months and at the 6th month were respectively 9% and 1.8%. While no complication or relapse was seen in the cases that were treated within 1-3 months, relapse was observed in those that were treated after 3 months (OR=0.93, 95% CI, p=0.014). In BOSCC, lesions treated surgically within the first 3 months have no complications and 2-year survival rates increase.
Keywords:
bovine; early diagnosis; immunohistochemistry; ocular squamous cell carcinoma; Real Time PCR
RESUMO
O objetivo deste estudo foi avaliar o papel do diagnóstico precoce e dos resultados cirúrgicos na determinação do prognóstico do BOSCC, que é frequentemente encontrado em bovinos e leva a perdas econômicas significativas. Os exames imuno-histoquímicos dos espécimes revelaram expressão grave de TNF-α, enquanto os testes de PCR em tempo real mostraram aumentos significativos nas expressões gênicas de IL-6 e TNF-α. Entre os 56 casos submetidos à cirurgia, foram realizados 31 procedimentos de excisão local, 19 de enucleação e 6 de exenteração, e os casos foram acompanhados por 2 anos. Várias complicações no local da ferida foram observadas em apenas 16,1% dos casos cirúrgicos. Nenhuma recidiva foi observada em 76,8% dos casos, a recidiva do tumor foi observada entre o 16º e o 23º mês em 12,6%, e as taxas de gado que foi removido do rebanho devido a complicações pós-operatórias nos primeiros 3 meses e no 6º mês foram, respectivamente, 9% e 1,8%. Embora nenhuma complicação ou recidiva tenha sido observada nos casos tratados dentro de 1-3 meses, a recidiva foi observada naqueles tratados após 3 meses (OR=0,93, 95% CI, p=0,014). No BOSCC, as lesões tratadas cirurgicamente nos primeiros 3 meses não apresentam complicações e as taxas de sobrevivência em 2 anos aumentam.
Palavras-chave:
bovino; diagnóstico precoce; imuno-histoquímica; carcinoma de células escamosas ocular; PCR em tempo real
INTRODUCTION
Squamous cell carcinomas are prevalently encountered in the ocular and periocular regions of domestic species (horses, dogs, cats, sheep, and pigs), and bovine ocular squamous cell carcinoma (BOSCC) cases constitute 80.3% of all lesions in the ocular and periocular regions (Martinz and Barros, 2014). BOSCC mostly affects the lower eyelid, lateral limbus, and third eyelid in cattle, and it is the most economically significant neoplasm in this species (Jones, 2019; Podarala et al., 2020). It is estimated that ocular squamous cell carcinoma in livestock leads to an annual loss of $20 million in the US alone (Pearce and Moore, 2014).
Although it is considered that the etiology of BOSCC can differ from region to region, it has been reported that multifactorial causes (bovine papillomavirus, bovine herpesvirus type 1 and 5, oxidative stress, high altitude, UV radiation and p53 gene mutation) are effective in its development (Fornazari et al., 2017; Podarala et al., 2020; Karakurt et al., 2021, 2024; Lakshmi et al., 2021). Cell damage plays a role in the pathogenesis of the disease. Following cell damage, inflammation occurs by the activation of some signal proteins. The method of treatment for the disease is determined by identifying the inflammation that occurs to achieve tissue standardization. Signal proteins have significant roles in all stages of inflammation, especially in its induction. Carcinogenesis refers to the formation of a tumor by normal cells and the development of a mass by the proliferation of these cells. Tumor necrosis factor-alpha (TNF-α) and Interleukin-6 (IL-6) are significant cytokines that mediate the differentiation of neoplastic cells and the induction of inflammation (Diaz et al., 2023; Kudo et al., 2023). BOSCC can have a heterogeneous population varying from histologically well-differentiated cases to anaplastic carcinoma, and cases of the same neoplasm can show different histological characteristics (Vala et al., 2020; Özcan-Martz et al., 2021). Therefore, it is believed that examining antigen expressions in different models would be beneficial for a better understanding of cellular behaviors and irregularities (Vala et al., 2020).
BOSCC lesions can be treated by surgical excision, enucleation, exenteration, cryotherapy, hyperthermia, photodynamic therapy, immunotherapy, chemotherapy, or a combination of these. In BOSCC cases, surgical intervention and a multimodal treatment approach can increase the probability of success and save the sight of the animal (Yavuz and Yumuşak, 2017; Podarala et al., 2020; Lakshmi et al., 2021; Ng et al., 2023).
Because all kinds of efforts to develop knowledge about the biology of BOSCC and investigate the factors that predict its prognosis are valuable, the purpose of this study is to reveal the importance of early diagnosis in the determination of prognosis in BOSCC cases using clinical, pathological, and molecular methods.
MATERIAL AND METHODS
The animal material of the study consisted of 56 cattle of different breeds, sexes, and ages that were brought to the surgery clinic of the Faculty of Veterinary Medicine at Harran University with complaints of mass formation in their ocular and periocular tissues between 1 January 2017 and 31 December 2021. Surgical interventions were performed on these 56 cattle, and their information, including breed, age, tumor localization, surgical technique, time of being brought to the clinic after the detection of the mass by the owner, relapse status, the time of relapse if any, and postoperative complications were recorded. Harran University Animal Experiments Local Ethics Committee (HRU-HADYEK 2023/007/08) approved the study, and the owners of the animals signed an approval form.
According to the anamneses, red-pink abnormal tissue masses and swellings were in the form of mildly protruding lesions that increased over time and became wider progressively. The cases were brought to our clinic within 1-12 months after the detection of the first signs with complaints of a reduction in milk yield, weight loss, and varying forms of eye discharge. The masses affected the function of ocular and periocular tissues and obstructed the vision of the animals to varying extents.
For treatment, local excision was performed in 31 cases, enucleation was performed in 19 cases, and exenteration was performed in 6 cases. In the postoperative period, eye pomade containing bacitracin 2500IU and neomycin sulfate 25mg (Thiocilline®, İbrahim Hayri, Türkiye) was administered for 5 days to the cases that underwent local excision, while those that underwent enucleation and exenteration procedures were administered procaine benzylpenicillin (200mg) and dihydrostreptomycin sulfate (200 mg) (Prolipen 20/20®, Vetaş, Türkiye) 22,000IU/kg through the intramuscular route for 5 days and meloxicam (Metacam®, B. Ingelheim, Türkiye) at 5mg/kg as a single intravenous injection. While short-term follow-ups in terms of postoperative complications and relapse status were carried out between the 14th and 21st days, when sutures were removed, long-term follow-ups (>21 days) were carried out by on-site examinations, telephone calls for 2 years, or when the cattle were brought back to the clinic for additional examination.
After the tissues that were fixated in 10% buffered formalin solution were washed under running water, they were subjected to routine tissue processing steps. The processed tissues were embedded in paraffin blocks. 5-μ-thick tissue slices from each paraffin block were put onto normal and adhesive-containing slides. Following deparaffinization and rehydration, the tissue specimens on the normal slides were stained with hematoxylin-eosin and Masson’s trichrome (Ulucan et al., 2019). Entellan drops were added to the tissue specimens subjected to histochemical staining, the specimens were covered using a cover slide, and they were examined under a light microscope. Pathologic lesions were scored based on severity as none (-), mild (+), moderate (++), or severe (+++) (Kutlu et al., 2022).
The tissue slices on the adhesive-containing slides were subjected to endogenous inactivation using 3% H2O2. To detect the presence of antigens in the tissues that were washed with PBS, the tissues were boiled in a retrieval solution and left to cool. To block non-specific binding sites in the tissues washed with PBS, incubation was performed with protein block. The tissues, onto which TNF-α (sc-52B83, Santa Cruz, USA) drops were added as the primary antibody, were left to incubate overnight at +4°C. The tissues were washed again with PBS and incubated with a biotinylated secondary antibody compatible with the primary antibody. The tissues were washed with PBS yet again and incubated with streptavidin-peroxidase. Finally, the tissues that were conjugated with 3,3′-Diaminobenzidine (DAB) chromogen were counterstained with Mayer’s hematoxylin (Çağlayan et al., 2019). Entellan was dripped onto the immunohistochemically stained tissues, and the tissues were covered with a cover slide and examined under a light microscope. The severity of the immunoreactions was rated as none (-), mild (+), moderate (++), or severe (+++) (Karakurt et al., 2021).
The mass-containing ocular and periocular tissues, which were kept at -80°C until the day of analyses, and control group tissue specimens that were collected from a slaughterhouse and were not administered any treatment or drug were subjected to mRNA isolation using a High Pure RNA Tissue isolation Kit (12033674001/Germany).
The synthesis of cDNA from the isolated mRNAs was carried out according to the instructions of the commercial OneScript Plus cDNA Synthesis Kit (ABM/Canada).
Using all components inside the kit, a mix composed of the following constituents was prepared for each specimen: 5X RT Buffer 4μl, DNTP 1μl, Random hexamer Primers 1μl, isolated RNA 2ng, OneScript Plus RTase 1μl, and Nuclease-Free Water to complete the volume to 20 μl. cDNA was obtained in a thermocycler device (BIORAD, USA) with a cycle of 15min at 55°C, 5min at 85°C, and 4°C, and measurements were made with the Nanodrop.
To amplify the cDNAs based on the reference gene and mark target and reference gene regions, 10μl of SYBR Green Master Mix compatible with the commercial FastStart Essential SYBR Green Master Mix (Roche, Germany) kit was prepared with Forward Primer 500nM 1μl, Reverse Primer 500nM 1μl, cDNA 5μl, and ddH20 3μl (Table 1).
After the prepared reference and target gene real-time PCR mixes were collected in 96-well plates along with compatible cDNAs, they were subjected to denaturation at 95°C for 10min, amplification at 95°C for 20s, at 60°C for 20s, and at 72°C for 20s for 45 cycles, holding at 95°C for 30s, a melting curve at 95°C for 30s and at 50°C for 1min, continuous measurement at 90°C and holding at 40°C for 1min, and they were loaded into the real-time PCR device (LightCycler, Roche, Germany). A system cycle protocol was created and run.
The reference and target Ct values of each specimen were obtained using the quantification analysis program of the Roche LightCycler 96 device. Calculations were made using the delta-delta Ct formula, and the results were analyzed.
The SPSS 22.1 (NC, USA) statistical analysis software was used for all analyses. Descriptive statistics were calculated to express the data. The normality of the distribution of the data was tested using the Shapiro-Wilk test. Mean±SD and median (range) values are reported for the normally distributed and non-normally distributed data, respectively. The relationships between the independent variables (age, breed) and the specific ocular localizations treated with surgical intervention were analyzed using chi-squared tests and Pearson’s correlation test. Then, the odds ratio (OR) and 95% confidence interval (95% CI) were calculated. Similarly, the relationships between the surgical techniques and independent variables such as complication development (yes or no) and relapse presence (yes or no) were analyzed using chi-squared tests and Pearson’s correlation tests, followed by the calculation of OR and 95% CI values. The relationship between an independent variable and ocular localization or surgical technique was accepted to be significant when OR>1 and p<0.05 (Schulz and Anderson, 2010; Karakurt et al., 2021).
RESULTS
The animal material of the study only included 56 cattle diagnosed with BOSCC based on histopathology and immunohistochemistry results. Table 2 presents information about the breeds and ages of the cattle, tumor localizations, surgical technique, time of visit after symptom detection, postoperative relapse, and postoperative complications.
All included cattle were female, and 5 of them (9%) were pregnant (3 Holstein, 2 Simmental). The highest rate of cattle was in the Holstein breed (46.5%), followed by the Simmental breed (42.9%), and Holstein hybrids (10.7%). While 1.8% of the cattle were 1-2 years old, 28.6% were 3-4 years old, 55.3% were 5-6 years old, 8.9% were 7-8 years old, and 5.4% were 9 years old or older. The most frequently observed tumor localization was the lower eyelid (32.1%), followed by the 3rd eyelid (30.4%), both the lower and 3rd eyelids (16.1%), the cornea-conjunctiva (10.7%), both the lower and upper eyelids (7.1%), and the upper eyelid alone (3.6%). The lower eyelid had a significantly higher rate of observation according to OR values (OR=0.21, 95% CI, p=0.001).
Breed, age, tumor localization, surgical technique, time of visit after symptom detection, postoperative relapse status, and postoperative complications in 56 cattle with bovine ocular squamous cell carcinoma (BOSCC)
After noticing the first signs of tumors, the owners of 58.9% of the cattle brought them to our clinic within 1-3 months, the owners of 17.9% brought them in 4-6 months, the owners of 16.1% brought them in 7-9 months, and the owners of 7.1% brought them in 10-12 months. The surgical techniques performed on the cattle were local excision at a rate of 55.4%, enucleation at a rate of 33.9%, and exenteration at a rate of 10.7%. No complication related to standing local anesthesia was encountered. The local excision technique was mostly performed in cases that were brought to the clinic in a short time after symptom detection (28 cases: 1-3 months, 3 cases: 4-6 months), the enucleation technique was mostly performed in cases that were brought in the medium term (5 cases: 1-3 months, 7 cases: 4-6 months, 7 cases: 7-9 months), and the exenteration technique was mostly performed in cases that were brought after a long time following symptom detection (2 cases: 7-9 months, 4 cases: 10-12 months) (OR=0.43, 95% CI, p=0.017).
While no postoperative complication was seen in 83.9% of the cases, there were only surgical site infections in 7.1%, wound dehiscence and excessive bleeding in addition to surgical site infections in 5.4%, and wound dehiscence accompanying surgical site infections in 3.6%. While no postoperative complications were observed in the cases treated with the local excision technique, there were complications in 5 cases treated with the enucleation technique and 4 treated with the exenteration technique (OR=0.96, 95% CI, p=0.078).
There was no relapse in 76.8% of the cases, there were tumor relapses between the 16th and 23rd months in 12.6%, and the rates of cattle that were removed from the herd due to postoperative complications within the first 3 months and at the 6th month were respectively 9% and 1.8%. All cattle removed from the herd within the first 6 months of treatment had been treated with the exenteration technique. While no relapse was observed in the cases treated with the local excision technique, there were 7 cases of relapse in the cattle treated with the enucleation technique (OR=1.39, 95% CI, p=0.012).
While no complication or relapse was seen in the cases that were treated within 1-3 months, relapse occurred between the 22nd and 23rd months in 2 cases which were treated between the 4th and 6th months. Two cases treated with the exenteration technique at 7-9 months after symptom detection were removed from the herd at 2 and 6 months, whereas relapse occurred at 16-21 months in 5 cases that were treated with enucleation. Four cases, which were brought at 10-12 months for intervention and underwent exenteration, were removed from the herd within 3 months (OR=0.93, 95% CI, p=0.014).
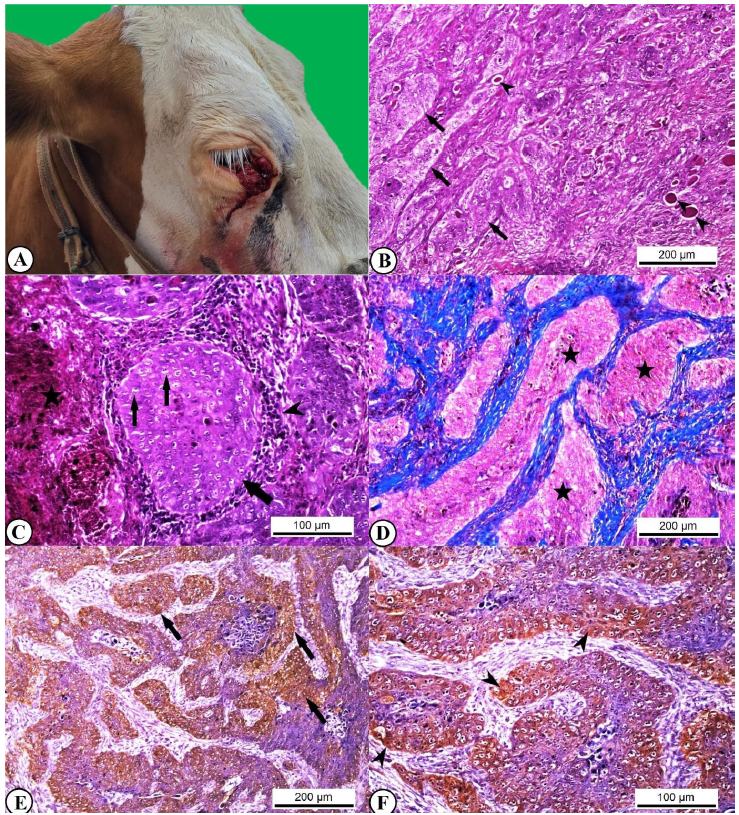
In the macroscopic examinations, the tissue specimens were observed to have a cauliflower-like appearance and non-uniform contours. Some of these specimens, which had a stiff and fragile character, showed necrotic, ulcerative, and hemorrhagic lesions. Gray-white color changes shaped by epithelial proliferations extending toward the deep parts were observed on the slice surfaces (Figure 1A). According to their macroscopic appearance, 30.4% of the lesions were verrucous, 21.4% were small nodular, 17.9% were dense plaques, 12.5% were multitype hyperemic plaques, 7.14% were crusted lesions, 7.14% were hemorrhagic ulcerative, and 3.6% were purulent-ulcerative.
In the microscopic examinations, high numbers of anaplastic epithelial cells with vesicular and discolored eosinophilic cytoplasm, hyperchromatic and large nucleolar nuclei, and a pleomorphic appearance were observed. Some of these cells had mitotic figures. It was determined that the poorly differentiated anaplastic cells showing weak spinous differentiation either did not produce much keratin or were in the form of separated keratinized cells. Among the well-differentiated atypical squamous cells, keratin pearls, in addition to keratin filaments that formed ties between these, were observed. There were occasional neoplastic cell invasions in the stroma in the form of cell islands or pseudo-filaments. Fibrous connective tissue proliferations were seen among some invading cells. In the ulcerated parts related to secondary infection development, there were necrosis, hemorrhage, and neutrophil infiltrations in the epidermis, and extensive mononuclear cell infiltrations around anaplastic cell islet (Figure 1B, 1C, 1D). The results of the histopathological examinations of the specimens are presented in Table 3.
In the immunohistochemical analyses, severe TNF-α expression was detected in the cytoplasm of anaplastic tumor cells, especially in tumor islets forming invasions into the stroma. The presence of TNF-α expression was also noted in the inflammatory cells of the specimens in which ulceration developed (Figure 1E, 1F). TNF-α expression was strong in aggressive tumors with poor differentiation and weak in tumors with good differentiation. The results of the specimens regarding TNF-α expression are shown in Table 3.
Macroscopic view of the right ocular region of a 3-year-old Simmental cattle with BOSCC originating from the 3rd eyelid (A). Neoplastic cell accumulations with the invasion of the stroma in the form of pseudo-filaments (arrows) and keratin formations (arrowheads), HE, x40 (B). Islet consisting of tumor cells (thick arrow), neoplastic cells with mitotic figures (thin arrows), dense mononuclear cell infiltrations around the area (arrowhead), hemorrhage and granulocytic cell infiltration in the ulcerated area (asterisk), HE, x100 (C). Tumor islets invading the stroma and pseudo-filamentous appendages (asterisks), MTC, x40 (D). Severe TNF-α expression in atypical cells in tumor islets (arrows), IHC, x40 (E). Severe TNF-α expression in the cytoplasm of tumor cells, IHC, x100 (F).
In the examinations of IL-6 and TNF-α gene expressions in the ocular and periocular tissues that contained masses by real-time PCR, in comparison to the tissue specimens in the control group that were supplied from the slaughterhouse and were not subjected to any surgery or substance administration, there were significant increases in the IL-6 and TNF-α gene expression levels of the treatment groups, and these results were compatible with the results of the pathological analyses (Fig. 2).
DISCUSSION
Several studies on tumors originating from the ocular and periocular regions in cattle have emphasized that BOSCC cases are the most prevalently encountered types of tumors (Gharagozlou et al., 2007; Ceylan et al., 2012; Chigerwe et al., 2017). It has been stated that BOSCC is seen after the age of 4, it is mostly encountered at the age of 7-9 in the Hereford, Simmental, and Holstein-Friesian breeds, and it is rarely observed in other breeds (Gharagozlou et al., 2007; Ceylan et al., 2012; Pugliese et al., 2014).
Genetic, phenotypic, and environmental (prolonged exposure to ultraviolet light) factors, eyelid pigmentation deficiency, age, viruses, and diet play an important role in the etiology of BOSCC (Fornazari et al., 2017, Podarala et al., 2020, Lakshmi et al., 2021). The prevalence of squamous cell cancers varies geographically, and it increases in cattle that are raised in hot and arid regions at high altitudes and low latitudes (Gharagozlou et al., 2007; Ceylan et al., 2012;Chigerwe et al., 2017). Ocular and periocular tumors are seen more frequently in cattle raised in regions where the average duration of solar radiation is 8-14 h as these cattle are exposed to high levels of UV light (Ceylan et al., 2012; Al-Asadi, 2012; Pugliese et al., 2014). Anderson and Badzioch (1991) also showed that the prevalence of BOSCC significantly increased along with the period of sunlight during the day and higher altitudes. Studies conducted on experimental animals demonstrated that high temperatures could also contribute to skin carcinogenesis by creating DNA damage through the same indirect oxidative stress process (Cerutti et al., 1990). The province of Şanlıurfa in Türkiye, where all cattle included in our study were kept, is located at an altitude of 550 m. The latitude of Şanlıurfa is 37.1608°, and its longitude is 38.7863°. The summer in Şanlıurfa is stiflingly hot and arid, with clear weather, while the winter is very cold and partly cloudy. Considering the daily average sunshine duration of 8.1 h in Şanlıurfa, exceeding 12 h in the summer months (mgm.gov.tr), these conditions are similar to those reported in other studies showing a higher prevalence of BOSCC. A limitation of this study was the fact that the role of other etiological factors and their interactions with secondary factors could not be examined.
In cattle, eyelid and corneoscleral pigment deficiencies are hereditary and genetically associated with each other. The effects of genetics on corneoscleral pigmentation determine the eye’s degree of susceptibility to some carcinogenic factors to a substantial extent. The most likely carcinogenic factor affecting the eye is the UV component of sunlight, and pigments in the eyes are important for protection against the harmful effects of UV radiation (Anderson, 1991; Anderson and Badzioch, 1991; Jara et al., 2022). Although previous studies have reported that BOSCC is the most frequently seen in breeds like Hereford with eyelid and corneoscleral pigment deficiencies, it is also frequently encountered in the Holstein and Simmental breeds that are characterized by facial hypopigmentation and/or pink skin around the eyes (Tsujita and Plummer, 2010; Chigerwe et al., 2017; Thiry et al., 2022). Because the extent of Holstein cattle farming is greater in the region where this study was carried out, this breed had a higher representation rate compared to Simmental cattle. The fact that cattle raised in the same geographical region and treated at one veterinary hospital were included in this study constituted one of the limitations of the study due to the existence of selection bias. Anderson and Badzioch (1991) emphasized that to facilitate protection against the harmful effects of UV radiation, control can be achieved by selective breeding with cattle having higher amounts of eyelid and corneoscleral pigmentation. Moreover, Davis et al. (2015) noted that the hybridization of breeds that have eyelid and corneoscleral pigment deficiencies with those that have darker pigmentation can increase pigmentation in their offspring, and the likelihood of cancer development in the eyes can thus be reduced. Considering the relationship between ocular pathologies and pigmentation, the selective breeding of cattle with certain eyelid pigmentation characteristics can lower the prevalence of ocular disorders. In any case, to lower the prevalence of the disease, the cattle diagnosed with BOSCC in purebred herds and the offspring of the affected cattle should not be used in breeding.
It is accepted that cattle constitute an advantageous model for the understanding of carcinogenesis in humans (Cappelleri et al., 2022). In tumor cases presenting inflammation, the inflammatory cells in the microenvironment cause cytokine expression, and these cytokines facilitate tumor growth while inhibiting antitumor responses (Larruskain et al., 2015; Xie et al., 2021). TNF-α, which is the most well-known among these cytokines, is also synthesized in malignant cells in the tumor microenvironment. In studies of TNF-α, which is a proinflammatory cytokine that has roles in important functions of the cell such as survival, differentiation, proliferation, and apoptosis, employing animal models, it has been found that this factor shows an antitumorigenic activity by the stimulation of an inflammatory response to protumorigenic tumor cells detected as foreign in terms of supporting epithelial-mesenchymal transition, angiogenesis, and invasion (Ohri et al., 2010; Shang et al., 2017; Gong et al., 2021). As biomarkers of the diagnosis of oral squamous cell carcinoma (SCC) in humans, IL-6, IL-8, and TNF-α are among the most researched cytokines, and the prognosis of patients with oral SCC is largely dependent on their stage at the time of diagnosis (Dikova et al., 2021; Ferrari et al., 2021). In a clinical study, Dikova et al. (2021) reported that IL-6 and TNF-α levels were significantly higher in advanced stages of oral SCC in comparison to earlier stages, and IL-6 and TNF-α could differentially demonstrate the progression of oral SCC in the presence of metastasis. According to Ferrari et al. (2021), there was a close interaction between saliva and oral dysplastic/neoplastic cells, the concentrations of these cytokines in the saliva of oral SCC patients were higher than those in healthy controls, and therefore, saliva could be an ideal candidate for the development of non-invasive diagnostic tests with high accuracy. In our study, it was seen that IL-6 and TNF-α, which are among the most studied cytokines as biomarkers of BOSCC, were at higher concentrations in the cattle with BOSCC than in the healthy controls. Kim et al. (2020) studied the TNF-α and IL-6 gene expressions in epithelial cells of the eyes. Similar to the result in our study, they reported that the expression levels of these two genes increased in the presence of diseases. Banasaz et al. (2023) examined miRNAs and the expression of IL-6 and TNF-α in eye cells and detected an increase in the expressions of these two genes, which was compatible with the result of our study. When the studies were analysed, Karakurt et al. (2021) investigated PCNA, MMP-9 and P53 expressions immunohistochemically in BOSCC cases and emphasized that the p53 gene could be used as a marker. In addition, Keizer et al. (2022) showed that mutations in TP53, CDKN2A, RB1 and TERT genes were observed in both tumour types in squamous cell carcinomas of the conjunctiva and ocular sebaceous carcinoma tumours in humans and miR-196b-5p and miR-107 were involved in the carcinogenesis of multiple conjunctival tumours. However, the effects of these two cytokines, which are effective in tumour cell proliferation, inhibition of apoptosis, metastasis and tumour spread to other organs and angiogenesis, have not been investigated molecularly at mRNA level by looking at IL-6 and TNF α levels in BOSCC cases in the literature. The results in this study, which supported the results reported in previous studies, showed the presence of severe TNF-α expression in tumor cells and suggested that this cytokine could be an important biomarker candidate for the diagnosis and follow-up of tumors (Kim et al., 2020; Banasaz et al., 2023). In immunohistochemical examination of BOSCC cases, an inverse correlation was observed between TNF-α expression and the degree of differentiation. In general, the information in the literature has indicated that tear samples can be used to develop non-invasive rapid diagnosis tests for the early diagnosis of BOSCC. Thus, with the early diagnosis of BOSCC, broad resections of tissues can be prevented, and survival rates can be increased without relapse.
The importance of the diagnosis process for the prognosis and treatment success of BOSCC is undeniable. Although the presence of a tumor can be macroscopically detected, histopathological examination is indispensable for the determination of tumor characteristics. Previous studies reported the prominence of differentiation, mitotic figures, and invasion, in addition to the presence of inflammatory reactions with substantial keratinization and fibrosis (Sözmen et al., 2019; Yıldız and Karakurt, 2022; Karakurt et al., 2023). Similar histopathological findings were encountered in this study. However, as opposed to previous studies, metastasis findings were not seen during the follow-up period.
The prognosis of oral SCC in humans is largely dependent on the stage of the disease at the time of diagnosis, and the 5-year survival rate of patients with advanced tumors was reported to be approximately 30% (Ferrari et al., 2021). In BOSCC, prognosis and outcomes depend on the early detection of clinical symptoms, the presence of metastases, and the timing of tumor removal (Jones, 2019; Özcan-Martz et al., 2021). The BOSCC of the third eyelid can have an aggressive locally invasive form, and this makes early diagnosis and resection critical for the life expectancy of animals (Jones, 2019). For animal welfare-related and economic reasons, tumors that are suitable for resection must be resected as soon as possible (Özcan-Martz et al., 2021). Chigerwe et al., (2017) claimed that the early clinical diagnosis of BOSCC can prevent the need for exenteration. In this study, it was observed that the cases that were diagnosed early within 1-3 months and treated with local excision had the highest success rates, and they did not show relapse or complications. It was discovered that relapse could develop between the 16th and 23rd months in all cases that were treated between the 4th and 9th months. Considering that all cases receiving delayed treatment (7-9 months and 10-12 months) with exenteration can be removed from the herd, interventions may not be preferred.
Surgical excision is still considered the gold standard treatment for cutaneous SCC in humans (Burns et al., 2022). It was highlighted that the removal of BOSCC localized in the eye or close to the eye by surgical excision resulted in a good prognosis (Podarala et al., 2020; Özcan-Martz et al., 2021). It was reported that the removal of one eye had little to no effect on the yield of cattle and their perceived quality of life, and most cattle owners (92%) were satisfied with the outcomes of the surgery (Thiry et al., 2022). While different treatment methods for BOSCC have been reported, excision and enucleation are still surgical methods that are valid today, low-cost, simple, and easily applicable with fewer pieces of equipment (Yavuz and Yumuşak, 2017; Podarala et al., 2020; Özcan-Martz et al., 2021; Thiry et al., 2022). In this study, it was determined that local excision and enucleation did not affect the postoperative yield performance of the animals or their perceived quality of life, whereas the 6 cases that underwent treatment with the exenteration technique due to the broad invasion of their tumors were removed from their herd within the first 6 months as complications that were economically detrimental persisted. These surgical techniques can be used to alleviate the pain in BOSCC cases localized in the ocular and periocular regions, increase the quality of life of the animal, reduce economic losses, and increase the animal’s duration of productivity. Nevertheless, cattle owners should be made aware of potential surgical complications after exenteration.
Schulz and Anderson (2010) stated that the relapse of OSCC is rare. Although Chigerwe et al. (2017) reported various complications and tumor relapses after enucleation/exenteration, they did not find a significant relationship between the emergence of postoperative complications and ocular diagnosis, age, anesthesia technique, or suture model. Yet another limitation of our study was that the relapse rate might have been underestimated as the cases were followed up for only 2 years, and the cattle that were diagnosed with relapse could not be monitored in the long term to evaluate their lifespan. Among the 56 cases followed up in this study for 2 years, 12.6% showed tumor relapse between the 16th and 23rd months, whereas various postoperative complications were observed in 10.8%.
CONCLUSION
Consequently, while the development of BOSCC has a multifactorial etiology, prolonged exposure to UV light in cattle that are kept in hot climates is the primary cause contributing to the pathogenesis of the disease. In BOSCC cases, the prognosis largely depends on the stage at the time of diagnosis, and early diagnosis should be facilitated using immunohistochemical and molecular methods. Complications are not observed in small BOSCC lesions that are treated early with surgical techniques alone, and the 2-year survival rates of these cases are considerably high. It is critical to lower the morbidity rate of the disease by the early diagnosis and treatment of lesions, as well as selective breeding.
REFERENCES
- AL-ASADI, R.N. A survey and treatment of ocular carcinomas in Iraqi dairy cows from (1987-2012). Kufa J. Vet. Med. Sci., v.3, p.66-77, 2012.
- ANDERSON, D.E. Genetic study of eye cancer in cattle. J. Hered., v.82, p.21-26, 1991.
- ANDERSON, D.E.; BADZIOCH, M. Association between solar radiation and ocular squamous cell carcinoma in cattle. Am. J. Vet. Res., v.52, p.784-788, 1991.
- BANASAZ, B.; ZAMZAM, R.; AGHADOOST, D. et al. Evaluation of expression pattern of cellular miRNAs (let-7b, miR-29a, miR-126, miR-34a, miR-181a-5p) and IL-6, TNF-α, and TGF-β in patients with pseudoexfoliation syndrome. Pathol. Res. Pract., v.249, p.1-10, 2023.
- BURNS, C.; KUBICKI, S.; NGUYEN, Q.B. et al. Advances in cutaneous squamous cell carcinoma management. Cancers, v.14, p.1-21, 2022.
- CAPPELLERI, A.; MINOLI, L.; PIGOLI, C. et al. Retrospective study of tumors from cattle slaughtered in Lombardy (Italy): Preliminary evaluation on the establishment of a bovine cancer registry. Vet. Ital., v.58, p.67-75, 2022.
- CERUTTI, P.; AMSTAD, P.; LARSSON, R. et al. Mechanisms of oxidant carcinogenesis. Prog. Clin. Biol. Res., v.347, p.183-186, 1990.
- CEYLAN, C.; OZYILDIZ, Z.; YILMAZ, R. et al. Clinical and histopathological evaluation of bovine ocular and periocular neoplasms in 15 cases in Sanliurfa Region. Kafkas Univ. Vet. Fak. Derg., v.18, p.469-474, 2012.
- CHIGERWE, M.; ANGELOS, J.A.; GAMSJÄEGER, L. et al. Transpalpebral exenteration in cattle: a retrospective study of 115 cases. Vet. Ophthalmol., v.20, p.435-440, 2017.
- ÇAĞLAYAN, C.; KANDEMIR, F.M.; DARENDELIOĞLU, E. et al. Rutin ameliorates mercuric chloride-induced hepatotoxicity in rats via interfering with oxidative stress, inflammation and apoptosis. J. Trace Elem. Med. Biol., v.56, p.60-68, 2019.
- DAVIS, K.M.; SMITH, T.; BOLT, B. et al. Digital quantification of eye pigmentation of cattle with white faces. J. Anim. Sci., v.93, p.3654-60, 2015.
- DIAZ, L.; BERNADEZ-VALLEJO, S.V.; VARGAS-CASTRO, R. et al. The phytochemical α-mangostin inhibits cervical cancer cell proliferation and tumor growth by downregulating E6/E7-HPV oncogenes and KCNH1 gene expression. Int. J. Mol. Sci., v.24, p.1-16, 2023.
- DIKOVA, V.; JANTUS-LEWINTRE, E.; BAGAN, J. Potential non-invasive biomarkers for early diagnosis of oral squamous cell carcinoma. J. Clin. Med., v.10, p.1-16, 2021.
- FERRARI, E.; PEZZI, M.E.; CASSI, D. et al. Salivary cytokines as biomarkers for oral squamous cell carcinoma: a systematic review. Int. J. Mol. Sci., v.22, p.1-14, 2021.
- FORNAZARI, G.A.; KRAVETZ, J.; KIUPEL, M. et al. Ocular squamous cell carcinoma in Holstein cows from the South of Brazil. Vet. World, v.10, p.1413-1420, 2017.
- GHARAGOZLOU, M.J.; HEKMATI, P.; ASHRAFIHELAN, J. A clinical and histopathological study of ocular neoplasms in dairy cattle. Vet. Arhiv., v.77, p.409-426, 2007.
- GONG, K.; GUO, G.; BECKLEY, N. et al. Tumor necrosis factor in lung cancer: Complex roles in biology and resistance to treatment. Neoplasia, v.23, p.189-96, 2021.
- JARA, E.; PEÑAGARICANO, F.; ARMSTRONG, E. et al. Revealing the genetic basis of eyelid pigmentation in Hereford cattle. J. Anim. Sci., v.100, p.1-8, 2022.
- JONES, M.L. Surgical eye procedures. Proc. Am. Assoc. Bovine Pract., v.52, p.264-267, 2019.
- KARAKURT, E.; AYDIN, U.; BEYTUT, E. et al. Expression of PCNA, MMP-9 and P53 in bovine ocular squamous cell carcinomas: An immunohistochemical study. J. Res. Vet. Med., v.40, p.98-105, 2021:
- KARAKURT, E.; AYDIN, U.; BEYTUT, E. et al. The role of oxidative and nitrosative stress in bovine ocular squamous cell carcinomas. Vet. Arh., v.94, p.109-118, 2024.
- KARAKURT, E.; COSKUN, N.; AYDIN, U. et al. An investigation of bovine papillomaviruses from ocular squamous cell carcinomas in cattle. Iran J. Vet. Res., v.24, p.51-7, 2023.
- KEIZER, R.O.; VRIENDS, A.L.; HOTTE, G.J. et al. MiR-196b-5p and miR-107 expression differentiates ocular sebaceous carcinoma from squamous cell carcinoma of the conjunctiva. Int. J. Mol. Sci., v.23, p.4877-4890, 2022.
- KIM, Y.H.; YANG, I.J.; NGUYEN, L.T.H. et al. Effect of diquafosol on hyperosmotic stress-induced tumor necrosis factor-Α and Interleukin-6 expression in human corneal epithelial cells. Korean J. Ophthalmol., v.34, p.1-10, 2020.
- KUDO, K.; KOBAYASHI, T.; KASAI, K. et al. Chondroitin sulfate is not digested at all in the mouse small intestine but may suppress interleukin 6 expression induced by tumor necrosis factor-α. Biochem. Biophys. Res. Commun., v.642, p.185-91, 2023.
- KUTLU, T.; KARAKURT, E.; AYDIN, U. et al. Apoptosis in bovine ocular squamouse cell carcinomas. Eurasian J. Vet. Sci., v.38, p.131-136, 2022.
- LAKSHMI, M.P.; VEENA, P.; KUMAR, R.V. et al. Efficacy of different treatment modalities for eye cancer in bovines. Indian J. Anim. Res., v.55, p.1101-1104, 2021.
- LARRUSKAIN, A.; ESPARZA‐BAQUER, A.; MINGUIJÓN, E. et al. SNP s in candidate genes MX dynamin‐like GTP ase and chemokine (C‐C motif) receptor‐5 are associated with ovine pulmonary adenocarcinoma progression in Latxa sheep. Anim. Genet., v.46, p.666-675, 2015.
- MARTINS, T.B.; BARROS, C.S. Fifty years in the blink of an eye: a retrospective study of ocular and periocular lesions in domestic animals. Pesqui. Vet. Bras., v.34, p.1215-1222, 2014.
- NG, A.T.J.; MCMULLEN, R.J.; SHAW, G.C. et al. Limbal squamous cell carcinoma in a black baldy cow: case report and surgical treatment. Case Rep. Vet. Med., v.2023, p.1-6, 2023.
- OHRI, C.M.; SHIKOTRA, A.; GREEN, R.H. et al. Tumour necrosis factor-alpha expression in tumour islets confers a survival advantage in non-small cell lung cancer. BMC Cancer, v.10, p.1-9, 2010.
- ÖZCAN-MARTZ, A.; MARTZ, P.; EBERT, F. et al. Case reports of squamous cell carcinoma in cows. Vet. Ital., v.57, p.161-168, 2021.
- PEARCE, J.W.; MOORE, C.P. Food animal ophthalmology. In: GELATT, K.N. (Ed.). Essentials of veterinary ophthalmology. 3.ed. Iowa, USA: John Wiley & Sons, Inc., 2014. p.449-484.
- PODARALA, V.; LAKSHMI, M.P.; VENKATA, S.K.R. et al. Efficacy of BCG vaccine and Mitomycin C for the treatment of ocular squamous cell carcinoma in bovines. Res. Vet. Sci., v.133, p.48-52, 2020.
- PUGLIESE, M.; MAZZULLO, G.; NIUTTA, P.P. et al. Bovine ocular squamous cellular carcinoma: a report of cases from the Caltagirone area, Italy. Vet. Arh., v.84, p.449-57, 2014.
- SCHULZ, K.; ANDERSON, D.E. Bovine enucleation: a retrospective study of 53 cases (1998-2006). Can. Vet. J., v.51, p.611-614, 2010.
- SHANG, G.S.; LIU, L.; QIN, Y.W. IL-6 and TNF-α promote metastasis of lung cancer by inducing epithelial-mesenchymal transition. Oncol. Lett., v.13, p.4657-4660, 2017.
- SÖZMEN, M.; DEVRIM, A.K.; SUDAGIDAN, M. et al. Significance of angiogenic growth factors in bovine ocular squamous cell carcinoma. J. Comp. Pathol., v.170, p.60-69, 2019.
- THIRY, C.; HOLZ, N.; VOELTER, K. et al. Eye enucleation and exenteration in cattle: a retrospective study of 38 cases (2013-2020). Schweiz. Arch. Tierheilkd., v.164, p.687-693, 2022.
- TSUJITA, H.; PLUMMER, C.E. Bovine ocular squamous cell carcinoma. Vet. Clin. North Am. Food Anim. Pract., v.26, p.511-529, 2010.
- ULUCAN, A.; YÜKSEL, H.; DÖRTBUDAK, M.B. et al. Comparative examination of commonly used some fixatives with routine histochemical staining’s for the optimal histological appearance in the gill tissue of zebrafish. Kocatepe Vet. J., v.12, p.158-167, 2019.
- VALA, H.; CARVALHO, T.; PINTO, C. et al. Immunohistochemical studies of cytokeratins and differentiation markers in bovine ocular squamous cell carcinoma. Vet. Sci., v.7, p.1-14, 2020.
- XIE, Q.; DING, J.; CHEN, Y. Role of CD8+ T lymphocyte cells: interplay with stromal cells in tumor microenvironment. Acta Pharm. Sin. B., v.11, p.1365-1378, 2021.
- YAVUZ, Ü.; YUMUŞAK, N. Clinical and histopathological evaluation of bovine ocular and periocular tumors. Harran Univ. Vet. Fak. Derg., v.6, p.73-78, 2017.
- YILDIZ, A.; KARAKURT, E. Determination of apoptosis and autophagy in bovine ocular squamous cell carcinomas by ımmunohistochemistry. Pak. Vet. J., v.42, p.147-152, 2022.
Publication Dates
-
Publication in this collection
14 July 2025 -
Date of issue
Jul-Aug 2025
History
-
Received
19 Nov 2024 -
Accepted
29 Jan 2025

 Evaluation of early diagnosis of bovine ocular squamous cell carcinoma (BOSCC) by clinical, pathological and molecular methods
Evaluation of early diagnosis of bovine ocular squamous cell carcinoma (BOSCC) by clinical, pathological and molecular methods