Abstracts
PURPOSE: To analyze, in vitro, the effects of acetylsalicylic acid (aspirin) and acetic acid solutions on VX2 carcinoma cells in the liver of rabbits with VX2 hepatic tumors; to determine the histolytic and anatomopathological characteristics of the solutions; and to evaluate the eventual biochemical and hepatic changes. METHODS: A total of 48 rabbits were evaluated. The animals were randomized into two groups, protocol 3 (study group) and protocol 4 (controls), and each group was then subdivided into 3 subgroups. Four days after implantation of the tumor in the liver, median laparotomy was performed with a 0.4-ml injection of a solution of either aspirin (5.0%), acetic acid (5.0%) or saline. The animals were sacrificed after 24 hours (protocol 3) or after 11 days (protocol 4). Body weight, clinical evolution and biochemical levels, as well as the abdominal and thoracic cavities, were evaluated, and liver microscopy was performed. RESULTS: No changes in clinical evolution, body weight or biochemical levels were reported. However, an increase in alkaline phosphatase was observed in protocol 4 (controls). The tumor was eliminated in both protocols. CONCLUSION: Acetylsalicylic acid and acetic acid solutions cause the destruction of experimental hepatic tumors.
Carcinoma; Aspirin; Acetic Acid; In vitro; In vivo; Rabitts
OBJETIVO: Analisar os efeitos das soluções de aspirina e de ácido acético, in vivo, em fígado de coelhos portadores de tumor hepático VX2, verificando o efeito histolítico e anatomo-patológico das soluções e eventuais alterações bioquímicas hepáticas. MÉTODOS: Utilizou-se 48 coelhos, divididos em 2 protocolos experimentais(3 e 4), subdivididos em 3 grupos cada. Após 4 dias da implantação do tumor no fígado, procedeu-se a laparotomia mediana, com injeção de 0,4 ml da solução de aspirina (5,0%), de ácido acético (5,0%) e solução salina; o sacrifício ocorreu apos 24 horas (protocolo 3) e 11 dias (protocolo 4); avaliou-se o peso, evolução clinica, dosagens bioquímicas, cavidade abdominal e torácica e microscopia do fígado. RESULTADOS: Não foram observadas alterações na evolução clinica, peso e nas dosagens bioquímicas, apenas elevação da fosfatase alcalina no grupo controle do protocolo 4. Observamos desaparecimento do tumor em ambos os protocolos. CONCLUSÃO: As soluções de ácido acético e ácido acetilsalicílico acarretam destruição do tumor hepático experimental.
Carcinoma; Aspirina; Ácido Acético; In vitro; In vivo; Coelhos
ORIGINAL ARTICLE
Effects of acetylsalicylic acid and acetic acid solutions on VX2 liver carcinoma in rabbits. In vivo analysis1 1 Research performed at Experimental Surgery Laboratory, Department of Surgery and Orthopedics, University of Botucatu Medical School (UNESP), Brazil.
Efeitos das soluções de aspirina e de ácido acético em fígado de coelhos portadores de tumor hepático VX2. Análise in vivo
Rogério Saad-HossneI; René Gamberini PradoI; William Saad HossneII
IPhD, Assistant Professor, Department of Surgery and Orthopedics, UNESP, Botucatu, Brazil
IIProfessor Emeritus, UNESP, Botucatu, Brazil
Correspondence Correspondence: Rogério Saad-Hossne Departamento de Cirurgia e Ortopedia Faculdade de Medicina de Botucatu - UNESP 18618-970 Botucatu SP Brazil saad@fmb.unesp.br
ABSTRACT
PURPOSE: To analyze, in vitro, the effects of acetylsalicylic acid (aspirin) and acetic acid solutions on VX2 carcinoma cells in the liver of rabbits with VX2 hepatic tumors; to determine the histolytic and anatomopathological characteristics of the solutions; and to evaluate the eventual biochemical and hepatic changes.
METHODS: A total of 48 rabbits were evaluated. The animals were randomized into two groups, protocol 3 (study group) and protocol 4 (controls), and each group was then subdivided into 3 subgroups. Four days after implantation of the tumor in the liver, median laparotomy was performed with a 0.4-ml injection of a solution of either aspirin (5.0%), acetic acid (5.0%) or saline. The animals were sacrificed after 24 hours (protocol 3) or after 11 days (protocol 4). Body weight, clinical evolution and biochemical levels, as well as the abdominal and thoracic cavities, were evaluated, and liver microscopy was performed.
RESULTS: No changes in clinical evolution, body weight or biochemical levels were reported. However, an increase in alkaline phosphatase was observed in protocol 4 (controls). The tumor was eliminated in both protocols.
CONCLUSION: Acetylsalicylic acid and acetic acid solutions cause the destruction of experimental hepatic tumors.
Key words: Carcinoma. Aspirin. Acetic Acid. In vitro. In vivo. Rabitts.
RESUMO
OBJETIVO: Analisar os efeitos das soluções de aspirina e de ácido acético, in vivo, em fígado de coelhos portadores de tumor hepático VX2, verificando o efeito histolítico e anatomo-patológico das soluções e eventuais alterações bioquímicas hepáticas.
MÉTODOS: Utilizou-se 48 coelhos, divididos em 2 protocolos experimentais(3 e 4), subdivididos em 3 grupos cada. Após 4 dias da implantação do tumor no fígado, procedeu-se a laparotomia mediana, com injeção de 0,4 ml da solução de aspirina (5,0%), de ácido acético (5,0%) e solução salina; o sacrifício ocorreu apos 24 horas (protocolo 3) e 11 dias (protocolo 4); avaliou-se o peso, evolução clinica, dosagens bioquímicas, cavidade abdominal e torácica e microscopia do fígado.
RESULTADOS: Não foram observadas alterações na evolução clinica, peso e nas dosagens bioquímicas, apenas elevação da fosfatase alcalina no grupo controle do protocolo 4. Observamos desaparecimento do tumor em ambos os protocolos.
CONCLUSÃO: As soluções de ácido acético e ácido acetilsalicílico acarretam destruição do tumor hepático experimental.
Descritores: Carcinoma. Aspirina. Ácido Acético. In vitro. In vivo. Coelhos.
Introduction
The human preoccupation with cancer is understandable in light of its high incidence worldwide. In Brazil, it is the second leading cause of death among the adult population1. Neoplasia is also the second leading cause of death in Brazilians over the age of 40 and the third leading cause of death at any age, accounting for approximately 16%, or 110,000, of the deaths that occur annually2. The most common types of neoplasia are skin, breast, lung, stomach, uterus, colon and rectum and prostate. Together, they account for 157,000 new cases every year2. The main factors affecting prognosis and recurrence of neoplasia are lymph node involvement, local recurrence, striation and, especially, the presence of distant metastases. One of the most commonly affected organs is the liver. Treatment options for liver neoplasia are limited by factors such as the number of metastases and their locations. The principal modalities of treatment for hepatic metastases currently include surgical resection, arterial ligation, embolization, chemotherapy and genetic therapy. Ablation techniques involving necrotizing and cytolytic substances, lasers, radiofrequencies, microwaves, hyperthermia and cryotherapy have also been used. Surgical resection is the main treatment for neoplasia and is the only one that offers the possibility of a cure. However, only small percentages (10 15%) of patients are good candidates for surgical intervention3. Among the palliative methods, ablation stands out. In order to destroy the lesion locally, cytolytic and necrotizing substances, including alcohol, are commonly used. Other ablation methods include cryotherapy, radiofrequency, laser and microwave. Ablation methods are generally reserved for inoperable cases. In the face of these obstacles and therapeutic considerations, new treatments for hepatic metastases should be developed. Such treatments should present high rates of efficacy, low cost, low occurrence of side effects and should be easily executed. In a study performed in 19964, we analyzed the effects of a solution consisting of acetic acid, glycerin, phenol and distilled water on Ehrlich ascites tumor cells in vitro and in vivo and observed that the solution caused tumor cell death in vitro. Intraperitoneal injection of the solution in vivo reduced the number of tumor cells and increased the number of inflammatory cells in suspension in the acetic acid solution4. In light of this observed effect, we decided to analyze the possible effects of one or more of the solution components in isolation. In the literature, there are no references to the use of acetic acid, or of its derivative, acetylsalicylic acid, in experimental tumors. Considering this fact and the results previously obtained, we decided to evaluate the effects of acetylsalicylic acid and acetic acid solutions on the livers of animals with VX2 carcinomas.
Purpose
To analyze the effects of bicarbonate acetylsalicylic acid solution and aqueous acetic acid solution, in vivo, in the livers of rabbits with experimental VX2 carcinoma. To determine 1) whether these solutions cause tumor necrosis or inhibit immediate (24-hour) tumor growth, 2) the histopathologic evolution 7 days after the experiment and 3) whether there are clinical or biochemical changes in hepatic function.
Methods
A total of 48 male albino rabbits, 69 weeks old and weighing 14002500 g, were studied. Experimental procedures commenced at 24 hours after the animals were delivered to the laboratory. The animals were deprived of food for 6 hours prior to the experiments but received water ad libitum.
Anesthesia and surgical technique
Animals were anesthetized by intravenous injection of 3% sodium pentobarbital (30 mg/kg) and subjected to median laparotomy via supraumbilical incision. Subsequently, 104 VX2 tumor cells in suspension were injected into the left lobe of the liver. The incision was closed, in layers, with 4-0 nonabsorbent nylon sutures. After four days, the animals were submitted to a second laparotomy for injection of the test solutions into the hepatic tumor. Injection volume ranged from 0.3 to 0.5 ml; both study protocols were carried out.
Test solutions
Both 5% acetic acid (aqueous) solution and 5% acetylsalicylic acid solution were tested. In order to obtain the desired concentration in the acetylsalicylic acid solution, 500 mg of acetylsalicylic acid was diluted into 10ml of sodium bicarbonate at 10%, which resulted in a bicarbonate acetylsalicylic acid solution. All solutions were prepared 2 minutes prior to use and were administered in doses of 0.3 to 0.5 ml, as was the saline solution administered to control animals.
Ponderal index and clinical evolution
The animals were weighed prior to each experimental procedure. Clinical evolution was evaluated through objective parameters such as post-surgical rehabilitation, food intake and activity. These were then subjectively classified as "good", "mediocre" or "poor".
Serum biochemical assessment
Over the course of each protocol and prior to each experiment, the animals were submitted to peripheral venous puncture with a 25x7 needle, and 5 ml of blood was collected. The following biochemical variables were analyzed: glycemia, alkaline phosphatase (AP), gamma-glutamyl transferase (GGT), aspartate aminotransferase (AST) and alanine aminotransferase (ALT).
Sacrifice
At the time of sacrifice, animals were submitted to lethal injection of the same anesthetic used during the surgical procedures
Macroscopic examination of the thoracic and abdominal cavity
Following sacrifice of the animals, the macroscopic aspects of the abdominal and thoracic cavities (serosa and organs) were evaluated.
Microscopic examination of liver tissue
The left hepatic lobe was embedded in paraffin. Sections were then cut, mounted on slides and randomly analyzed in a bright field optic microscope. The technician had no previous knowledge concerning the corresponding protocol.
Statistical analysis of the results
Ponderal indices and biochemical levels were analyzed by ANOVA, considering three experiments conducted at different times. Comparison of means was performed using the Tukey's test. Statistical significance was set at less than or equal to 5% (p < 0.05).
Protocols
Protocol 1
A total of 24 animals were randomly divided into three groups (8 animals per group): Group A (VX2 + Saline Solution), Group B (VX2 + 5% Acetylsalicylic acid) and Group C (VX2 + 5% Acetic Acid). The animals were weighed and biochemical levels were determined at three points: prior to tumor implantation (day 0), on the day of injection (day 4) and at sacrifice (24 hours after injection, on day 5).
Protocol 2
This protocol was created with the aim of evaluating the prolonged effects of injection of the solutions into the hepatic tumor (VX2).
A total of 24 animals were randomly divided into three groups (8 animals per group): Group D (VX2 + Saline Solution), Group E (VX2 + 5% Acetylsalicylic acid) and Group F (VX2 + 5% Acetic Acid). The animals were weighed and biochemical levels were determined at three points: prior to tumor implantation (day 0), on the day of injection (day 4) and at sacrifice (7 days after injection, on day 11).
Results
Protocol 1
Clinical evolution
In all groups (A, B and C), the animals present good clinical evolution and there were no deaths.
Ponderal index
In all three groups (A, B and C), ponderal indices increased from day 0 to day 4, although there was general weight reduction (due to being fasted) between days 4 and 5 (during the 24 hours following injection and preceding sacrifice).
Biochemical levels
Glycemia, alkaline phosphatase, gamma-glutamyl transferase and aspartate aminotransferase. All Glycemia, AP and GGT levels were within reference values. There was a progressive increase in AST levels over time, although no statistically significant differences were found in any of these levels at any of the various time points evaluated.
Alanine aminotransferase
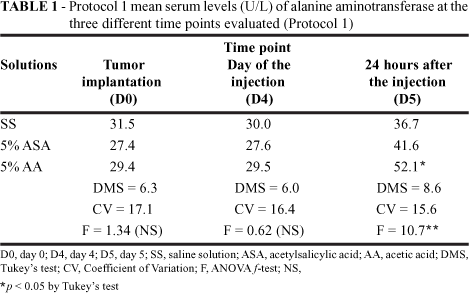
Analysis of the findings demonstrates that, in this protocol, all ALT levels were within reference values (1060 U/L). In addition, there was a larger and more evident increase of these values in group C. There were statistically significant differences between the group receiving 5% acetic acid solution and the groups receiving either saline (controls) or 5% acetylsalicylic acid (Table 1).
Macroscopic aspects of thoracic and abdominal cavities
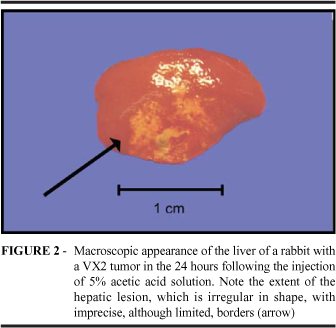
There were no changes to the thoracic cavity of any of the animals studied. Analysis of the abdominal cavity revealed the hepatic lesion in all animals. In the control group (Group A) animals, the hepatic lesion was explicit, showing yellowish-white coloration and hardening, with a diameter between 0.8 and 1.2 cm. There were no signs of adjacent hepatic lesions (Figure 1). In the livers of animals treated with 5% acetylsalicylic acid (Group B), we observed that lesions were irregular-shaped (although limited), reddish-white in coloration, hardened and larger (0.8 to 1.0 cm in diameter) than those observed in the livers of control group animals. In addition, these lesions were cystic in appearance and there were indications of adjacent hepatic lesions. In the group treated with 5% acetic acid solution (Group C), the lesioned area was slightly more extensive (1.2 to 1.5 cm in diameter), but similar to that observed in Group B, with imprecise borders (although limited), reddish-white in coloration, hardened and indications of adjacent hepatic lesions (Figure 2).
Microscopic appearance of the liver
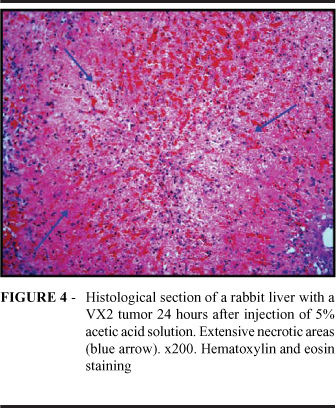
In all the animals treated with saline solution (Group A), we identified a large tumor nodule. The adjacent hepatic tissue presented normal characteristics, with no areas or signs suggestive of necrosis (Figure 3). In 6 of the 8 animals treated with 5% acetylsalicylic acid solution (Group B), we observed large areas of coagulative necrosis were observed in the hepatic parenchyma, with hemorrhage, intraparenchymal inflammatory infiltrate and lack of viable focal tumor cells. In the remaining 2 animals, similar necrotic areas were identified, although with tumor cell foci. In 6 of the 8 animals treated with 5% acetic acid solution (Group C), we also observed large areas of coagulative necrosis in the parenchyma, without tumor lesions (Figure 4). In the remaining 2 animals, we observed small tumor cell foci adjacent to the necrotic areas (Figure 5), similar to those observed in Group B.
Protocol 2
Clinical evolution
All animals presented favorable clinical evolution. No deaths occurred during the study period.
Ponderal indices
No statistically significant differences among groups were found at any of the three time points.
Biochemical dosagens
Glycemia, gamma-glutamyl transferase, aspartate aminotransferase and alanine aminotransferase. Glycemia, GGT, AST and ALT values were all within reference values. No statistically significant difference was found in any of the levels at any of the three time points evaluated.
Alkaline phosphatase
All AP values obtained on the first day following the implant, as well as at the second time point (day of injection, day 4) were within reference limits (90146 U/L). At the third time point (day of sacrifice, day 11), 6 animals in the control group presented values which exceeded reference limits, whereas these values remained within normal reference limits in the other groups (treated with acetylsalicylic acid or acetic acid). Statistical analysis showed that this difference between the control group and the other groups was statistically significant (Table 2).
Macroscopic appearance of thoracic and abdominal cavities
No changes to the thoracic cavity were observed in any animals evaluated in Protocol 2. Abdominal cavity examination of animals treated with saline solution (Group D) clearly revealed tumor nodules, yellowish-white in coloration, 1.0 to 1.6 cm in diameter, hardened and surrounded by small, multiple punctiform lesions that were nodular in appearance and consistency (Figure 6). Macroscopic examination of the histological sections showed well-delimited hardened oval-shaped tumor nodules. These lesions were restricted to the affected lobe. There were no lesions in the other hepatic lobe or in the rest of the cavity. In the group treated with 5% acetylsalicylic acid solution (Group E), one or more (maximum, three) irregular (although limited) areas (0.3 to 0.5 cm in diameter) that were yellowish-white in color and hardened, presenting punctiform or circular patterns, were observed. These were restricted to the left lobe and there were no apparent signs of tumor lesion. In the macroscopic sections, we observed characteristics similar to those described above, due to the superficial nature of the lesions. No other changes were observed in the other areas of the abdominal cavity. In most group F animals, irregular areas of yellowish-white color were observed at the injection sites (Figure 7). These areas measured from 0.7 to 1.3 cm in diameter, were limited to the left lobe and were cicatricial in appearance. There were no signs of tumor lesion. In macroscopic sections, we observed intraparenchymal lesions of cicatricial and fibrous appearance, with no signs of tumor lesions. In the remaining 3 animals, the hepatic lesions presented punctiform or circular appearance and yellowish-white coloration, similar to those observed in group E. No other changes were observed in the cavity.
Microscopic appearance of the liver
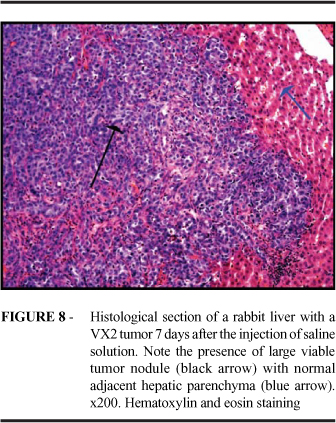
In all the animals within the group treated with saline solution (Group D), a large tumor nodule, with normal adjacent hepatic parenchyma, was found. No signs suggesting necrosis or tumor nonviability were found in any of the slides (Figure 8). In the group treated with 5% acetylsalicylic acid solution (Group E), 75% of the animals presented no signs of tumor. Microscopy demonstrated the presence of a fibrous-necrotic nodule with a proliferation of ducts and no signs of tumor lesion (Figure 9). In the remaining 25%, in addition to the lesions previously described, (two to three) microscopic nodules were identified. These were restricted to a small area within that of the necrosis and hepatic fibrosis. In the group treated with 5% acetic acid solution (Group F), we identified fibrous-necrotic nodules with intense foreign-body type gigantocellular immune response, with innumerable foci of calcification and no tumor cells. As in group E, we identified, in 2 animals, small tumor cell nodules (two to three) between the area of inflammatory response and that of the hepatic necrosis (Figure 10).
Discussion
Overview
The VX2 tumor has long been employed in radiological studies for the detection of tumors, with the aim of improving the sensitivity of diagnostic techniques such as tomography5,6, nuclear magnetic resonance7,8 and PET scans, and developing new drugs for use in chemotherapy9-14, as well as improving other kinds of treatment, such as chemoembolization8-15 and radiofrequency16,17,18. Tumor cell concentrations used to maintain the tumor are classically equal to or higher than 107 cells per ml. In our study, the same concentration (107 cells) was used in order to evaluate the in vitro effect. In a previous study, we used a solution composed of acetic acid, glycerin and phenol in the treatment of Ehrlich ascites tumor cells. We observed that the in vitro solution destroyed tumor cells and increased the number of inflammatory cells4. Therefore, it seemed appropriate to test one or more of these components separately in the livers of animals with tumors. In a pilot experiment, we observed that acetic acid actually has a destructive effect when it infiltrates the liver. It occurred to us to study the effect of acetylsalicylic acid as well because it would afford us the opportunity to study both of its components. The acetyl component (which can be analyzed through observation of the lytic effect of acetic acid) and the salicylic component (salicylic acid and the salicylates are, traditionally, applied externally as cytolytic agents) could be individually analyzed. Extremely positive reinforcement of the idea was provided by the results of our in vitro companion study, in which acetylsalicylic acid was determined to have an intense cytolytic action.
Protocol 1 Animals sacrificed 24 hours after injection
Clinical evolution and ponderal indices were considered favorable in the animals with tumors that were sacrificed 24 hours after injection. There were no deaths, which suggests that, despite having malignant hepatic lesions and having undergone treatment, these animals suffered no major clinical consequences. The lack of changes in the biochemical levels indicates that the implanted tumor did not provoke any major functional changes that would be detectable in biochemical testing. Mean GGT in all three Protocol 1 groups increased progressively from the time of implantation (day 0) to the time of sacrifice (24 hours after injection; day 5). However, the values all remained within the normal range, and there were no statistically significant differences among groups at any of the studied time points. These elevated GGT levels may have been the result of tumor growth. Statistical analysis of ALT levels (Table 1) demonstrated that there were differences between the 5% acetic acid group and the two other groups (saline solution and 5% acetylsalicylic acid). These were probably due to the fact that acetic acid has a more intense effect on the hepatic parenchyma than does acetylsalicylic acid, causing more cellular destruction and, as a consequence, liberation of ALT. However, all ALT values were within normal limits. The elevation in aminotransferase levels was an expected response, since serum aminotransferase levels always rise when there is tissue destruction and acute necrosis of the hepatic cells. Macroscopic evaluation of the thoracic cavity revealed, first, that there was no macroscopically visible tumor dissemination in the thoracic cavities of control group or study animals and, second, that the drugs used caused no pulmonary alterations (clotting or condensation), indicating that these drugs are innocuous in this aspect. Macroscopically, the livers presented reddish-white, localized lesions associated to the irregularly shaped, slightly vegetative tumor lesion. Under microscopy, we observed that there was no necrosis of the tumor tissue in the livers of control group animals. In these animals, the tumor nodules were large, intact and viable. In the two study groups, the effects differed greatly from those observed in the controls. In 75% of the animals treated with 5% acetylsalicylic acid, we identified large necrotic areas and nonviable tumors. In the remaining 25%, the tumor necrosis was partial, suggesting that this solution is effective in tumor destruction, although the dose employed (0.3 to 0.5 ml), or other factors, may have prevented infiltration of the entire area. Similarly, in 75% of the animals treated with 5% acetic acid, no tumor cell foci were found outside the area of necrosis. In two of these animals, however, we identified small viable tumor cell foci, also likely resulting from an insufficient quantity of the solution injection. These results suggest that both drugs are efficacious and provoke tumor necrosis. However, in some cases, the doses administered seemed insufficient to destroy the tumor. It is possible that the drugs injected did not reach all areas of the tumor.
Protocol 2 Animals sacrificed 7 days after injection
In animals implanted with tumor cells and sacrificed 7 days after injection with the solutions, clinical evolution and ponderal indices were considered favorable. There were no deaths. For the parameters evaluated, we have demonstrated that these drugs can be safely used for this purpose. As for the biochemical levels, we observed that AP values increased (exceeding normal levels) in 75% of the control group animals, whereas no such increases were observed in animals treated with acetylsalicylic acid or acetic acid. Statistical analysis showed that the differences between the control group and the two study groups were statistically significant.
This increase in control group AP values is suggestive of intrahepatic cholestasis, as well as bile canalicular contraction, resulting from tumor growth. Since this increase was not observed in the two treated groups, it might indicate that the drugs used exert partial or total control over tumor growth and thereby prevent cholestasis. Statistical analysis of the other biochemical levels revealed no differences among the groups, indicating that those values are unaffected by either the drugs or the tumor growth. As in the Protocol 1 groups, we observed no macroscopic changes in the thoracic cavity, providing additional evidence of the previously described innocuous effects of the drugs, as well as of the characteristics of the evolution of this type of tumor. In the macroscopic evaluation of the hepatic tissue, the differences between the control group and the groups treated with the two solutions were evident. In control group animals, the lesions had characteristics that were typical of tumors, whereas, in the animals treated with acetic acid or acetylsalicylic acid, there was no evidence of tumor lesion, only irregular, whitish or reddish-white areas that were all similar. This demonstrates that these solutions were able, either partially or totally, to inhibit the evolution of tumor growth and destroy the tumors. In all of the animals in both protocols, the lesions in the abdominal cavities were limited to the hepatic parenchyma. This demonstrates that the solutions used had an isolated local effect. There were no other alterations or inflammatory processes away from the site of application, nor were there any other lesions or extrahepatic changes during the study period. We may, therefore, attest to the low toxicity of acetic acid and acetylsalicylic acid solutions, evidenced as well by the lack of other biological changes, when used in animals with or without tumors according to the techniques employed in this study. Macroscopic analysis of the hepatic tissue revealed uncontrolled tumor growth in control group animals, whereas no tumor lesions were found in the majority of treated animals. Similarly to what was observed in Protocol 1, 75% of the animals treated with acetylsalicylic acid presented no signs of tumor lesion, whereas areas of tumor growth were identified in the remaining 25%. In the animals treated with acetic acid, 6 out of the 8 animals presented no evidence of tumor and, in the remaining 2 animals, viable microscopic tumor nodules were seen at the periphery, suggesting that the drugs did not reach these areas of the tumor. The limited response in 25% of the animals treated with acetylsalicylic acid or acetic acid indicates that the solutions, in the dosages employed, may have only a partial effect in some animals, probably as a consequence of the dosages being insufficient to affect the entire area of the tumor.
Conclusion
In the animals with VX2 carcinoma, both solutions caused necrosis of the tumor tissue within 24 hours after injection. At 7 days after injection, the areas destroyed were found to be free of tumor tissue and showed regeneration of the hepatic tissue, presenting fibrotic foci and inflammatory infiltrate. No clinical alterations were observed. The only statistically significant biochemical alterations were the increases in ALT levels at 24 hours after injection in animals receiving 5% acetic acid and the increases in AP levels in control group animals at 14 days after injection.
Acknowledgments
Prof. Dr. Carlos Eduardo Bacchi and Profa. Dra. Sheila Zambello de Pinho.
Received: March 12, 2007
Review: May 15, 2007
Accepted: June 14, 2007
Conflict of interest: none
Financial source: none
-
1World Health Organization-WHO. [on line]. Geneva, 1998. [cited 2001 Fev 16]. Available from: http:// www.who.org
-
2Brasil. Ministério da Saúde. Datasus. Sistema de Informações sobre mortalidade, 1997. Brasília, 2001. [acesso em 2001 Fev 16]. Disponível em: url: http://www.datasus.gov.br
- 3. Schlag PM, Benhidjeb T, Kilpert B. Surgical and multimodality treatment of colorectal liver metastases. Onkologie. 1999; 22:92-7.
- 4. Saad-Hossne R. Efeitos da solução aquosa de fenol, ácido acético e glicerina sobre a celularidade no líquido ascítico do tumor de Ehrlich em camundongo [Dissertação - Mestrado]. Universidade Estadual Paulista, Faculdade de Medicina de Botucatu; 1997.
- 5. De Baere T, Zhang X, Aubert B, Leander P, Harry G, Ropers J, Ducreux M, Roche A. Quantification of tumor uptake of rodized oils and emulsions of iodized oils: experimental study. Radiology. 1996; 201:731-6.
- 6. Leander P. An imaging study in a rabbit tumor model. Acta Radiol. 1996; 37:63-8.
- 7. Pauser S, Wagner S, Lippmann J, Pohlen U, Rezka R, Wolf KJ, Berger G. Evaluation of efficient chemoembolization mistures by magnetic resonance imaging therapy monitoring: An experimental study on the VX-2 tumor in rabbit liver. Cancer Res. 1996;56:1863-96.
- 8. Yamada K, Jinbo T, Miyahara K, Inoue Y, Tateno Y, Ikehira H, Furuhama K. Contrast-Enhanced MRI with gadodiamine injection in rabbit carcinoma models. J Vet Med Sci. 1996;58:389-96.
- 9. Iwai K, Maeda H, Konno T. Tumor targeting by arterial administration of lipids: rabbits model with VX-2 carcinoma in the liver. Anticancer Res. 1987;7:321-8.
- 10. Izumi B, Tashiro S, Miyauchi Y. Anticancer effects of local administration of mitomycin C via artery or portal vein on implantation and growth of VX-2 cancer injection in rabbit liver. Cancer Res. 1986;46:4167-70.
- 11. Roberson PL, Mc Shan DL, Mc Keever PE, Ensminger WD. Three-dimensional tumor dosimetry for hepatic microsphere therapy. J Nucl Med. 1992; 33:735-8.
- 12. Zhao Z, Ramirez LN, Simenauer N. Pharmacokinetics of intra-arterial hepatic adriamycin-lipiodol in rabbits with VX-2 tumor. Reg Cancer Treat. 1993; 4:213-7.
- 13. Kuwata Y, Hirota S, Sako M. Treatment of metastatic liver tumors by intermittent repetitive injection of and angiogenesis inhibitor using and implantable port system in a rabbit model [abstract]. Kobe J Med Sci. 1997; 43:83-98.
- 14. Hanuro M, Nakamura K, Sakai Y, Nakata M, Yamada R. New oil agent for targeting chemoembolization for hepatocellular carcinoma. Cardiovasc Intervent Radiol. 1999; 22:130-4.
- 15. Beppu T, Ohara C, Yamaguchi Y. A new chemoembolization therapy in rabbits liver with VX-2. Reg Cancer Treat. 1992; 5:33-5.
- 16. Goldberg SN, Gazelle GS, Compton CC. Radiofrequency tissue ablation of VX-2 tumor nodules in the rabbit lung. Acad Radiol. 1996; 3:929-35.
- 17. Kuszyk BS, Boitnott JK, Choiti MA, Magee CA, Horton KM, Fishman EK. Local tumor recurrence following hepatic cryoablation. Radiology. 2000; 217:477-86.
Publication Dates
-
Publication in this collection
23 July 2007 -
Date of issue
Aug 2007
History
-
Reviewed
15 May 2007 -
Received
12 Mar 2007 -
Accepted
14 June 2007