Abstracts
PURPOSE: To evaluate the effects of aspirin 10% and 20% on mesenteric lymph nodes of rabbits as basis for its use on lymph nodes metastases. METHODS: A total of 20 lymph nodes from 20 rabbits (randomized in four groups) were evaluated. Aspirin solutions 10% (groups A and C) and 20% (groups B and D) were injected into mesenteric lymph nodes of healthy rabbits and had its gross and histological effects evaluated at 24 hours (groups A and B) and at seven days (groups C and D). RESULTS: In the groups A and B evaluated at 24 hours it was observed extensive necrosis and hemorrhage, a significant increase in apoptosis throughout the lymph node with medullary sinuses enlargement and an increase in germinal centers. In the groups C and D evaluated at seven days of solution injection there was also an increase in apoptosis with higher elevation of histiocytes and a significant decrease of necrosis and an increase of giant cells was noticed causing a foreign body chronic inflammation. In all comparisons, there were no differences between the concentrations used (10 and 20%). CONCLUSIONS: The injection of aspirin on lymph nodes caused necrosis and an increase of apoptosis after 24 hours and after seven days of treatment there was regeneration of the lymph nodes, with intense decrease of necrosis and a great elevation of apoptosis. These experimental results support future clinical studies on application of aspirin in the treatment of lymphatic metastases, since the increase of apoptosis is one of the pillars of cancer therapy.
Aspirin; Lymph Nodes; Neoplasm Metastasis; Apoptosis; Therapeutics; Rabbits
OBJETIVO: Avaliar os efeitos do ácido acetilsalicílico a 10% e 20% em linfonodos mesentéricos de coelhos para posterior embasamento e uso em metástases linfonodais. MÉTODOS: Um total de 20 linfonodos de 20 coelhos (divididos aleatoriamente em quatro grupos) foi avaliado. As soluções de aspirina a 10% (grupos A e C) e 20% (grupos B e D) foram injetadas em linfonodos mesentéricos de coelhos sadios e seus efeitos macroscópicos e histológicos foram avaliados em 24 horas (grupos A e B) e em sete dias (grupos C e D). RESULTADOS: Nos grupos avaliados em 24 horas (A e B) foi verificada intensa necrose e hemorragia, aumento importante de apoptose em todo o linfonodo, com alargamento dos seios medulares e aumento dos centros germinativos. Nos grupos avaliados em sete dias (C e D) também houve aumento da apoptose, com maior elevação de histiócitos e diminuição importante da necrose; a hemorragia foi ausente e aumento de células gigantes foi visualizado, conferindo processo inflamatório crônico do tipo corpo estranho. Não houve diferença entre as concentrações utilizadas (10 e 20%) em nenhuma das comparações. CONCLUSÕES: A injeção de aspirina em linfonodos causou necrose e um aumento de apoptose após 24 horas e após sete dias de tratamento, houve regeneração dos gânglios linfáticos, com diminuição intensa de necrose e grande aumento de apoptose. Uma vez que o aumento de apoptose é um dos pilares dos tratamentos antineoplásicos, estes resultados experimentais embasam eventual aplicação clínica da aspirina no tratamento de metástases linfonodais.
Aspirina; Linfonodos; Metástase Neoplásica; Apoptose; Terapêutica; Coelhos
9 - ORIGINAL ARTICLE
EFFECTS OF DRUGS
Effects of aspirin on mesenteric lymph nodes of rabbits as basis for its use on lymph nodes metastases1 1 Research performed at Division of Coloproctology, Department of Surgery, Botucatu Medical School (FMB), State University of Sao Paulo (UNESP), Botucatu-SP, Brazil.
Efeitos da aspirina em linfonodos mesentéricos de coelhos como base para o uso em metástases linfonodais
Rodrigo Peduti BatistaI; Rafael DenadaiII; Rogério Saad-HossneIII
IMD, Gastroenterologist, Division of Coloproctology, Department of Surgery, FMB-UNESP, Botucatu-SP, Brazil. Main author. Acquisition and interpretation of data, technical procedures, manuscript writing
IIGraduate student, School of Medical Sciences, UNIMAR, Marilia-SP, Brazil. Interpretation of data, manuscript writing
IIIPhD, Associate Professor, Division of Coloproctology, Department of Surgery, FMB-UNESP, Botucatu-SP, Brazil. Supervised all phases of the study; conception, design, intellectual and scientific content of the study; manuscript writing; critical revision
Correspondence Correspondence: Rogério Saad-Hossne Distrito de Rubião Júnior, s/n 18618-000 Botucatu SP Brasil Tel./Fax: (55 14)3882-5475 saad@fmb.unesp.br
ABSTRACT
PURPOSE: To evaluate the effects of aspirin 10% and 20% on mesenteric lymph nodes of rabbits as basis for its use on lymph nodes metastases.
METHODS: A total of 20 lymph nodes from 20 rabbits (randomized in four groups) were evaluated. Aspirin solutions 10% (groups A and C) and 20% (groups B and D) were injected into mesenteric lymph nodes of healthy rabbits and had its gross and histological effects evaluated at 24 hours (groups A and B) and at seven days (groups C and D).
RESULTS: In the groups A and B evaluated at 24 hours it was observed extensive necrosis and hemorrhage, a significant increase in apoptosis throughout the lymph node with medullary sinuses enlargement and an increase in germinal centers. In the groups C and D evaluated at seven days of solution injection there was also an increase in apoptosis with higher elevation of histiocytes and a significant decrease of necrosis and an increase of giant cells was noticed causing a foreign body chronic inflammation. In all comparisons, there were no differences between the concentrations used (10 and 20%).
CONCLUSIONS: The injection of aspirin on lymph nodes caused necrosis and an increase of apoptosis after 24 hours and after seven days of treatment there was regeneration of the lymph nodes, with intense decrease of necrosis and a great elevation of apoptosis. These experimental results support future clinical studies on application of aspirin in the treatment of lymphatic metastases, since the increase of apoptosis is one of the pillars of cancer therapy.
Key words: Aspirin. Lymph Nodes. Neoplasm Metastasis. Apoptosis. Therapeutics. Rabbits.
RESUMO
OBJETIVO: Avaliar os efeitos do ácido acetilsalicílico a 10% e 20% em linfonodos mesentéricos de coelhos para posterior embasamento e uso em metástases linfonodais.
MÉTODOS: Um total de 20 linfonodos de 20 coelhos (divididos aleatoriamente em quatro grupos) foi avaliado. As soluções de aspirina a 10% (grupos A e C) e 20% (grupos B e D) foram injetadas em linfonodos mesentéricos de coelhos sadios e seus efeitos macroscópicos e histológicos foram avaliados em 24 horas (grupos A e B) e em sete dias (grupos C e D).
RESULTADOS: Nos grupos avaliados em 24 horas (A e B) foi verificada intensa necrose e hemorragia, aumento importante de apoptose em todo o linfonodo, com alargamento dos seios medulares e aumento dos centros germinativos. Nos grupos avaliados em sete dias (C e D) também houve aumento da apoptose, com maior elevação de histiócitos e diminuição importante da necrose; a hemorragia foi ausente e aumento de células gigantes foi visualizado, conferindo processo inflamatório crônico do tipo corpo estranho. Não houve diferença entre as concentrações utilizadas (10 e 20%) em nenhuma das comparações.
CONCLUSÕES: A injeção de aspirina em linfonodos causou necrose e um aumento de apoptose após 24 horas e após sete dias de tratamento, houve regeneração dos gânglios linfáticos, com diminuição intensa de necrose e grande aumento de apoptose. Uma vez que o aumento de apoptose é um dos pilares dos tratamentos antineoplásicos, estes resultados experimentais embasam eventual aplicação clínica da aspirina no tratamento de metástases linfonodais.
Descritores: Aspirina. Linfonodos. Metástase Neoplásica. Apoptose. Terapêutica. Coelhos.
Introduction
Cancer is the leading cause of death in economically developed countries and the second leading cause of death in developing countries1. Once a large number of patients have distant metastases on lymph nodes adjacent to the tumor at initial diagnosis and the lymph node spread is a major determinant for the staging and the prognosis of most human malignancies2, their treatment is needed.
Resection surgery is considered the main type of treatment for cure of lymphatic metastases but in some situations it presents limiting factors such as number and location of lymph nodes involved and especially the adherence to adjacent organs and structures3,4.
Considering such difficulties and therapeutic characteristics of lymphatic metastases3,4, new treatment options need to be developed5. In order to have applicability it's expected that these new treatment options have effectiveness, low cost, low frequency of side effects and they should be easily executed.
In our research group, we had the opportunity to study and scientifically prove cytolytic effects of aspirin (acetylsalicylic acid) and its derivatives, either in vitro or in vivo on several experimental models6-10. Following this line of research, the purpose of this experimental study was to evaluate the effects of aspirin on normal lymph nodes of rabbits as basis for its later use on lymph nodes affected by tumor.
Methods
The experimental protocol was approved by the Ethics Committee on Animal Experiments of the Institution. The rabbits were kept according to the guidelines of the Guide for the Care and Use of Laboratory Animals (Institute for Laboratory Animal Research, 1996) and according to the ethical principles of the Brazilian College on Animal Experimentation (COBEA).
Animal housing, anesthesia, surgical procedures and test solution application
A total of 20 male, rabbits, 14-18 weeks old and weighting 3400-4000g, were studied. The animals were kept in light-dark cycles (12/12h) with free access to food and water; they were deprived of food for six hours prior to the experiments but received water ad libitum. All animals were anesthetized by intravenous injection of 10% ketamine hydrochloride (100mg/kg) and 2% xylazine hydrochloride (10mg/kg) and subjected to median laparotomy via supraumbilical incision for direct visualization of the mesenteric lymph nodes.
Subsequently, all animals were randomly allocated via a computer generated process into four groups of five rabbits each and 0,5ml of the test solutions were injected on mesenteric lymph nodes: groups A and C received intranodal injection of 10% acetylsalicylic acid and groups B and D received intranodal injection of 20% acetylsalicylic acid. In order to obtain the desired concentrations, 1g or 2g of acetylsalicylic acid (Pharma Nostra, Anapolis-GO, Brazil) were dissolved in 10ml of 10% sodium bicarbonate, forming bicarbonate acetylsalicylic acid. The solutions were prepared just before use. Finally, the incision was closed, in layers, with 4-0 nonabsorbent nylon sutures.
Early and late evaluations, and clinical evolution
The early effects (groups A and B) of solutions on mesenteric lymph nodes were evaluated by sacrificing rabbits 24h after the end of treatment. The late effects (groups C and D) of solutions on mesenteric lymph nodes were evaluated by sacrificing rabbits seven days after the end of treatment. All rabbits were killed by a lethal intravenous anesthetic dose.
During the study period (variable according to the group), were assessed the clinical outcomes of all animals through objective parameters, including recovery from surgery, food, and activity, being then classified into good, fair, or poor progress.
Gross analysis, collection of specimen and histopathogical examination
After 24 hours (groups A and B) or seven days (Groups C and D) of treatment, all animals were submitted to a second laparotomy in order to dissect the lymph nodes (1 per animal) for histopathogical study. During the laparotomies were carried out gross analysis of all abdominal cavities.
All lymph nodes collected were kept in buffered formol for two days; it was then prepared for mounting on histological slides and colored to hematoxylin eosin. These were analyzed by an experienced pathologist who was blind to the study, in a bright field optic microscope (qualitative analysis). The histological findings were graduated, by pathologist, into cross from the minimum of one cross (+1) to the maximum of four crosses (+4).
Results
Clinical evolution and gross examination of abdominal cavity
Animals present good clinical evolution in all groups without deaths. There was no significant change in weight of the animals. There were no abnormalities in all abdominal cavities.
Histopathogical evaluation
Table 1 shows the descriptions of the histopathological findings for each group.
Group A (10%)
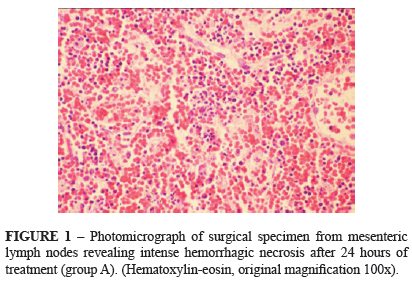
Necrosis was intense (+3) as hemorrhage (+2) spread in the lymph nodes (Figure 1). Around the necrosed areas were identified histiocytes and eosinophils (+1), and a significant increase in apoptosis (Figure 2). There was an enlargement of medullary sinuses.
Group B (20%)
Necrosis was identified (+2) in medullar and cortical regions. Hemorrhage varied in intensity from +1 to +2/+4 and histiocytes (+2). The number of germinal centers varied from 18 to 28. As in group A, apoptosis was increased around the necrosed areas (Figure 3).
Group C (10%)
Necrosis varied from absent to +2 in the medullary sinuses. Hemorrhage was absent. Around necrosed areas there was an increase in histiocytes (+3). Eosinophils were observed but with just +1 in intensity. There was an enlargement in medullary sinuses (+2) (Figure 4). In 3 cases there were giant cells (+3). Apoptosis increased in medullary sinuses and in germinal centers (Figure 5).
Group D (20%)
There was a decrease in necrosis (Figure 6), varying since absent until +1 and a decrease in eosinophils (absent to +1). Histiocytes were evident (+3 to +4) (Figure 7). There were 6 germinal centers. Giant cells, as in group C, were observed (+3) (Figure 8). Apoptosis was also increased, especially in medullary sinuses.
Discussion
Starting with the assumption that lymphatic metastases are determinants of a poor prognosis and their treatment often is not possible2-4, the search for new therapeutic approaches becomes necessary5. Thus, the main focus of this study was to evaluate the effects of aspirin on mesenteric lymph nodes of rabbits, as a basis for therapeutic method for the treatment of on lymphatic metastases.
In this experimental research, there were no differences in the histological results of groups A and B, they were gathered to be discussed. In these groups was evident the necrotizing effects of the aspirin solution and in all lymph nodes there were necrosis. Ohnishi et al.11 found the same lesions using acetic acid on liver and Saad-Hossne et al.7 also found coagulative necrosis on liver of rabbits after the injection of acetic acid and aspirin. In a common pattern of cell death caused by oxygen loss, the necrotic cells show an increase of eosinophils. In the group B the amplification of eosinophils was very important, as a characteristical necrotic process and acute inflammatory response. This occurs by the increased attachment of eosine to the denaturated citoplasmatic proteins and, also, to basophilic loss usually provided by ribonucleid acid in cytoplasm12,13. It was verified the enlargement of medullary sinuses, demonstrating progressive elevation of macrophage activity and inflammatory cell response.
In these groups (A and B), in which the acute response to antigenic stimulus was evaluated, there was an increase of germinal centers, more intense in group B. This is characteristically a follicular hyperplasia, with the germinal centers forming the place of transformation and multiplication of lymphocytes that reacts to antigenic stimulus. The reactive germinal centers are formed by a mixture of cells, like the centroblasts, centrocytes, dendritic cells, small T lymphocytes CD4+ / CD57+ and macrophages, as B lymphocytes in maturation13-15.
The event considered as of greatest value in this experimental study was the elevation of apoptosis. In groups A and B the elevation of apoptosis occurred in all parts of the lymph nodes. The induction to apoptosis may be caused by DNA damage, whether by direct lesion or by free radicals production, by mitochondrial lesions and also by cellular influx of calcium (ATP dependent) with caspases activation16,17. Apoptosis may occur in many situations and has the function to eliminate harmful cells and useless cells16,17. It may also be considered a pathological situation when cells suffer injury beyond the repair capacity, mainly when DNA or cells' proteins are affected, time when injured cells are eliminated16,17.
In groups C and D the effects of aspirin solution were evaluated in 7 days, and were observed the alterations compatible to later inflammatory processes, without difference between both. There was an important decrease of necrosis, with regenerative process, but without fibrosis. As a consequence of apoptosis elevation, the number of histiocytes was also high. The sinusal histiocytes are macrophages found in the spleen and lymph nodes. The macrophages are responsible for phagocytosis18. Still compatible to later inflammatory processes, in these groups hemorrhage and eosinophils were absent. Finally, there was an increase in giant cells, with aggregated epithelioid macrophages surrounded by linphocytes, featuring a cronic foreign body inflammation.
The knowledgement of apoptotic pathways has a great applicability in medicine and pharmacology, whether in developing new drugs to induces apoptosis, as a therapeutic strategy, or understanding the mechanisms of radiotherapy or chemotherapy resistance19-21. Researches related to apoptosis are really important in practical application of discoveries in diseases related to homeostatic balance loss, such as cancer and neurovegetative diseases16.
In a recent review22, the importance of apoptosis in cancer cells was discussed; this can be induced by hypoxia, shortage of nutrients or growth factors, and radiotherapy, or chemotherapy. As a means of protecting the host, physiologic apoptosis is rapidly and specifically recognized by phagocytic cells. Apoptotic bodies are silently removed by phagocytosis; this event is associated with the release of potent antiinflammatory mediators like transforming growth factor-b (TGF-b), prostaglandin E2, or platelet-activating factor to avoid local inflammatory reactions. Apoptosis phenomenon eliminates cells that have accumulated DNA damage without causing inflammation and prevents tumor formation first and tumor growth later.
Once the main result of this study was that in all lymph nodes evaluated were found aspirin-induced apoptosis, and activation of apoptosis is a major goal of cancer therapy19-21, the authors can assume that aspirin could be beneficial for the treatment of lymphatic metastases.
The regular intake of non-steroidal antiinflammatory drugs, inhibitors of cyclooxygenase (COX), such as aspirin, can reduce the risk of development of some cancers23-26 and also induce apoptosis in various cancer cell lines27. However, the molecular mechanisms through which these drugs induce apoptosis are not well understood27; there are several mechanisms proposed, including inhibition of proteasome function, activation of ceramide pathway, activation of caspases, up-regulation of several proapoptotic proteins, down-regulation of NF-κB activity, and generation of endoplasmic reticulum and oxidative stress27,28.
The overexpression of COX-2 is commonly found in many cancers29,30. Several mechanisms by which COX-2 contributes to progression of cancer have been reported, including stimulation of proliferation and inhibition of apoptosis of cancer cells, stimulation of cancer cell invasion and angiogenesis, and suppression of immune responses29,30. Targeting COX-2 provides an important opportunity in cancer research; indeed, the expression of COX-2 has been reported as an indicator of poor prognosis in a wide variety of tumors29,30. The COX-2 overexpression is causally linked to the suppression of the host's immune system, with an enhanced resistance to apoptosis and stimulation of cancer cell growth and invasion29,30. So we may assume that the aspirin use in lymph nodes with tumor cells could stimulate apoptosis and destroy these cells (as observed in this experimental research) and also inhibit the activity of tumor by inhibition of COX 1 and 2. This ability to destroy tumor cells has already been investigated by our group6-10 in other reports using experimental tumors (Ehrlich and VX-2) and by others27,31-38.
Conclusions
Aspirin solution cause necrosis and an increase in apoptosis after 24 hours and that after seven days of treatment there was regeneration of the lymph nodes, with intense decrease of necrosis and a great elevation of apoptosis without difference in the concentration of solution (10 and 20%). Further clinical studies are warranted to evaluate the efficacy of this strategy on lymphatic metastases.
Received: June 28, 2012
Review: August 29, 2012
Accepted: September 27, 2012
Conflict of interest: none
Financial source: none
- 1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69-90.
- 2. Leong SP, Cady B, Jablons DM, Garcia-Aguilar J, Reintgen D, Jakub J, Pendas S, Duhaime L, Cassell R, Gardner M, Giuliano R, Archie V, Calvin D, Mensha L, Shivers S, Cox C, Werner JA, Kitagawa Y, Kitajima M. Clinical patterns of metastasis. Cancer Metastasis Rev. 2006;25(2):221-32.
- 3. Principe A, Del Gaudio M, Ercolani G, Golfieri R, Cucchetti A, Pinna AD. Radical surgery of gallblader carcinoma: possibilities of survival. Hepatogastroenterology. 2006;53(71):6604.
- 4. Minagawa M, Yamamoto J, Miwa S, Sakamoto Y, Kokudo N, Kosuge T, Miyagawa S, Makuu Chi M. Selection criteria for simultaneous resection in patients with synchronous liver metastasis. Arch Surg. 2006;141(10):1006-12.
- 5. Zwaans BM, Bielenberg DR. Potential therapeutic strategies for lymphatic metastasis. Microvasc Res. 2007;74(2-3):145-58.
- 6. Saad-Hossne R, Prado RG, Hossne WS. Effects of acetylsalicylic acid and acetic acid solutions in VX2 carcinoma cells: in vitro analysis. Acta Cir Bras. 2006;21(3):151-4.
- 7. Saad-Hossne R, Prado RG, Hossne WS. Efeito da solução de ácido acetilsalicílico e de ácido acético em fígado de coelhos. Acta Cir Bras. 2004;19(6):677-86.
- 8. Saad-Hossne R, Hossne WS, Prado RG. Efeito da solução aquosa de fenol, ácido acético e glicerina sobre o tumor ascítico de Erlich. Estudo experimental in vitro. Acta Cir Bras. 2004;19(1):54-8.
- 9. Saad-Hossne R, Hossne WS, Prado RG. Ascite neoplásica. Efeito da solução aquosa de fenol, ácido acético e glicerina sobre o tumor ascítico de Erlich. Acta Cir Bras. 2003;18(3):534-6.
- 10. Siqueira JM, Barreto AB, Saad-Hossne R. Treatment of endometriosis with local acetylsalicylic acid injection: experimental study in rabbits. J Minim Invasive Gynecol. 2011;18(6):800-6.
- 11. Ohnishi K, Ohyama N, Ito S, Fujiwara K. Ultrasound guided intratumor injection of acetic acid for the treatment of small hepatocellular carcinoma. Radiology. 1994;193(3):743-52.
- 12. Kita H. Eosinophils: multifaceted biological properties and roles in health and disease. Immunol Rev. 2011;242(1):161-77.
- 13. Favaloro B, Allocati N, Graziano V, Di Ilio C, De Laurenzi V. Role of apoptosis in disease. Aging (Albany NY). 2012;4(5):330-49.
- 14. Wolniak KL, Shinall SM, Waldschmidt TJ. The germinal center response. Crit Rev Immunol. 2004;24(1):39-65.
- 15. McHeyzer-Williams LJ, Driver DJ, McHeyzer-Williams MG. Germinal center reaction. Curr Opin Hematol. 2001;8(1):52-9.
- 16. Ulukaya E, Acilan C, Yilmaz Y. Apoptosis: why and how does it occur in biology? Cell Biochem Funct. 2011;29(6):468-80.
- 17. Kataoka S, Tsuruo T. Physician education: apoptosis. Oncologist. 1996;1(6):399-401.
- 18. Erwig LP, Henson PM. Clearance of apoptotic cells by phagocytes. Cell Death Differ. 2008;15(2):243-50.
- 19. Debatin KM. Apoptosis pathways in cancer and cancer therapy. Cancer Immunol Immunother. 2004;53(3):153-9.
- 20. Fischer U, Schulze-Osthoff K. Apoptosis-based therapies and drug targets. Cell Death Differ. 2005;12(Suppl 1):942-61.
- 21. Ghobrial IM, Witzig TE, Adjei AA. Targeting apoptosis pathways in cancer therapy. CA Cancer J Clin. 2005;55(3):178-94.
- 22. Demaria S, Pikarsky E, Karin M, Coussens LM, Chen YC, El-Omar EM, Trinchieri G, Dubinett SM, Mao JT, Szabo E, Krieg A, Weiner GJ, Fox BA, Coukos G, Wang E, Abraham RT, Carbone M, Lotze MT. Cancer and inflammation: promise for biologic therapy. J Immunother. 2010;33(4):335-51.
- 23. Rothwell PM, Fowkes FG, Belch JF, Ogawa H, Warlow CP, Meade TW. Effect of daily aspirin on long-term risk of death due to cancer: analysis of individual patient data from randomised trials. Lancet. 2011;377(9759):31-41.
- 24. Cuzick J, Otto F, Baron JA, Brown PH, Burn J, Greenwald P, Jankowski J, La Vecchia C, Meyskens F, Senn HJ, Thun M. Aspirin and non-steroidal anti-inflammatory drugs for cancer prevention: an international consensus statement. Lancet Oncol. 2009;10(5):501-7.
- 25. Rothwell PM, Price JF, Fowkes FG, Zanchetti A, Roncaglioni MC, Tognoni G, Lee R, Belch JF, Wilson M, Mehta Z, Meade TW. Short-term effects of daily aspirin on cancer incidence, mortality, and non-vascular death: analysis of the time course of risks and benefits in 51 randomised controlled trials. Lancet. 2012;379(9826):1602-12.
- 26. Harris RE. Cyclooxygenase-2 (cox-2) blockade in the chemoprevention of cancers of the colon, breast, prostate, and lung. Inflammopharmacology. 2009;17(2):55-67.
- 27. Dikshit P, Chatterjee M, Goswami A, Mishra A, Jana NR. Aspirin induces apoptosis through the inhibition of proteasome function. J Biol Chem. 2006;281(39):29228-35.
- 28. Jana NR. NSAIDs and apoptosis. Cell Mol Life Sci. 2008;65(9):1295-301.
- 29. Iwata C, Kano MR, Komuro A, Oka M, Kiyono K, Johansson E, Morishita Y, Yashiro M, Hirakawa K, Kaminishi M, Miyazono K. Inhibition of cyclooxygenase-2 suppresses lymphonode metastasis via reduction of lymphangiogenesis. Cancer Res. 2007;67(21):10181-9.
- 30. de Groot DJ, de Vries EG, Groen HJ, de Jong S. Non-steroidal anti-inflammatory drugs to potentiate chemotherapy effects: from lab to clinic. Crit Rev Oncol Hematol. 2007;61(1):52-69.
- 31. Iglesias-Serret D, Piqué M, Barragán M, Cosialls AM, Santidrián AF, González-Gironès DM, Coll-Mulet L, de Frias M, Pons G, Gil J. Aspirin induces apoptosis in human leukemia cells independently of NF-kappaB and MAPKs through alteration of the Mcl-1/Noxa balance. Apoptosis. 2010;15(2):219-29.
- 32. Tian Y, Ye Y, Gao W, Chen H, Song T, Wang D, Mao X, Ren C. Aspirin promotes apoptosis in a murine model of colorectal cancer by mechanisms involving downregulation of IL-6-STAT3 signaling pathway. Int J Colorectal Dis. 2011;26(1):13-22.
- 33. Raza H, John A, Benedict S. Acetylsalicylic acid-induced oxidative stress, cell cycle arrest, apoptosis and mitochondrial dysfunction in human hepatoma HepG2 cells. Eur J Pharmacol. 2011;668(1-2):15-24.
- 34. Kumar A, Vishvakarma NK, Tyagi A, Bharti AC, Singh SM. Anti-neoplastic action of aspirin against a T cell lymphoma involves alteration in tumor microenvironment and regulation of tumor cell survival. Biosci Rep. 2012;32(1):91-104.
- 35. Hossain MA, Kim DH, Jang JY, Kang YJ, Yoon JH, Moon JO, Chung HY, Kim GY, Choi YH, Copple BL, Kim ND. Aspirin enhances doxorubicin-induced apoptosis and reduces tumor growth in human hepatocellular carcinoma cells in vitro and in vivo. Int J Oncol. 2012;40(5):1636-42.
- 36. Im SR, Jang YJ. Aspirin enhances TRAIL-induced apoptosis via regulation of ERK1/2 activation in human cervical cancer cells. Biochem Biophys Res Commun. 2012;424(1):65-70.
- 37. Hossain MA, Kim DH, Jang JY, Kang YJ, Yoon JH, Moon JO, Chung HY, Kim GY, Choi YH, Copple BL, Kim ND. Aspirin induces apoptosis in vitro and inhibits tumor growth of human hepatocellular carcinoma cells in a nude mouse xenograft model. Int J Oncol. 2012;40(4):1298-304.
- 38. De Luna-Bertos E, Ramos-Torrecillas J, García-Martínez O, Díaz-Rodríguez L, Ruiz C. Effect of aspirin on cell growth of human MG-63 osteosarcoma line. Scientific World Journal. 2012;2012:834246
Publication Dates
-
Publication in this collection
29 Oct 2012 -
Date of issue
Nov 2012
History
-
Received
28 June 2012 -
Accepted
27 Sept 2012 -
Reviewed
29 Aug 2012