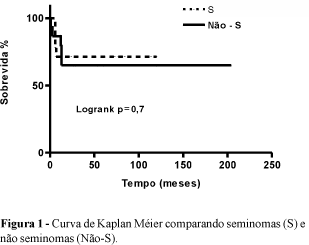
OBJECTIVE: The aim of the study is to analyze the characteristics and the evolution of patients with testicular germ cell tumors. METHODS: We analyzed 29 patients: 14 seminomas and 15 non-seminomas. All of them underwent orquiectomy. Patients with seminomas stage II and III received adjuvant treatment with raditherapy, and those with non-seminomas stage II and III received neoadjuvant chemotherapy followed by lymphadenectomy. Mean followup for seminomas was 56 months and for non-seminomas it was 40 months. RESULTS: The most common complain was an increase in testis volume (57%) and pain (30%). The mean age in seminomas was 41.2 years and in non-seminomas it was 29.2 years. Previous criptorquidy was refered by 28.5% of patients with seminomas and 15.5% with non-seminomas. The respective proportions of stages I, II and III were 79%, 14% and 7% in seminomas, and 40%, 27% and 33% in non-seminomas. Patients with seminomas did not show serum rise of alfa-fetoprotein or b-HCG while those with non-seminomas such tumor markes were elevated respectively in 46.6% and 33.3% of the sample. Disease specific death occurred in 1 patient with seminoma and in 3 with non-seminoma tumor, but survival curves were similar for both groups. CONCLUSION: In spite of the earlier treatment of criptorquidy such an antecedent stand as an important risk factor for the development of testicular germ cell tumor. Even though non-seminomas presents with higher stages the survival curves are similar for both groups of tumors. Analyze 29 patients that were submitted to orquiectomy (14 seminomas e 15 non seminomas), concerning their age, signs and symptoms, risk factors, clinical staging, tumor markers follow up and survival rates. RESULTS: There was no statistically difference in survival rates of patients with seminomas and non-seminomas tumors.
testicular tumor; seminoma; non-seminoma; germ cell tumor