Abstract
PURPOSE: To investigate the protective effects of ischemic pre and postconditioning, as well as the association of both methods, in skeletal muscle injury produced by ischemia and reperfusion in rats. METHODS: An experimental study was designed using 40 Wistar rats divided in four groups (n=10): Control - rats submitted to ischemia for 240 minutes (min) and reperfusion for 60 min; Ischemic preconditioning (Pre) - animals submitted to three cycles of clamping and releasing the aorta for five min before being submitted to the ischemia/reperfusion procedure; Ischemic postconditioning (Post) - rats submitted to three cycles of clamping and releasing the aorta for one min after the 240-minute ischemic phase; Ischemic pre and postconditioning (Pre-post) - animals submitted to the same procedures of Pre and Post groups. Skeletal muscle injury was evaluated by measuring serum levels of aspartate aminotransferase (AST), lactate dehydrogenase (LDH) and creatine phosphokinase (CPK); and muscular levels of malondialdehyde (MDA) and glycogen. RESULTS: AST levels were significantly higher in Pre and Pre-post groups (P<.01). There were no differences in LDH and CPK levels. Muscular MDA levels were similar. Glycogen levels were significantly higher in Pre and Pre-post groups (P<.01). CONCLUSIONS: Both preconditioning and its association with postconditioning had a protective effect by avoiding glycogen depletion in skeletal muscle in rats submitted to ischemia and reperfusion. Association of pre and postconditioning did not show advantage compared to preconditioning alone. Postconditioning alone did not show protective effect.
Ischemic Preconditioning; Ischemic Postconditioning. Ischemia; Reperfusion; Muscle, Skeletal; Models, Animal; Glycogen; Rats
7 ORIGINAL ARTICLE
ISCHEMIA-REPERFUSION
Ischemic pre and postconditioning in skeletal muscle injury produced by ischemia and reperfusion in rats1 1 Research performed at Division of Vascular and Endovascular Surgery, Department of Surgery and Anatomy, Faculty of Medicine of Ribeirao Preto, University of Sao Paulo (FMRP/USP), Brazil.
José Alves LintzI; Marcelo Bellini DalioII; Edwaldo Edner JovilianoIII; Carlos Eli PiccinatoIV
IMaster, Vascular Surgeon, Division of Vascular and Endovascular Surgery, Department of Surgery and Anatomy, FMRP/USP, Ribeirao Preto-SP, Brazil. Conception and design of the study; acquisition, analysis and interpretation of data; manuscript writing
IIPhD, Assistant Physician, Division of Vascular and Endovascular Surgery, Department of Surgery and Anatomy, FMRP/USP, Ribeirao Preto-SP, Brazil. Intellectual and scientific content of the study, manuscript writing, critical revision
IIIPhD, Associate Professor, Division of Vascular and Endovascular Surgery, Department of Surgery and Anatomy, FMRP/USP, Ribeirao Preto-SP, Brazil. Manuscript writing, critical revision
IVPhD, Chairman and Head, Division of Vascular and Endovascular Surgery, Department of Surgery and Anatomy, FMRP/USP, Ribeirao Preto-SP, Brazil. Conception and design of the study, critical revision
Correspondence Correspondence: Carlos Eli Piccinato Faculdade de Medicina de Ribeirão Preto, Campus Universitário 14049-900 Ribeirão Preto SP Brasil Tel/Fax: (55 16)3602-2593 cepiccin@fmrp.usp.br
ABSTRACT
PURPOSE: To investigate the protective effects of ischemic pre and postconditioning, as well as the association of both methods, in skeletal muscle injury produced by ischemia and reperfusion in rats.
METHODS: An experimental study was designed using 40 Wistar rats divided in four groups (n=10): Control rats submitted to ischemia for 240 minutes (min) and reperfusion for 60 min; Ischemic preconditioning (Pre) animals submitted to three cycles of clamping and releasing the aorta for five min before being submitted to the ischemia/reperfusion procedure; Ischemic postconditioning (Post) rats submitted to three cycles of clamping and releasing the aorta for one min after the 240-minute ischemic phase; Ischemic pre and postconditioning (Pre-post) animals submitted to the same procedures of Pre and Post groups. Skeletal muscle injury was evaluated by measuring serum levels of aspartate aminotransferase (AST), lactate dehydrogenase (LDH) and creatine phosphokinase (CPK); and muscular levels of malondialdehyde (MDA) and glycogen.
RESULTS: AST levels were significantly higher in Pre and Pre-post groups (P<.01). There were no differences in LDH and CPK levels. Muscular MDA levels were similar. Glycogen levels were significantly higher in Pre and Pre-post groups (P<.01).
CONCLUSIONS: Both preconditioning and its association with postconditioning had a protective effect by avoiding glycogen depletion in skeletal muscle in rats submitted to ischemia and reperfusion. Association of pre and postconditioning did not show advantage compared to preconditioning alone. Postconditioning alone did not show protective effect.
Key words:Ischemic Preconditioning. Ischemic Postconditioning. Ischemia. Reperfusion. Muscle, Skeletal. Models, Animal. Glycogen. Rats.
Introduction
Tissular injury after ischemia and reperfusion has been intensively studied in the last years1-3. Ischemic preconditioning, which consists on producing short periods of ischemia followed by reperfusion before a sustained ischemic period, exerts a protective effect against ischemia and reperfusion injury in many animal species4. In humans, this effect is described in the heart5, brain6, kidneys7, liver8 and skeletal muscle9. Ischemic postconditioning, which consists on producing short periods of ischemia and reperfusion just before sustained reperfusion, also produced protective effects in rats10, rabbits11, pigs12 and humans13.
The optimal protocol for applying ischemic pre and postconditioning aiming maximal muscular tissue protection is yet undetermined. Motivated by this issue, we employed an experimental model of partial ischemia in rats that simulate the clinical condition found in acute arterial occlusion in humans to test ischemic pre and postconditioning.
The purpose of this study was to analyze the protective effects of ischemic pre and postconditioning, as well as the association of both methods, in skeletal muscle injury produced by ischemia and reperfusion in rats.
Methods
All protocols were approved in accordance with the Animal Experimentation Ethics Committee at University of Sao Paulo Ribeirao Preto, Brazil. Forty male Wistar rats (University of Sao Paulo, Ribeirao Preto, Brazil), weighing 250 to 450 g and clinically healthy were used for this study. Animals were maintained in a room controlled for temperature and light and were provided food and water ad libitum. Animal care complied with the Principles of Laboratory Animal Care (formulated by the National Society for Medical Research) and the Council for International Organization of Medical Sciences (CIOMS) ethical code for animal experimentation14.
Ischemia and reperfusion model
Anesthesia was induced with intraperitonial sodium thiopental (50mg/Kg body weight). Ischemia and reperfusion injury was induced by clamping the infra-renal aorta, following the surgical procedure described in detail elsewhere15. Abdominal aorta was exposed through a midline incision and occluded with an atraumatic vascular clamp just below the renal arteries, after full heparinization. After 240 minutes (min) of aortic occlusion (ischemic phase), the clamp was removed for more 60 min (reperfusion phase). The wound was closed with interrupted cotton 3-0 between the phases. At the end of the experiment, blood samples were collected from the inferior vena cava and a biopsy from the right hindlimb gastrocnemius muscle was performed.
Experimental design
Animals were randomly divided in four groups (n=10): Control rats submitted to ischemia for 240 min and reperfusion for 60 min; Ischemic preconditioning (Pre) animals submitted to three cycles of clamping the aorta for five min and releasing for more five min before being submitted to the ischemia/reperfusion procedure; Ischemic postconditioning (Post) rats submitted to three cycles of clamping the aorta for one min and releasing for more one minute after the 240-minute ischemic phase; Ischemic pre and postconditioning (Pre-post) animals submitted to three cycles of clamping the aorta for five min and releasing for more five minutes before the 240-minute ischemic phase and also submitted to three cycles of clamping the aorta for one minute and releasing for one more min after the 240-minute ischemic phase. Figure 1 represents the experimental protocol.
Skeletal muscle injury was evaluated by measuring serum levels of released cytoplasmic enzymes (aspartate aminotransferase AST, lactate dehydrogenase - LDH and total creatine phosphokinase -CPK); and tissular levels of a cell membrane degradation product (malondialdehyde - MDA) and muscular energetic storage (muscular glycogen).
Measurement of serum and muscular markers
Blood samples were used for determination of serum levels of AST, LDH and CPK. All determinations were performed with appropriate spectrophotometric essays. Results were expressed in Units/L.
Muscular samples were used for determination of muscular MDA and glycogen levels. For MDA determinations, muscular samples were homogenized, centrifuged (3000×g, 10 min, 4°C) and the supernatant was submitted to a spectrophotometric essay (Lipid Peroxidation Assay Kit Calbiochem, San Diego, CA). Results were expressed in µM. For glycogen determinations, muscular samples were treated with KOH 30%. Levels of glycogen were measured by spectrophotometry using the anthrone method. Results were expressed in mg%.
Statistical analysis
Data were expressed as the mean ± the standard deviation. Statistical comparisons among groups were done with KruskalWallis one-way analysis of variance with Bonferroni post test. A P value of less than .05 was considered statistically significant.
Results
Serum AST, LDH and CPK levels
AST levels were significantly higher in Pre and Pre-post groups, compared with control group (P<.01). Post group AST levels were similar to control group (Figure 2).
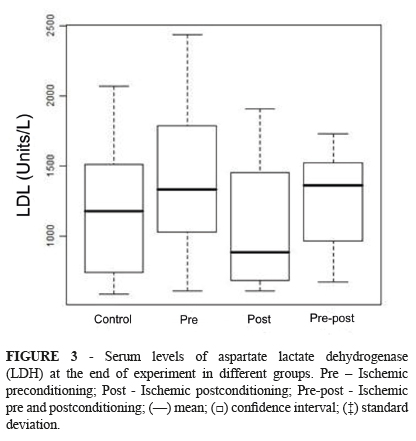
There were no differences in LDH (Figure 3) and CPK (Figure 4) levels among the four groups.
Muscular MDA and glycogen levels
MDA levels were not different in the four groups (Figure 5).
Glycogen levels were significantly higher in Pre and Pre-post groups, compared with control group (P<.01). Post group presented increased glycogen levels, not significantly different form control groups (Figure 6).
Discussion
The protective effect of ischemic preconditioning in skeletal muscle was first demonstrated in a swine model, in which three cycles of ten minutes of ischemia and reperfusion was followed by four hours of ischemia16. Several experimental protocols were later developed aiming to maximize the protective effect. The most studied preconditioning protocols used sequential clamping times of five to ten minutes, and one to five cycles of ischemia and reperfusion. Pang et al.9, using preconditioning of three cycles of ischemia and reperfusion with ten minutes of clamping, followed by four hours of ischemia and 90 minutes of reperfusion, obtained preserved muscular levels of phosphocreatine and adenosine triphosphate and low serum lactate levels. Mattei et al.17 investigated the efficiency of different preconditioning protocols in skeletal muscle in rats, comparing three cycles of 2.5, five and ten minutes. The best protective effects were found using three cycles of five minutes. Efficacy of preconditioning seems to be less dependent on clamping duration than on the number of cycles of ischemia and reperfusion.
Zhao et al.18 presented the concept of ischemic postconditioning18, and McAllister et al.12 first tested it in skeletal muscle. The supposed molecular mechanism of tissue protection of ischemic postconditioning involved the inhibition of opening of mitochondrial permeability transition pores (mPTP). Szijártó et al.19 tested ischemic postconditioning in a model of partial ischemia using infra-renal aortic clamping. Rats were submitted to 180 min of ischemia followed by four hours of reperfusion. The postconditioning group was submitted to six cycles of ten seconds of reperfusion and ischemia, just before the beginning of the sustained reperfusion. Postconditioning group had a decreased systemic inflammatory response (TNF-α) and a marked reduction in reperfusion-related organ dysfunctions (lungs and kidneys).
The experimental model employed in the present study differs from most described in the literature. The latter employed muscular flaps, in which a single pedicle was clamped, generating total ischemia. In our model partial ischemia was generated in skeletal muscle, since the collateral pathways were not occluded. Partial ischemia model simulates the clinical condition commonly found in acute arterial occlusion. These conditions are also encountered in arterial surgery, when clamping is necessary.
The findings of the present study demonstrated that in Pre and Pre-post groups there was an increase in serum AST, but no alterations in CPK levels during reperfusion phase. Castro e Silva Jr. et al.8, studying postconditioning and hepatic ischemia observed similar results. These observations could be explained by the fact that serum enzyme levels correlate with timing of an ischemic process. During an ischemic injury, CPK usually increases before AST, but also decreases first. Increased CPK levels associated with decreased AST levels indicate recent injury. Persistent increased levels of both enzymes indicate continued injury and decreased CPK levels associated with increased AST levels indicate recovery form an ischemic insult20. As preconditioning and also its association with postconditioning promoted a significant increase in AST levels, which was not shared by CPK and LDH, one can say that when blood was collected, skeletal muscle was under recovery from the ischemic injury.
Previous experiments involving hepatic ischemia demonstrated that postconditioning caused a decrease in lipid peroxidation10. Lipid peroxidation produces a number of metabolites, whose combination with proteins and DNA results in toxic substances, such as MDA. Most damage caused by lipid peroxidation occurs in cell membrane10. Kin et al.21 demonstrated that the protective effect of postconditioning was mediated by endogenous adenosine, through activation of receptors A2A and A3AR. This effect was responsible for a decreased lipidic peroxidation. Sudden reperfusion removes from tissues endogenous protective substances that were generated during ischemia, such as adenosine. With gradual reperfusion, these substances are kept in the tissue, exerting a protective effect10. In the present experiment, as MDA levels were similar in all groups, the protective effect of preconditioning, postconditioning and their association in skeletal muscle was not demonstrated.
In the present study, muscular glycogen levels in Pre and Pre-post groups were significantly greater than in the control group. Thus, preconditioning, as well as its association with postconditioning prevented the depletion of glycogen storage caused by ischemic process. These results are similar to those of Weiss et al.22 and Pescador et al.23. The exact molecular mechanism to explain this phenomenon is yet to be understood.
During hypoxia, anaerobic glycolysis occurs and tissue demands of glucose are increased. In this scenario, maintenance of glycogen storage and also the occurrence of glycogen synthesis seem to be paradoxal. In our study, ischemic preconditioning and its association with postconditioning caused this phenomenon. One possible explanation is the role of hypoxia-inducible factors (HIF). These factors are dependent on genic expression and would stimulate glycogen synthase isoform 1 (GSY-1) during the short hypoxic periods. Glycogen synthesis would replenish cell glycogen storages, which would be utilized in posterior periods. Thus, GYS-1 induction by HIF would consist in a cellular adaptation to hypoxia, in which cells prepare themselves for future oxygen shortage. A better comprehension of this process could explain physiologic adaptations to hypoxia, and also help to develop therapeutic strategies against diseases which hypoxia plays a major role23.
In current surgical and clinical practice, the protective effect of ischemic preconditioning over skeletal muscle has the potential to be applied in several situations. Good examples are procedures that require increased clamping time. In general surgery, it may be used in complex trauma operations. In vascular surgery, preconditioning may benefit aortic and extremity reconstructive arterial procedures. In plastic surgery, muscular and myocutaneuos flaps24, and also techniques of replantation, free tissue transfer, and composite tissue allotransplantation25 may be favored.
Another practical application is that ischemic preconditioning, even in remote sites can protect distant tissues and organs. Remote ischemic preconditioning reduces myocardial injury after major cardiovascular surgery26. A trial in patients undergoing abdominal aortic aneurysm repair reported a significant reduction in perioperative myocardial infarctions27. However, large-scale trials testing the technique are required in patients undergoing major vascular surgery. Postconditioning has a potential advantage over preconditioning, since it can be performed in the end of the ischemic period. Thus, it could be applied in clinical situations where intervention is performed in the end of the ischemic period, such as trauma surgery.
Both ischemic pre and postconditioning are techniques with great potential of being applied in clinical and surgical practice. However, more studies are required to clarify diverse results published with different animal species and organs. Remote pre and postconditioning need to be more studied, as well as its relation with drugs that could produce the same protective effects.
Conclusions
Both preconditioning and association of pre and postconditioning had a protective effect by avoiding glycogen depletion in skeletal muscle in rats submitted to ischemia and reperfusion. Association of pre and postconditioning did not show advantage compared to preconditioning alone. Postconditioning alone did not show protective effect.
Received: February 14, 2013
Review: April 15, 2013
Accepted: May 13, 2013
Conflict of interest: none
Financial source: FAEPA-HC/FMRP-USP
- 1. Grisotto PC, dos Santos AC, Coutinho-Netto J, Cherri J, Piccinato CE. Indicators of oxidative injury and alterations of the cell membrane in the skeletal muscle of rats submitted to ischemia and reperfusion. J Surg Res. 2000;92(1):1-6.
- 2. Carden DL, Granger DN. Pathophysiology of ischaemia-reperfusion injury. J Pathol. 2000;190(3):255-66.
- 3. Gillani S, Cao J, Suzuki T, Hak DJ. The effect of ischemia reperfusion injury on skeletal muscle. Injury. 2012;43(6):670-5.
- 4. Pasupathy S H-VS. Surgical implications of ischemic preconditioning. Arch Surg. 2005;140(4):405-9.
- 5. Sanada S, Komuro I, Kitakaze M. Pathophysiology of myocardial reperfusion injury: preconditioning, postconditioning, and translational aspects of protective measures. Am J Physiol Heart Circ Physiol. 2011;301(5):H1723-41.
- 6. Schaller B, Graf R. Cerebral ischemic preconditioning. An experimental phenomenon or a clinical important entity of stroke prevention? J Neurol. 2002;249(11):1503-11.
- 7. Torras J, Herrero-Fresneda I, Lloberas N, Riera M, Ma Cruzado J, Ma Grinyo J. Promising effects of ischemic preconditioning in renal transplantation. Kidney Int. 2002;61(6):2218-27.
- 8. Castro e Silva Jr. O, Centurion S, Pacheco EG, Brisotti JL, Oliveira AF, Sasso KD. Basics aspects of the ischemia reperfusion injury and of the ischemic preconditioning. Acta Cir Bras. 2002;17:96-100.
- 9. Pang CY, Yang RZ, Zhong A, Xu N, Boyd B, Forrest CR. Acute ischaemic preconditioning protects against skeletal muscle infarction in the pig. Cardiovasc Res. 1995;29(6):782-8.
- 10. Teixeira AR, Molan NT, Kubrusly MS, Bellodi-Privato M, Coelho AM, Leite KR, Machado MA, Bacchella T, Machado MC. Postconditioning ameliorates lipid peroxidation in liver ischemia-reperfusion injury in rats. Acta Cir Bras. 2009;24(1):52-6.
- 11. Huang H, Zhang L, Wang Y, Yao J, Weng H, Wu H, Chen Z, Liu J. Effect of ischemic post-conditioning on spinal cord ischemic-reperfusion injury in rabbits. Can J Anaesth. 2007;54(1):42-8.
- 12. McAllister SE, Ashrafpour H, Cahoon N, Huang N, Moses MA, Neligan PC, Forrest CR, Lipa JE, Pang CY. Postconditioning for salvage of ischemic skeletal muscle from reperfusion injury: efficacy and mechanism. Am J Physiol Regul Integr Comp Physiol. 2008;295(2):R681-9.
- 13. Loukogeorgakis SP, Panagiotidou AT, Yellon DM, Deanfield JE, MacAllister RJ. Postconditioning protects against endothelial ischemia-reperfusion injury in the human forearm. Circulation. 2006;113(7):1015-9.
- 14. Howard-Jones N. A CIOMS ethical code for animal experimentation. WHO Chronicle. 1985;39(2):51-6.
- 15. Perry MO, Fantini G. Ischemia: profile of an enemy. Reperfusion injury of skeletal muscle. J Vasc Surg. 1987;6(3):231-4.
- 16. Mounsey RA, Pang CY, Forrest C. Preconditioning: a new technique for improved muscle flap survival. Otolaryngol Head Neck Surg. 1992;107(4):549-52.
- 17. Mattei A, Sutter PM, Marx A, Stierli P, Heberer M, Gurke L. Preconditioning with short cycles improves ischemic tolerance in rat fast- and slow-twitch skeletal muscle. Eur Surg Res. 2000;32(5):297-304.
- 18. Zhao ZQ, Corvera JS, Halkos ME, Kerendi F, Wang NP, Guyton RA, Vinten-Johansen J. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2003;285(2):H579-88.
- 19. Szijarto A, Gyurkovics E, Aranyi P, Onody P, Stangl R, Tatrai M, Lotz G, Mihaly Z, Hegedus V, Blazovics A, Kupcsulik P. Effect of postconditioning in major vascular operations on rats. Magy Seb. 2009;62(4):180-7.
- 20. Antonio LG, Evora PR, Piccinato CE. Use of alprostadil, a stable prostaglandin E1 analogue, for the attenuation of rat skeletal muscle ischemia and reperfusion injury. Minerva Chir. 2009;64(6):559-64.
- 21. Kin H, Zhao ZQ, Sun HY, Wang NP, Corvera JS, Halkos ME, Kerendi F, Guyton RA, Vinten-Johansen J. Postconditioning attenuates myocardial ischemia-reperfusion injury by inhibiting events in the early minutes of reperfusion. Cardiovasc Res. 2004;62(1):74-85.
- 22. Weiss RG, de Albuquerque CP, Vandegaer K, Chacko VP, Gerstenblith G. Attenuated glycogenolysis reduces glycolytic catabolite accumulation during ischemia in preconditioned rat hearts. Circ Res. 1996;79(3):435-46.
- 23. Pescador N, Villar D, Cifuentes D, Garcia-Rocha M, Ortiz-Barahona A, Vazquez S, Ordonez A, Cuevas Y, Saez-Morales D, Garcia-Bermejo ML, Landazuri MO, Guinovart J, del Peso L. Hypoxia promotes glycogen accumulation through hypoxia inducible factor (HIF)-mediated induction of glycogen synthase 1. PLoS One. 2010;5(3):e9644.
- 24. Harder Y, Amon M, Laschke MW, Schramm R, Rucker M, Wettstein R, Bastiaanse J, Frick A, Machens HG, Kuntscher M, Germann G, Vollmar B, Erni D, Menger MD. An old dream revitalised: preconditioning strategies to protect surgical flaps from critical ischaemia and ischaemia-reperfusion injury. J Plast Reconstr Aesthet Surg. 2008;61(5):503-11.
- 25. Wang WZ, Baynosa RC, Zamboni WA. Update on ischemia-reperfusion injury for the plastic surgeon: 2011. Plast Reconstr Surg. 2011;128(6):685e-92e.
- 26. Walsh SR, Tang TY, Sadat U, Gaunt ME. Remote ischemic preconditioning in major vascular surgery. J Vasc Surg. 2009;49(1):240-3.
- 27. Ali ZA, Callaghan CJ, Lim E, Ali AA, Nouraei SA, Akthar AM, Boyle JR, Varty K, Kharbanda RK, Dutka DP, Gaunt ME. Remote ischemic preconditioning reduces myocardial and renal injury after elective abdominal aortic aneurysm repair: a randomized controlled trial. Circulation. 2007;116(11 Suppl):I98-105.
Publication Dates
-
Publication in this collection
04 June 2013 -
Date of issue
June 2013
History
-
Received
14 Feb 2013 -
Accepted
13 May 2013 -
Reviewed
15 Apr 2013