ABSTRACT
PURPOSE:
To compare two different experimental models of osteoarthritis in rabbits: intra-articular collagenase injection and anterior cruciate ligament transection.
METHODS:
Ten adult rabbits were randomly divided in two groups: COLL (collagenase group) and ACLT (anterior cruciate ligament transection). The COLL group was treated with 0.5 ml collagenase solution (2mg collagenase/0.5 ml sterile PBS), and the ACTL group was subjected to anterior cruciate ligament. After six and twelve weeks, respectively, the animals in the COLL and ACTL groups were euthanized. The gross appearance and histological examinations conducted in the cartilage articular surface was blindly scored according to the criteria developed by Yoshimi et al. (1994) and Mankin et al. (1971), respectively.
RESULTS:
The gross morphologic observation, macroscopic score and histological examinations have demonstrated that the ACTL group presented the highest scores, and lesions more severe than those in the COLL group.
CONCLUSIONS:
Both methods, anterior cruciate ligament transection and collagenase, applied to the stifle joint of the rabbits have effectively induced degenerative changes in the cartilage tissue, through statistically significant analysis (p≤0.05). The ACTL method has presented more severe lesions.
Key words:
Joint Diseases; Osteoarthritis; Anterior Cruciate Ligament; Collagenases; Rabbits
Introduction
Osteoarthritis (OA) is a degenerative joint disease most commonly occurring in the knee and seen in middle-aged and elderly adults. Knee OA is one of the major causes of pain and disability; it significantly affects the patients' quality of life11. Iannitti T, Elhensheri M, Bingol AO, Palmieri B. Preliminary histopathological study of intra-articular injection of a novel highly cross-linked hyaluronic acid in a rabbit model of knee osteoarthritis. J Mol Hist. 2013 April;44(2):191-201. doi: 10.1007/s10735-012-9457-4.
https://doi.org/10.1007/s10735-012-9457-...
. According to the World Health Organization22. World Health Organization. The World Health Report 2002. Reducing risks, promoting healthy life. Geneva; 2002., OA is the fourth leading cause of years lost to disability worldwide, due to the increasing number of individuals suffering from this disease. Understanding OA is a key process to develop better treatment and prevention strategies against it.
Animal models have proved to be of considerable importance in elucidating mechanisms underlying joint damage due to OA and in providing proof of concept in the development of pharmacological and biological agents able to change the structural damage in the OA-affected joint33. Brandt KD. Animal models of osteoarthritis. Biorheology. 2002;39(1-2):221-35.. These models were designed using different mechanisms through which stress leads to OA development, namely: the transection of the meniscus and/or ligaments44. Kamekura S, Hoshi K, Shimoaka T, Chung U, Chikuda H, Yamada T, Uchida M, Ogata N, Seichi A, Nakamura K, Kawaguchi H. Osteoarthritis development in novel experimental mouse models induced by knee joint instability. Osteoarthritis Cartilage. 2005 Jul;13(7):632-41. doi: 10.1016/j.joca.2005.03.004
https://doi.org/10.1016/j.joca.2005.03.0...
, the intra-articular injection of a chemical substance such as papain55. Farkas T, Bihari-Varga M, Biro T. Thermoanalytical and histological study of intra-articular papain-induced degradation and repair of rabbit cartilage. II. Mature animals. Ann rheum Dis. 1976 Feb;35(1):23-6. PMID: 1275577. or collagenase66. Kikuchi T, Sakuta T, Yamaguchi T. Intra-articular injection of collagenase induces experimental osteoarthritis in mature rabbits. Osteoarthritis Cartilage . 1998 May;6(3):177-86. doi: 10.1053/joca.1998.0110.
https://doi.org/10.1053/joca.1998.0110...
. The knee dynamics stability is affected by both the passive (ligamentous) and active (neuromuscular) joint restraints. Among all knee joint stability contributors regarding the femur77. Kiapour AM, Wordeman SC, Paterno MV, Quatman CE, Levine JW, Goel VK, Demetropoulos CK, Hewett TE. Diagnostic value of knee arthrometry in the prediction of anterior cruciate ligament strain during landing. Am J Sports Med. 2014 Feb;42(2):312-9. doi: 10.1177/0363546513509961.
https://doi.org/10.1177/0363546513509961...
, the anterior cruciate ligament (ACL) has long been considered the primary passive restraint to the anterior translation of the tibia. The anterior cruciate ligament transection (ACTL) model has been widely described and has shown the appropriate histological and biochemical changes associated with OA progression88. Ameye LG, Young MF. Animal models of osteoarthritis: lessons learned while seeking the "Holy Grail". Curr Opin Rheumatol. 2006;18(5):537-47. doi: 10.1097/01.bor.0000240369.39713.af
https://doi.org/10.1097/01.bor.000024036...
. The ACTL model has resulted in joint instability; thus, it has induced cartilage degeneration, subchondral bone sclerosis and osteophyte formation, which mimics the pathological changes observed in human OA99. Hayami T, Pickarski M, Zhuo Y, Wesolowski GA, Rodan GA, Duong LT. Characterization of articular cartilage and subchondral bone changes in the rat anterior cruciate ligament transection and mini-sectomized models of osteoarthritis. Bone. 2006;38(2):234-43. PMID: 16185945..
The collagenase-induced OA model is based on joint-instability induction through intra-articular collagenase injection. Such induction procedure weakens the ligaments and leads to an OA-like pathology, including cartilage matrix erosion and osteophyte formation within 6 weeks1010. Blom AB, van Lent PL, Holthuysen AE, van der Kraan PM, Roth J, van Rooijen N, van den Berg WB. Synovial lining macrophages mediate osteophyte formation during experimental osteoarthritis. Osteoarthritis Cartilage . 2004 Aug;12(8):627-35. doi: 10.1016/j.joca.2004.03.003.
https://doi.org/10.1016/j.joca.2004.03.0...
. Despite being a convenient model (rapidity, ease application and consistency in cartilage damage induction), it is still unclear whether the tissue characteristics of the animal subjected to collagenase injection are comparable to that of the ACTL model.
The aim of the present study is to compare OA development between the anterior cruciate ligament transection model and the intra-articular collagenase injection through the gross morphological observations-macroscopic score and the histological evaluation of the cartilage articular surface.
Methods
The experimental protocol was approved by the Ethics Committee on Animal Use (CEUA) of UNESP, under no. 027839/12, at a meeting held on December 12th, 2012. The experiment was conducted in compliance with the ethical principles adopted by the Brazilian College of Animal Experimentation (COBEA).
Ten healthy white New Zealand adult male rabbits weighing 2.8-3.4 kg were used in the experiment. The animals were randomly distributed in two groups with five animals each, namely: COLL (collagenase group) and ACLT (anterior cruciate ligament transection). The animals were individually housed and handled according to the institutional Animal Care and Use Committee. The environment was enriched with objects such as burrows and nests in order to assure the animals' welfare.
Induction of the experimental OA
The collagenase group
The collagenase solution was prepared in laminar flow hood using type II Collagenase from Clostridium histolyticum (enzyme activity 425 U/mg-Sigma-Aldrich, Germany). Two milligrams were dissolved in 0.5 ml sterile phosphate-buffered saline solution and filtered through a 0.22 µm membrane. The animals were anesthetized using an intra-muscular injection of 2 mg/kg xylazine hydrochloride and 30 mg/kg ketamine hydrochloride. The solution was intra-articularly injected into the right stifle joint after the right stifle joint was clipped and aseptically prepared. The injection was administered at days 1 and 4, according to the method by Kikuchi et al (19986. Kikuchi T, Sakuta T, Yamaguchi T. Intra-articular injection of collagenase induces experimental osteoarthritis in mature rabbits. Osteoarthritis Cartilage . 1998 May;6(3):177-86. doi: 10.1053/joca.1998.0110.
https://doi.org/10.1053/joca.1998.0110...
)6. The rabbits were kept in their cages at the end of the anesthetic recovery, with free movement for 6 weeks.
Surgical group
The surgical OA model was induced through anterior cruciate ligament transection (ACLT), according to the method by Vignon et al.1111. Vignon E, Bejui J, Mathieu P, Hartmann JD, Ville G, Evreux JC, Descotes J. Histological cartilage changes in a rabbit model of osteoarthritis. J Rheumatol. 1987 May;14 Spec Nº:104-6. PMID: 3625662.. The animals were anesthetized using an intra-muscular injection of 2 mg/kg xylazine hydrochloride and 30 mg/kg ketamine hydrochloride in combination with the cephalic vein catheterization and with intravenous propofol maintenance. The right stifle was prepared in a surgically sterile fashion. The patella was medially dislocated through lateral parapatellar arthrotomy in order to achieve the optimal visualization of the anterior cruciate ligament and of the knee positioned in full flexion. The ACL was visualized and transected with a scalpel. The stifle joint was irrigated with sterile saline and the joint capsule and subcutaneous tissue were closed using 4-0 polydioxanone suture. The skin was closed using 3-0 nylon surgical suture. Prophylactic antibiotics added with enrofloxacin 15 mg/kg were administered every 24 hours for 5 days after surgery. Analgesic therapy was conducted through the administration of tramadol 2 mg/kg every 12 hours for 3 days. The animals were kept in their cages at the end of the anesthetic recovery, with free movement for 12 weeks.
Gross morphological observations and macroscopic score
Rabbits were euthanized using pharmacological thiopental overdose followed by potassium chloride. The animals from the collagenase group were euthanized at the 6th week, those from the surgical group were euthanized at the 12th week, according to the respective methods. The right stifle joints were dissected and the lateral and medial femoral condyles were examined for gross morphological changes. Cartilage degeneration was assessed and measured according to the Yoshimi scoring system1212. Yoshimi T, Kikuchi T, Obara T, Yamaguchi T, Sakakibara Y, Itoh H, Iwata H, Miura T. Effects of high-molecular-weight sodium hyaluronate on experimental osteoarthrosis induced by the resection of rabbit anterior cruciate ligament. Clin Orthop Relat Res. 1994 Jan;(298):296-304. PMID: 8118990. (0, normal cartilage; 1, softened cartilage; 2, fibrillation; 3, erosion; 4, ulceration; and 5, cartilage loss). All assessments were graded by a blinded pathologist.
Histological evaluation and score
The lateral and femoral condyles were fixed with 10% neutral buffered formalin and decalcified with 20% ethylenediaminetetraacetic acid right after the macroscopic score. The calcified condyle was embedded in paraffin and the standard frontal micro-sections (5 μm width) were prepared and stained in hematoxylin, eosin and toluidine blue. The degree of cartilage degradation was assessed through the scoring system by Mankin et al.1313. Mankin HJ, Dorfman H, Lippiello L, Zarins A. Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips. II. Correlation of morphology with biochemical and metabolic data. J Bone Joint Surg Am. 1971 Apr;53(3):523-37. PMID: 5580011., with modification. The histological evidence of cartilage degeneration was assessed through the structural change in articular cartilage (0, normal; 1, surface irregularities; 2, pannus and surface irregularities; 3, clefts to transitional zones; 4, clefts to radial zones; 5, clefts to calcified zones; and 6, complete disorganization), as well as through the cell status (0, normal; 1, diffuse hypercellularity; 2, cloning; and 3, hypocellularity). The total cartilage degeneration score ranged from 0 (normal) to 9 (complete disorganization and hypocellularity of the articular cartilage). All cartilage sections were graded by a blinded pathologist.
Statistical analyses
The statistical analysis was conducted in the Instat software at significance level p<0.05. The data collected through macroscopic and histomorphological scores were subjected to nonparametric Mann-Whitney Test.
Results
Gross morphological observations and macroscopic score
There were significant differences between the groups. The mean of the COLL group was 2.417±0.5149 and that of the ACTL group was 3.750±0.7538. The two-tailed P value was 0.0006, and it was considered extremely significant. Gross surface damage signs were more apparent in the ACTL group, thus exhibiting more severe lesions. Ulceration areas and cartilage losses were found in this group, whereas there was discoloration, fibrillation and erosion in the COLL group.
Histological examinations
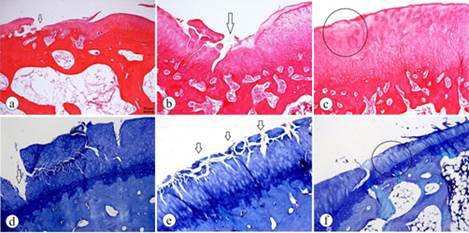
The ACTL group showed more severe signs of degenerative alterations. The mean histological scores were: COLL, 3.833±1.624; ACTL, 6.583±1.165. The two-tailed P value was 0.0008, which was considered extremely significant. As shown in Figure 1, the structural change in the articular cartilage of animals in the ACTL group showed severe degradation and disorganization, given that the fissures were deeper and that there were losses in the superficial and deep layers in some areas and, consequently, the exposure of the calcified zone. The severe hypocellularity of the chondrocytes was noticed in the cell status.
The histological assessment of the ACTL group. A-C (hematoxylin and eosin staining); D-F (Toluidine Blue staining). A. Clefts in superficial and transitional zones (arrows). B. Structural disorganization and clefts in superficial, transitional, deep and calcified zones (arrow). C. Hypocellularity of chondrocytes (ellipse). D. Loss of superficial and deep layers and the consequent exposure of the calcified zone (arrow). E. Clefts in superficial and transitional zones (arrows). F. Hypocellularity of the chondrocytes and mild surface irregularity (ellipse).
In the COLL group, the structural change in the articular cartilage showed irregular surface layer with fibrillation, swelling and lysis sings in the matrix. Pannus, diffuse hipercelularity and clustering were also noticed in this group (Figure 2).
The histological assessment of the COLL group. A-C (hematoxylin and eosin staining); D-F (Toluidine Blue staining). A-B. Mild surface irregularity (arrow) and clustering of chondrocytes (ellipse). C. Pannus (ellipse). D. Mild surface irregularity, clefts in superficial and transitional zones (arrow) and clustering (ellipse). E. Diffuse hypercellularity (ellipse). F. Clustering of chondrocytes (ellipse).
Discussion
The rabbit model appeared to be practical for early therapy evaluation stages due to its relative cost effectiveness, easy handling and to the animal's joint size, which is suitable for surgical procedures1414. Chu CR, Szczodry M, Bruno S. Animal models for cartilage regeneration and repair. Tissue Eng Part B. 2010 Feb;16 (1):105-15. doi: 10.1089/ten.TEB.2009.0452.
https://doi.org/10.1089/ten.TEB.2009.045...
, as shown in the present study. Nonetheless, the welfare during experimental procedures is of concern in most research centers worldwide. Thus, the aim of the present study was to compare the effectiveness of two different models for osteoarthritis induction in rabbits. Both methods were capable of producing consistent lesions, which were compatible with osteoarthritis.
The present study has demonstrated evident degenerative changes in the articular cartilage of the collagenase group. However, these changes were different to those in the ACLT. Nevertheless, both methods have induced the development of OA, although in different grades. Probably the time-dependent progression may have resulted from the different action modes applied to the knee joints. Notwithstanding, it is important highlighting that the evaluation period was different between the two groups, because the methodology was applied exactly as described in the herein referred literature. Both methods were able to induce joint instability through ligament rupture or weakening the ligaments due to the collagenase effect; thus leading to an OA-like pathology. Melo et al.1515. Melo EG, Nunes VA, Rezende CMF, Gomes MG, Malm C, Gheller VA. Sulfato de condroitina e hialuronato de sódio no tratamento da doença articular degenerativa em cães. Estudo histológico da cartilage articular e membrana synovial. Arq Bras Med Vet Zootec. 2008;60(1):83-92. have stated that changes are expected in both groups due to joint instability. The imbalance in the distribution of forces on the articular surface led to the breakdown of proteoglycan arrangements, to the consequent increase in cartilage hydration (swelling) and to the exposure of collagen fibrils (fibrillation).
The results have shown that the chemical osteoarthritis induction through intra-articular collagenase injection in the rabbit model resulted in cartilage degenerative changes. However, these changes were superficial and showed early osteoarthritis stages due to the collagenase concentration, according to Kikuchi et al.66. Kikuchi T, Sakuta T, Yamaguchi T. Intra-articular injection of collagenase induces experimental osteoarthritis in mature rabbits. Osteoarthritis Cartilage . 1998 May;6(3):177-86. doi: 10.1053/joca.1998.0110.
https://doi.org/10.1053/joca.1998.0110...
. The ACLT method has created degenerative cartilage lesions after 12 weeks, as well as more severe degradation44. Kamekura S, Hoshi K, Shimoaka T, Chung U, Chikuda H, Yamada T, Uchida M, Ogata N, Seichi A, Nakamura K, Kawaguchi H. Osteoarthritis development in novel experimental mouse models induced by knee joint instability. Osteoarthritis Cartilage. 2005 Jul;13(7):632-41. doi: 10.1016/j.joca.2005.03.004
https://doi.org/10.1016/j.joca.2005.03.0...
,1515. Melo EG, Nunes VA, Rezende CMF, Gomes MG, Malm C, Gheller VA. Sulfato de condroitina e hialuronato de sódio no tratamento da doença articular degenerativa em cães. Estudo histológico da cartilage articular e membrana synovial. Arq Bras Med Vet Zootec. 2008;60(1):83-92.,1616. Desando G, Cavallo C, Sartoni F, Martini L, Parrilli A, Veronesi F, Fini M, Giardino R, Facchini A, Grigolo B. Intra-articular delivery of adipose derived stromal cells attenuates osteoarthritis progression in an experimental rabbit model. Arthritis Res Ther. 2013 Jan 29;15(1):R22. doi: 10.1186/ar4156.
https://doi.org/10.1186/ar4156...
.
Although the lesions in animals of the COLL group were more superficial, the high instability of the ACTL model was the disadvantage of this method. In order to evaluate the effectiveness of different osteoarthritis treatments it is necessary stabilizing the joint through the surgical correction of the ruptured ligament, although the complete stability is impossible to be reached. Furthermore, since ACLT presented instability based on the biomechanically-induced model, factors such as cage dimensions and flooring could interfere in the development of the disease, because they influence the activity of the animal's affected limb1717. Laverty S, Girard CA, Williams JM, Hunziker EB, Pritzker KPH. The OARSI histopathology initiative - recommendations for histological assessments of osteoarthritis in the rabbit. Osteoarthritis Cartilage . 2010 Oct;18 Suppl 3:S53-65. doi: 10.1016/j.joca.2010.05.029.
https://doi.org/10.1016/j.joca.2010.05.0...
. According to Kikuchi et al.66. Kikuchi T, Sakuta T, Yamaguchi T. Intra-articular injection of collagenase induces experimental osteoarthritis in mature rabbits. Osteoarthritis Cartilage . 1998 May;6(3):177-86. doi: 10.1053/joca.1998.0110.
https://doi.org/10.1053/joca.1998.0110...
, the intra-articular injection can be conveniently performed, the surgical procedures to develop an OA model are complicated and the cartilage degeneration induction takes longer than the intra-articular injection of chemical substances. Furthermore, it has the advantage of being minimally invasive, less painful, reproducible and relatively easy to conduct. Thus, the intra-articular injection can be considered an alternative more practical than the ACLT to be applied to an OA animal model.
Despite the importance of the current study, some limitations were noticed in it. The main limitation was the small number of rabbits in each group. However, it is important calling the attention to the concern with the reduced number of animals participating in the experiments. Besides, more objective and sophisticated outcome measurement systems could have been adopted such as gene expression and type I and II collagen analysis through immunohistochemistry applied to the animal's cartilage. It could have helped elucidating the molecular difference levels between techniques. Nevertheless, it is worth stating that the present study offers valuable data to promote the further application of both methods.
Conclusions
The anterior cruciate ligament transection and collagenase methods applied to the rabbit knee joint have effectively induced degenerative changes in the cartilage tissue, although in different grades. The ACTL method has presented more severe lesions. This result may be due to that the surgical model provides more joint instability, moreover, the establishment of osteoarthritis be longer, six weeks more than the COLL group.
References
-
1Iannitti T, Elhensheri M, Bingol AO, Palmieri B. Preliminary histopathological study of intra-articular injection of a novel highly cross-linked hyaluronic acid in a rabbit model of knee osteoarthritis. J Mol Hist. 2013 April;44(2):191-201. doi: 10.1007/s10735-012-9457-4.
» https://doi.org/10.1007/s10735-012-9457-4 -
2World Health Organization. The World Health Report 2002. Reducing risks, promoting healthy life. Geneva; 2002.
-
3Brandt KD. Animal models of osteoarthritis. Biorheology. 2002;39(1-2):221-35.
-
4Kamekura S, Hoshi K, Shimoaka T, Chung U, Chikuda H, Yamada T, Uchida M, Ogata N, Seichi A, Nakamura K, Kawaguchi H. Osteoarthritis development in novel experimental mouse models induced by knee joint instability. Osteoarthritis Cartilage. 2005 Jul;13(7):632-41. doi: 10.1016/j.joca.2005.03.004
» https://doi.org/10.1016/j.joca.2005.03.004 -
5Farkas T, Bihari-Varga M, Biro T. Thermoanalytical and histological study of intra-articular papain-induced degradation and repair of rabbit cartilage. II. Mature animals. Ann rheum Dis. 1976 Feb;35(1):23-6. PMID: 1275577.
-
6Kikuchi T, Sakuta T, Yamaguchi T. Intra-articular injection of collagenase induces experimental osteoarthritis in mature rabbits. Osteoarthritis Cartilage . 1998 May;6(3):177-86. doi: 10.1053/joca.1998.0110.
» https://doi.org/10.1053/joca.1998.0110 -
7Kiapour AM, Wordeman SC, Paterno MV, Quatman CE, Levine JW, Goel VK, Demetropoulos CK, Hewett TE. Diagnostic value of knee arthrometry in the prediction of anterior cruciate ligament strain during landing. Am J Sports Med. 2014 Feb;42(2):312-9. doi: 10.1177/0363546513509961.
» https://doi.org/10.1177/0363546513509961 -
8Ameye LG, Young MF. Animal models of osteoarthritis: lessons learned while seeking the "Holy Grail". Curr Opin Rheumatol. 2006;18(5):537-47. doi: 10.1097/01.bor.0000240369.39713.af
» https://doi.org/10.1097/01.bor.0000240369.39713.af -
9Hayami T, Pickarski M, Zhuo Y, Wesolowski GA, Rodan GA, Duong LT. Characterization of articular cartilage and subchondral bone changes in the rat anterior cruciate ligament transection and mini-sectomized models of osteoarthritis. Bone. 2006;38(2):234-43. PMID: 16185945.
-
10Blom AB, van Lent PL, Holthuysen AE, van der Kraan PM, Roth J, van Rooijen N, van den Berg WB. Synovial lining macrophages mediate osteophyte formation during experimental osteoarthritis. Osteoarthritis Cartilage . 2004 Aug;12(8):627-35. doi: 10.1016/j.joca.2004.03.003.
» https://doi.org/10.1016/j.joca.2004.03.003 -
11Vignon E, Bejui J, Mathieu P, Hartmann JD, Ville G, Evreux JC, Descotes J. Histological cartilage changes in a rabbit model of osteoarthritis. J Rheumatol. 1987 May;14 Spec Nº:104-6. PMID: 3625662.
-
12Yoshimi T, Kikuchi T, Obara T, Yamaguchi T, Sakakibara Y, Itoh H, Iwata H, Miura T. Effects of high-molecular-weight sodium hyaluronate on experimental osteoarthrosis induced by the resection of rabbit anterior cruciate ligament. Clin Orthop Relat Res. 1994 Jan;(298):296-304. PMID: 8118990.
-
13Mankin HJ, Dorfman H, Lippiello L, Zarins A. Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips. II. Correlation of morphology with biochemical and metabolic data. J Bone Joint Surg Am. 1971 Apr;53(3):523-37. PMID: 5580011.
-
14Chu CR, Szczodry M, Bruno S. Animal models for cartilage regeneration and repair. Tissue Eng Part B. 2010 Feb;16 (1):105-15. doi: 10.1089/ten.TEB.2009.0452.
» https://doi.org/10.1089/ten.TEB.2009.0452 -
15Melo EG, Nunes VA, Rezende CMF, Gomes MG, Malm C, Gheller VA. Sulfato de condroitina e hialuronato de sódio no tratamento da doença articular degenerativa em cães. Estudo histológico da cartilage articular e membrana synovial. Arq Bras Med Vet Zootec. 2008;60(1):83-92.
-
16Desando G, Cavallo C, Sartoni F, Martini L, Parrilli A, Veronesi F, Fini M, Giardino R, Facchini A, Grigolo B. Intra-articular delivery of adipose derived stromal cells attenuates osteoarthritis progression in an experimental rabbit model. Arthritis Res Ther. 2013 Jan 29;15(1):R22. doi: 10.1186/ar4156.
» https://doi.org/10.1186/ar4156 -
17Laverty S, Girard CA, Williams JM, Hunziker EB, Pritzker KPH. The OARSI histopathology initiative - recommendations for histological assessments of osteoarthritis in the rabbit. Osteoarthritis Cartilage . 2010 Oct;18 Suppl 3:S53-65. doi: 10.1016/j.joca.2010.05.029.
» https://doi.org/10.1016/j.joca.2010.05.029
-
Financial source: none
-
1
Research performed at Division of Operative Technique and Experimental Surgery, Department of Surgery, Universidade Federal do Mato Grosso do Sul (UFMS), Brazil
Publication Dates
-
Publication in this collection
Sept 2016
History
-
Received
19 May 2016 -
Reviewed
21 July 2016 -
Accepted
23 Aug 2016