Abstracts
The objective was to standardize an experimental model of parabiotic isolated heart in rabbits, testing their stability and durability for use in cardiovascular research. Sixty-six Norfolk-2000 rabbits were used and divided in 2 groups: donors of the isolated heart and support animals, in a total of 33 preparations. Circulatory support for the isolated heart was established with the aid of peristaltic pumps and the flow was kept constant (16ml/min.). An intraventricular balloon was inserted in the left ventriculum, and was adjusted to produce diastolic pressure of ± 10mmHg. Heart rate was established at 120 beats per minute with the use of a pacemaker. Hemodynamic, laboratory and histopathological parameters were evaluated. Of the 33 preparations, 13 were excluded according to preestablished criteria. Of the 20 remaining preparations, 10 completed the maximum protocol time (180 minutes). There was progressive hemodynamic deterioration with decrease of mean blood pressure (89.30±6.09mmHg->47.50±6.35mmHg) in the support animal. There was hemodynamic stability of the isolated heart for the 10 preparations that completed the 180 minutes of the protocol. Laboratory parameters showed progressive decrease of sodium, potassium and hemoglobin, which is compatible with hemodilution. Histopathology showed greater distance among the fibers, compatible with edema. The present model showed 100% stability and activity of the preparations within 60 minutes, and 50% of the active preparations were progressively lost within 180 minutes. The present model is viable for cardiovascular research.
Heart; Casts; Ventricular function
O objetivo foi a padronização de modelo experimental de coração isolado parabiótico em coelhos, testando sua estabilidade e durabilidade, para fins de pesquisa cardiovascular. Foram utilizados 66 coelhos raça Norfolk-2000 divididos em grupo doador do coração isolado e animais suporte, totalizando 33 preparações. Mediante auxilio de bombas peristálticas estabeleceu-se suporte circulatório para o coração isolado mantendo-se fluxo constante(16ml/min.). Um balão intraventricular foi inserido no ventrículo esquerdo, sendo ajustado para gerar pressão diastólica de ± 10mmHg. A freqüência foi fixada em 120 batimentos por minuto mediante o uso de marcapasso. Foram avaliadas variáveis hemodinâmicas, laboratoriais e anatomopatológicas. Das 33 preparações, 13 foram excluídas mediante critérios pré-estabelecidos. Das 20 restantes, 10 cumpriram o tempo máximo do protocolo(180 minutos). Com relação ao animal suporte houve deterioração hemodinâmica progressiva c/ queda da pressão arterial média(89,30±6,09mmHg->47,50±6,35mmHg). Com relação ao corações isolado, das 10 preparações que cumpriram os 180 minutos de protocolo, houve estabilidade hemodinâmica. As variáveis laboratoriais mostraram queda progressiva do sódio, potássio e hemoglobina, sendo compatível com hemodiluição. O exame anatomopatológico mostrou espaçamento maior entre fibras, compatível com edema. O presente modelo mostrou estabilidade e atividade de 100% das preparações em 60 minutos, havendo perdas progressivas chegando a 50% das preparações em atividade em 180 minutos. O presente modelo, dentro das limitações estabelecidas é viável para pesquisas cardiovasculares.
Coração; Modelos cirúrgicos; Função ventricular
STANDARDIZATION OF AN EXPERIMENTAL MODEL OF PARABIOTIC ISOLATED HEART IN RABBITS1 1 . Experimental LaboratoryDepartment of Surgery FMB-UNESP;
Antônio Sérgio Martins2 1 . Experimental LaboratoryDepartment of Surgery FMB-UNESP;
Marcos Augusto de Moraes Silva2 1 . Experimental LaboratoryDepartment of Surgery FMB-UNESP;
Beatriz Bojokian Matsubara3 1 . Experimental LaboratoryDepartment of Surgery FMB-UNESP;
Flávio Aragon4 1 . Experimental LaboratoryDepartment of Surgery FMB-UNESP;
Carlos Roberto Padovani4 1 . Experimental LaboratoryDepartment of Surgery FMB-UNESP;
Martins AS, Moraes-Silva MA, Matsubara BB, Aragon F, Padovani CR. Standardization of an experimental model of parabiotic isolated heart in rabbits. Acta Cir Bras [serial online] 2000 Jul-Sept;15(3). Available from: URL: http://www.scielo.br/acb.
ABSTRACT: The objective was to standardize an experimental model of parabiotic isolated heart in rabbits, testing their stability and durability for use in cardiovascular research. Sixty-six Norfolk-2000 rabbits were used and divided in 2 groups: donors of the isolated heart and support animals, in a total of 33 preparations. Circulatory support for the isolated heart was established with the aid of peristaltic pumps and the flow was kept constant (16ml/min.). An intraventricular balloon was inserted in the left ventriculum, and was adjusted to produce diastolic pressure of ± 10mmHg. Heart rate was established at 120 beats per minute with the use of a pacemaker. Hemodynamic, laboratory and histopathological parameters were evaluated. Of the 33 preparations, 13 were excluded according to preestablished criteria. Of the 20 remaining preparations, 10 completed the maximum protocol time (180 minutes). There was progressive hemodynamic deterioration with decrease of mean blood pressure (89.30±6.09mmHg->47.50±6.35mmHg) in the support animal. There was hemodynamic stability of the isolated heart for the 10 preparations that completed the 180 minutes of the protocol. Laboratory parameters showed progressive decrease of sodium, potassium and hemoglobin, which is compatible with hemodilution. Histopathology showed greater distance among the fibers, compatible with edema. The present model showed 100% stability and activity of the preparations within 60 minutes, and 50% of the active preparations were progressively lost within 180 minutes. The present model is viable for cardiovascular research.
SUBJECT HEADINGS: Heart. Casts, surgical. Ventricular function.
INTRODUCTION
Reliable experimental models are important for successful experiments. In the cardiovascular field, the isolated heart maintained by nutrient solutions first introduced by LANGENDORFF1 has gained importance due to its simplicity. HEYMANS and KOCHMANN, in 19042, published a variation of LANGENDORFF's model using a support animal. The lack of effective anticoagulation has limited the use of the method. The use of nutrient solutions is limited by the size of the heart and by the intense edema produced3,4. The use of blood as perfusate has physiological advantages such as less edema, however support animals have to be used 5. The isolated heart model may be used in almost all laboratory animals, and has been reported in dogs6,7, mice8, guinea pigs9, pigs10 and rabbits11,12. The rabbits showed advantages when compared to other smaller animals, such as a larger heart, which makes surgical instrumentation easier, composition of myosins and calcium kinetics similar to humans, as opposed to mice13.
OBJECTIVE
Standardize the experimental model of parabiotic isolated heart in rabbits, testing their stability and durability in cardiovascular research.
METHODS
Sixty-six Norfolk-2000 rabbits of both genders, weighing 2270 to 2700 grams were used. The animals were prepared according to the "Guide for Care Use of Laboratory Animals."14 written by the United States National Science Academy.
Two animals were randomly used in each experiment. One as support animal and the other as a donor of the isolated heart.
Experimental times
After 15 minutes of perfusion, time zero was established (T0) and then after every 30 minutes(in a total of 180 minutes)establishing times T1, T2, T3, T4, T5 and T6.
Experimental model
The animals were anesthestized with pentobarbital sodium (30mg/Kg i.v.) and heparinized (3mg/Kg).
The isolated heart was perfused, at the root of the aorta, with blood from the support rabbit, obtained from the cannulation of the right carotid artery and pumped by a peristaltic infusion pump. Venous return was carried out by gravity, through the pulmonary artery of the isolated heart, which was previously cannulated in the right jugular vein of the support animal. A latex balloon was inserted in the left ventriculum to measure its function. Two pacemaker electrodes for stimulation (120epm) and a 3 mm polyvinyl cannula to read right atrial pressure were placed in the right atrium. The isolated heart was immersed in a basin with saline solution and circulation of heated water to maintain the temperature at 38oC.
The left carotid artery of the support animal was catheterized to obtain blood samples and for mean blood pressure monitoring (MBP). The jugular vein was catheterized for drug infusion (Ringer 5ml/kg and 8.4% sodium bicarbonate at 1.0mEq/Kg/hour) and central venous pressure monitoring (CVP).The support animal was placed on a thermal mattress to maintain rectal temperature at 38o C. The support animal was artificially ventilated with 35 cycles per minute. The isolated heart and support animal were connected to a polygraph (EEP-12 by Electronics for Medicine).
At the end of the experiment, fragments of the left ventriculum of the isolated heart and support animal were obtained for histopathological analysis under light microscopy and H-E staining.
Statistical analysis
Repeated multivariate analysis were performed for the ten preparations completing the protocol time15.
RESULTS
Of the 33 initial preparations, 13 were excluded according to criteria shown in Table 1.
Of the 20 remaining preparations, 10 completed the total protocol time (180 minutes). From T3 (90 minutes) there was progressive loss of the preparations due to death of the support animal (Table 2.).
Hemodynamic parameters of the support animal.
Mean Blood Pressure (MBP) and central venous pressure (CVP) .
Mean and respective standard deviations at the studied experimental times, as well as the statistical analysis results, are shown in Table 3.
Hemodynamic parameters of the isolated heart.
Developed pressure (DP), diastolic pressure (DP), peak first time derivative of intraventricular pressure (dp/dt+), coronary resistance (CR), right atrial pressure (RAP) and perfusion pressure(PP)
Mean and respective standard deviation at the studied experimental time as well as the statistical analysis results are shown in Table 4.
Laboratory Parameters
Sodium, potassium, calcium, glycose, hemoglobin.
Mean and respective standard deviations at the studied experimental times, as well as the statistical analysis results are shown in Table 5.
pHA, PaO 2, PaCO2, HCO3, Sata.
Mean and respective standard deviations at the studied experimental times, as well as the statistical analysis results are shown in Table 6.
Somatic parameters.
Rectal temperature of the support animal, temperature of the isolated heart, heart rate of the support animal.
Mean and respective standard deviations at the studied experimental times, as well as the statistical analysis results are shown in Table 7.
Histopathology
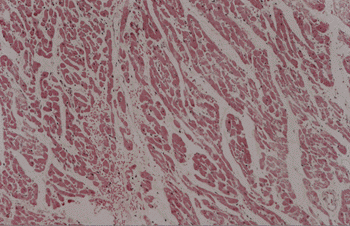
All of the isolated hearts showed the fibers were further apart and 8 of them had signs of mild interstitial hemorrhage(fig.1).
DISCUSSION
The rabbit has advantages when compared to smaller animals, such as easy racial, age and gender standardization, and availability in vivarium. Due to their size, the surgical manipulation of the heart is easier. Myosin composition and calcium kinetics are similar to human myocardium13. Therefore, the rabbit is an adequate choice for cardiovascular research. The isolated heart method may be carried out using nutrient solutions as perfusate. Adequate preparation and stability are required for the results obtained in the experiment to be valid. However, it does not require the use of another animal, which has to be done in the parabiotic model. The blood avoids severe edema, which is usual in nutrient solutions and the support animal works as a perfect homeostatic control16.
The flow system or constant pressure system may be used, each of them with advantages, disadvantages and in the present study, constant flow system was used according to DORING e DEHNERT's formula17.
The intraventricular balloon method was used to asses ventricular function18 with a preestablished diastolic pressure of ± 10mmHg.
Fixed ventricular rate is required for a comparison of the experimental parameters throughout the different experimental times. Based on literature, we chose a rate of 120 beats per minute19, 20.
The major exclusion cause was related to respiratory infection of the support animals, a severe condition leading to early death of the animal. In the present study, 90% of the preparations were active 120 minutes after the beginning of the experiment, an adequate result for most of the existing experimental protocols.21 Hemodynamic parameters of the support animal showed progressive decrease of mean blood pressure. Since volemia was kept constant (CVP p>0.05) and knowing that the death of the support animal was the major cause of failure of the preparations, it may be concluded that other factors could explain this condition, such as progressive vasoplegia resulting from extended extracorporeal circulation periods, i.e., the exposure of blood elements to non biological surfaces produces the inflammatory syndrome whose final expression is severe hypotension and death22.
Hemodynamic parameters of the isolated heart were compatible with literature reports23 and showed stability throughout the protocol.
Laboratory parameters showed progressive hemodilution, probably due to blood loss resulting from manipulation and sample collection. Calcium stability, which was continuously replaced reinforces this idea.
Histopathology showed greater distance among the fibers, indicating edema(fig.1). Signs of interstitial hemorrhage may be explained by perfusion pressure slightly higher than average for the flow used in the study, and heparinization.
CONCLUSIONS
The present model of parabiotic isolated heart showed adequate stability and reliability for use in cardiovascular research.
Acknowledgements
We wish to thank Ms.Rosemary Cristina da Silva for technical support.
Martins AS, Moraes-Silva MA, Matsubara BB, Aragon F, Padovani CR. Padronização de modelo experimental de coração isolado parabiótico em coelhos. Acta Cir Bras [serial online] 2000 Jul-Sept;15(3). Available from: URL: http://www.scielo.br/acb.
RESUMO: O objetivo foi a padronização de modelo experimental de coração isolado parabiótico em coelhos, testando sua estabilidade e durabilidade, para fins de pesquisa cardiovascular. Foram utilizados 66 coelhos raça Norfolk-2000 divididos em grupo doador do coração isolado e animais suporte, totalizando 33 preparações. Mediante auxilio de bombas peristálticas estabeleceu-se suporte circulatório para o coração isolado mantendo-se fluxo constante(16ml/min.). Um balão intraventricular foi inserido no ventrículo esquerdo, sendo ajustado para gerar pressão diastólica de ± 10mmHg. A freqüência foi fixada em 120 batimentos por minuto mediante o uso de marcapasso. Foram avaliadas variáveis hemodinâmicas, laboratoriais e anatomopatológicas. Das 33 preparações, 13 foram excluídas mediante critérios pré-estabelecidos. Das 20 restantes, 10 cumpriram o tempo máximo do protocolo(180 minutos). Com relação ao animal suporte houve deterioração hemodinâmica progressiva c/ queda da pressão arterial média(89,30±6,09mmHg->47,50±6,35mmHg). Com relação ao corações isolado, das 10 preparações que cumpriram os 180 minutos de protocolo, houve estabilidade hemodinâmica. As variáveis laboratoriais mostraram queda progressiva do sódio, potássio e hemoglobina, sendo compatível com hemodiluição. O exame anatomopatológico mostrou espaçamento maior entre fibras, compatível com edema. O presente modelo mostrou estabilidade e atividade de 100% das preparações em 60 minutos, havendo perdas progressivas chegando a 50% das preparações em atividade em 180 minutos. O presente modelo, dentro das limitações estabelecidas é viável para pesquisas cardiovasculares.
DESCRITORES: Coração. Modelos cirúrgicos. Função ventricular.
Address for correspondence:
Caixa Postal 539 Campus Unesp Botucatu
Botucatu - São Paulo
18618-000
e-mail:amartins@fmb.unesp.br
Data do recebimento: 31/05/2000
Data da revisão: 16/06/2000
Data da aprovação: 14/07/2000
2. Department of Surgery FMB-UNESP;
3. Department of Cardiology FMB-UNESP;
4. Department of Statistics IB-UNESP.
- 1. Langendorff O. Untersuchungen am uberleden sougethierherzen. Pflugers Arch Ges Physiol 1895;61:291-320.
- 2. Heymans JF, Kochmann M. Une nouvelle methodo de circulation artificialle a travers de couer isole de mammifere. Arch Int. Pharmacodyn Ther 1904;13:379-86.
- 3. Woo-Ming M, Mawu N, Lester L, Malinin TI. Water content of hearts with crystalloid and nutrient solutions. J Mol Cell Cardiol 1980;12:371-86.
- 4. Zornoff LAM, Paiva, SAR, Tornero, MTT, Zornoff LM, Carvalho MSS, Tucci PJF. Influęncia do acréscimo de manitol a soluçőes nutrientes no desempenho mecânico e no grau de edema miocárdico de coraçőes isolados de ratos. Arq Bras Cardiol 1995;64:225-9.
- 5. Shandu R, Diaz RJ, Wilson G. Comparision of ischemic preconditioning in blood perfused and buffer perfused isolated heart models. Cardiovasc Res 1993;27:602-7.
- 6. Osher WJ. Pressure-flow relation of the coronary system. Am J Physiol 1953;172:403-16.
- 7. Cannon MB, Vine AJ, Kantor HL, Lahorra, JA, Nickell SA, Hahn JW, Teplick RS, Titus, JS, Torchiana DF. et al. Warm and cold blood cardioplegia. Circulation 1994;90:328-38.
- 8. Nydhal S, Frebelius S, Swedenborg J. Thrombin inactivation and effects of antithrombim and heparin in a recirculating Langendorff preparation. Thromb Res 1992;65:365-76.
- 9. Boban M, Stowe DF, Buljubasic N, Kampine JP, Bosnjak ZJ. Direct comparative effects of isoflurane and desflurane in isolated guinea pig hearts. Anesthesiology 1992;76:775-80.
- 10. Ascuitto RJ, Ascuitto NT, Ranage D, McDonough KH. Importance of fatty acid oxidation in the neonatal pig heart with hypoxia and reoxygenation. J Dev Physiol 1990;14:291-4.
- 11. Sage MD, Gavin JB. Microvascular function at the margins of early experimental myocardial infarcts in the isolated rabbits hearts. Heart Vessels 1986;2:81-6.
- 12. Palmisano BW, Mehner RW, Baker JE, Stowe DF, Bosnjak ZJ, Kampine JP. Direct affects of halothane and isoflurane in the fat rabbit heart with rigth ventricular hypertrophy secondary to chronic hypoxemia. Anesth Analog 1995;80:1122-8.
- 13. Hasenfuss G. Animal models of human cardiovascular disease, heart failure and hypertrophy. Cardiovasc Res 1998;39:60-76.
- 14. Wolfle TL: Guide for the care and use of laboratory animals. Washington: National Academy Press; 1996.
- 15. Morrison DF. Multivariate statistical methods. 2ed. New York: McGraw Hill;1976.
- 16. Qiu Y, Hearse DJ. Comparison of ischemic vulnerability and responsiveness to cardioplegic protection in crystalloid perfused versus blood-perfused hearts. J Thorac Cardiovasc Surg 1992;103:960-8.
- 17. Doring HJ, Dehnert H. The isolated perfused heart to Langendorff. Germany: Biomesstechnik-verlag; 1988.
- 18. Goto Y, Slinker BK, Le Winter MM. Acuracy of volume measurement of rabbit left ventricle by ballon method. Am J Physiol 1988;255:H394-6.
- 19. Vargas F, Blackshear G. Transcapillary osmotic flows in the in vitro perfused heart. Am J Physiol 1981;240:H448-56.
- 20. Chen CC, Morishige N, Masuda M, Lin W, Thone F, Mugagwa K. R56865, a Na+ and Ca2+ overload inhibitor, reduces myocardial ischemia reperfusion injury in blood perfused rabbits hearts. J Moll Cell Cardiol 1993;25:1445-59.
- 21. Smith RE, Palmer RM, Bucknall CA, Moncada S. Role of nitric oxide synthesis in the regulation of coronary vascular tone in the isolated perfused rabbit heart. Cardiovasc Res 1992;26:508-12.
- 22. Weselcouch E, Luneau CJ, Willians KJ, Gosselin RE. The failure of serum albumin to affect capillary permeability in the isolated rabbit heart. Microvasc Res 1984;28:373-86.
- 23. Serizawa T, Vogel WM, Apstein CS, Grossman W. Comparison of acute alterations in left ventricular relaxation and diastolic chamber stiffness induced by hypoxia and ischemia. Role of myocardial oxygen supply-demand imbalance. J Clin Invest 1981;68:91-102.
Publication Dates
-
Publication in this collection
04 Sept 2000 -
Date of issue
Sept 2000
History
-
Accepted
14 July 2000 -
Reviewed
16 June 2000 -
Received
31 May 2000