Abstracts
PURPOSE: To investigate whether N-acetylcysteine has a protective effect against renal injury as a remote organ after skeletal muscle ischemia-reperfusion in rats. METHODS: Twenty Wistar male rats were divided randomly into two experimental groups: group ischemia-reperfusion (group I) and group ischemia-reperfusion + N-acetylcysteine (group II). After ketamine and xylazine anesthesia, femoral artery was exposed. All animals were undergone 2h of ischemia by occlusion femoral artery and 24h of reperfusion. Rats that were treated with N-acetylcysteine given IV at a dose of 150 mg/kg-¹, immediately before reperfusion. After 24h of reperfusion, the blood samples were collected and submitted for evaluation of plasmatic urea, creatinine values and then rats were euthanized and left kidney harvested for histopathological analysis under light microscopy. RESULTS: The urea (35±7.84 mg.dL-1), creatinine (1.46±0.47 mg.dL-1) values were significantly lower in group II (P=0.000). Renal histopathologic study in group I showed extensive distal and proximal tubular cells necrosis and sloughing of epithelial cells into the tubular lumen, cast formation in tubule and glomerul, glomerul fibrosis and hemorrhage. Histopathologically, there was a significant difference (p=0.037) between two groups. CONCLUSION: The N-acetylcysteine was able to decrease renal injury induced by skeletal muscle ischemia reperfusion in rats.
Acetylcysteine; Muscle, Skeletal; Ischemia; Reperfusion; Kidney; Rats
OBJETIVO: Investigar se a N-acetilcisteína tem um efeito protetor contra a lesão renal como um órgão remoto músculo esquelético após isquemia-reperfusão em ratos. MÉTODOS: Vinte ratos Wistar machos foram distribuídos aleatoriamente em dois grupos experimentais: grupo isquemia-reperfusão (grupo I) e grupo isquemia-reperfusão N-acetilcisteína (grupo II). Após a anestesia de ketamina e xilazina, a artéria femoral foi exposta. Todos os animais foram submetidos a 2h de isquemia pela oclusão da artéria femoral e 24h de reperfusão. Os ratos que foram tratados com N-acetilcisteína administrados IV na dose de 150 mgkg-1, imediatamente antes da reperfusão. Após 24h de reperfusão, as amostras de sangue foram coletadas e submetidas para avaliação de uréia, creatinina e, em seguida, os ratos foram sacrificados e rim esquerdo retirados para estudo histopatológico em microscopia de luz. RESULTADOS: A uréia (35 ± 7,84 mg.dL-1), creatinina (1,46 ± 0,47 mg.dL-1) os valores foram significativamente menores no grupo II (p=0,000). Estudo histopatológico renal do grupo I mostrou extensa necrose distal e proximal, células tubular e descamação das células epiteliais para o lúmen tubular, formação de elenco no túbulo e glomerulo, fibrose glomerular e hemorragia. Histopatologicamente houve uma diferença significativa (p=0,037) entre os dois grupos. CONCLUSÃO: A N-acetilcisteína foi capaz de diminuir a lesão renal induzida por reperfusão de isquemia do músculo esquelético em ratos.
Acetilcisteína; Músculo Esquelético; Isquemia; Reperfusão; Rim; Ratos
4 - ORIGINAL ARTICLE
ISCHEMIA-REPERFUSION
Protective effect of N-acetylcysteine on kidney as a remote organ after skeletal muscle ischemia-reperfusion1 1 Research performed at the Department of Surgery, Faculty of Specialized Veterinary Sciences, Science and Research Branch, Islamic Azad University, Tehran, Iran.
Efeito protetor da N-acetilcisteína no rim como um órgão remoto músculo esquelético após isquemia-reperfusão
Mohammad Ashrafzadeh TakhtfooladiI; Amirali JahanshahiII; Gholamreza JahanshahiIII; Amir SotoudehIV; Hamed Ashrafzadeh TakhtfooladiV; Mohammadreza KhansariVI
IFellow PhD degree, Department of Surgery, Faculty of Specialized Veterinary Sciences, Science and Research Branch, Islamic Azad University, Tehran, Iran. Conception, design and scientific content of study; analysis and interpretation of data
IIFellow PhD degree, Department of Surgery, Faculty of Specialized Veterinary Sciences, Science and Research Branch, Islamic Azad University, Tehran, Iran. Helped with technical procedures, collection and processing of study information
IIIAssociate Professor, Department of Oral & Maxillofacial Pathology, School of Dentistry, Isfahan University of Medical Sciences, Isfahan, Iran. Design, supervised all phases of the study; histological analysis; manuscript writing
IVAssistant Professor, Faculty of Veterinary Science, Kahnooj Branch, Islamic Azad University, Kerman, Iran. Helped with technical procedures, collection and processing of study information
VGraduate student, Faculty of Veterinary Sciences, Karaj Branch, Islamic Azad University, Alborz, Iran. Helped with technical procedures
VIMaster, Department of Physiology, Faculty of Specialized Veterinary Sciences, Science and Research Branch, Islamic Azad University, Tehran, Iran. Biochemical analysis, statistical analysis
Correspondence Correspondence: Gholamreza Jahanshahi Department of Oral & Maxillofacial Pathology, School of Dentistry, Isfahan University of Medical Sciences Isfahan, Iran Phone: +983117922877-79 Fax:+983116687080 ahanshahi@dnt.mui.ac.ir
ABSTRACT
PURPOSE: To investigate whether N-acetylcysteine has a protective effect against renal injury as a remote organ after skeletal muscle ischemia-reperfusion in rats.
METHODS: Twenty Wistar male rats were divided randomly into two experimental groups: group ischemia-reperfusion (group I) and group ischemia-reperfusion + N-acetylcysteine (group II). After ketamine and xylazine anesthesia, femoral artery was exposed. All animals were undergone 2h of ischemia by occlusion femoral artery and 24h of reperfusion. Rats that were treated with N-acetylcysteine given IV at a dose of 150 mg/kg-1, immediately before reperfusion. After 24h of reperfusion, the blood samples were collected and submitted for evaluation of plasmatic urea, creatinine values and then rats were euthanized and left kidney harvested for histopathological analysis under light microscopy.
RESULTS: The urea (35±7.84 mg.dL-1), creatinine (1.46±0.47 mg.dL-1) values were significantly lower in group II (P=0.000). Renal histopathologic study in group I showed extensive distal and proximal tubular cells necrosis and sloughing of epithelial cells into the tubular lumen, cast formation in tubule and glomerul, glomerul fibrosis and hemorrhage. Histopathologically, there was a significant difference (p=0.037) between two groups.
CONCLUSION: The N-acetylcysteine was able to decrease renal injury induced by skeletal muscle ischemia reperfusion in rats.
Key words: Acetylcysteine. Muscle, Skeletal. Ischemia. Reperfusion. Kidney. Rats.
RESUMO
OBJETIVO: Investigar se a N-acetilcisteína tem um efeito protetor contra a lesão renal como um órgão remoto músculo esquelético após isquemia-reperfusão em ratos.
MÉTODOS: Vinte ratos Wistar machos foram distribuídos aleatoriamente em dois grupos experimentais: grupo isquemia-reperfusão (grupo I) e grupo isquemia-reperfusão N-acetilcisteína (grupo II). Após a anestesia de ketamina e xilazina, a artéria femoral foi exposta. Todos os animais foram submetidos a 2h de isquemia pela oclusão da artéria femoral e 24h de reperfusão. Os ratos que foram tratados com N-acetilcisteína administrados IV na dose de 150 mgkg-1, imediatamente antes da reperfusão. Após 24h de reperfusão, as amostras de sangue foram coletadas e submetidas para avaliação de uréia, creatinina e, em seguida, os ratos foram sacrificados e rim esquerdo retirados para estudo histopatológico em microscopia de luz.
RESULTADOS: A uréia (35 ± 7,84 mg.dL-1), creatinina (1,46 ± 0,47 mg.dL-1) os valores foram significativamente menores no grupo II (p=0,000). Estudo histopatológico renal do grupo I mostrou extensa necrose distal e proximal, células tubular e descamação das células epiteliais para o lúmen tubular, formação de elenco no túbulo e glomerulo, fibrose glomerular e hemorragia. Histopatologicamente houve uma diferença significativa (p=0,037) entre os dois grupos.
CONCLUSÃO: A N-acetilcisteína foi capaz de diminuir a lesão renal induzida por reperfusão de isquemia do músculo esquelético em ratos.
Descritores: Acetilcisteína. Músculo Esquelético. Isquemia. Reperfusão. Rim. Ratos.
Introduction
Ischemic and reperfusion injury of the extremities may result in a systemic, severe and complex metabolic syndrome, manifested by acute renal failure, myoglobinuria, metabolic acidosis, hypercalemia and free radicals release1. The risk of revascularization in ischemic extremities may result to renal failure and a clinical entity as myonephropathic-metabolic syndrome2. The pathogenesis of acute renal failure following rhabdomyolysis has been attributed to several mechanisms: 1) Myoglobin nephrotoxicity impair renal function, mainly when dehydratation, acidemia, or both coxists; 2) Primary reduction of glomerular filtration rate due to cortical and glomerular hemodynamic changes due to hypotension after restoration of the blood flow to the extremity; 3) Myoglobin cast producing tubular obstruction and tubular acute necrosis; 4) Release of oxygenderived free radicals mediating back leakage of filtrate through damaged tubular renal epithelium, with loss of renal excretory function1.
Multiple pharmacological agents such as iloprost, vitamin C, pentoxifylline and L-alanyl-glutamin are proposed to be useful against renal injury as a remote organ after hindlimb ischemia-reperfusion2-4.
N-acetyl cystenie is a small molecule containing a thiol group, which has antioxidant properties5. It has been suggested that N-acetylcystenie replenishes glutathione stores, increases superoxide dismutase activity, scavenges hydroxyl free radical and interferes autocatalytic lipid peroxidation6. N-acetylcysteine has proven to be renoprotective in experimental models of both toxic, and ischemic acute renal failure7-9. Recent clinical trials suggest that N-acetylcysteine may be effective in preventing contrast nephropathy10-12.
However its role in reducing the damage in kidney after skeletal muscle ischemia-reperfusion has not been addressed completely yet.
In this experimental study, we aimed to examine the protective effect of N-acetylcysteine on kidney as a remote organ damage after the skeletal muscle ischemia-reperfusion by assessing histopathological and functional analysis in rat model.
Methods
All rats of the present research were cared according to the norms of the Islamic Azad University Faculty of Specialized Veterinary Sciences Tehran Iran laboratory of animal experimentations; this investigation was approved by the Committee of Ethics in Research with animals in Department of Veterinary Surgery too.
Twenty Wistar male rats weighing 250-300 g (12-15 weeks old) were used in this study. All rats were kept at a constant room temperature under standard conditions with food and water ad libitum in individual plastic cages with soft bedding. Animals were divided randomly into two experimental groups of ten rats each: group ischemia-reperfusion (group I) and group ischemia-reperfusion + N-acetylcysteine (group II).
Anesthesia was induced using intramuscular ketamine (50 mg/kg-1) plus xylazine (10 mg/kg-1). After induction of anesthesia, the left hind limb was completely clipped with an electric shaver. After clipping, disinfecting and dropping (using a sterile technique), a skin incision was made on medial surface of the left hind limb. After isolated the femoral artery and vein from the surrounding structures, femoral artery was exposed and clamped with a mini bulldog forceps. Before clamped the femoral artery, 250 IU heparin was administered via the jugular vein to prevent clotting. All animals were undergone 2h of ischemia by occlusion femoral artery with a vascular clamp and 24h of reperfusion13. Rats were maintained in a dorsal recumbency and kept anesthetized throughout the duration of the ischemic period. Additional doses were given as necessary to maintain anesthesia during the experiment. Body temperature was maintained with a heating pad under anesthesia. In group II N-acetylcysteine (150 mg/kg-l) was injected intravenous immediately before reperfusion. Following the ischemic period, the vascular forceps was removed and then surgical site was routinely closed. After surgery, fluid losses were replaced by administration of 5ml of warm (37˚C) isotonic saline i.p, and rats were returned to their cages with food and water ad libitum during the reperfusion period. The analgesic nalbuphine hydrochloride (2 mg/kg-1) was used via subcutaneous during observation time.
After 24h of reperfusion, the blood samples were collected from jugular vein and submitted for evaluation of plasmatic urea, creatinine values and all animals were submitted to laparotomy and the left nephrectomy was performed for histopathological analysis under light microscopy, then rats were euthanized by overdose of intraperitoneal pentobarbital injection (300 mg/kg-l).
The blood samples were analyzed by Automatic DADE AnalyzerTM to determine the biochemical plasmatic measures of urea, creatinine. Renal tissues were placed in 10% formalin solution and processed routinely by embedding in paraffin then tissues were sectioned in 4 µm pieces and stained with Hematoxylin-Eosin stain.
Grading of severity of renal injury was carried out by a pathologist who was blinded to the experiment and data. renal injury was graded into four grades as follows: grade 0 represents no diagnostic change; grade 1 demonstrated tubular cell swelling, brush border loss, nuclear condensation with up to 1/3 of tubular profile showing nuclear loss; grade 2 is as grade 1, but greater than 1/3 and less than 2/3 of tubular profile showing nuclear loss; and grade 3 displayed greater than 2/3 of tubular profile showing nuclear loss14. A total of four slides from each renal sample were randomly screened and the mean was accepted as the representative value of the sample.
Statistical analyses were carried out using SPSS statistical software (version 11.2). Results were expressed as the mean +/- standard deviation. The Mann-Whitney U-test and T-Test were employed to analyze two groups consecutively. Values of P<0.05 were considered as statistically significant.
Results
All of rats tolerated operation and survived until the final study period. data belonging to plasma urea and creatinine measurements from blood samples after reperfusion are shown in table 1. plasma urea and creatinine concentrations were significantly increased in rats group i, compared with group ii (p=0.000).
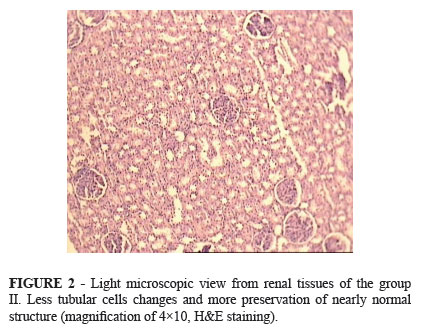
Figure 1 illustrates representative photomicrograph of the renal tissues from group I and Figure 2 illustrates representative photomicrograph of the renal tissues from group II that obtained 24h after reperfusion. Renal histopathologic study in group I showed extensive distal and proximal tubular cells necrosis and sloughing of epithelial cells into the tubular lumen, cast formation in tubule and glomerul, glomerul fibrosis and hemorrhage. Histopathologic examination confirmed the extent of renal change in the group II (1.6±0.84) was significantly lower than group I (2.4±0.70).
Discussion
Ischemic injury occurs in the lower extremities during surgery or trauma. During ischemia, muscle cells cannot keep their membrane integrity and this causes releasing of calcium, phospholipid A2, formation of polyunsaturated fatty acids and fatty acids radicals. If the oxygenation is re-established at that stage of ischemia, fatty acid radicals react with oxygen and undergo lipid peroxidation reaction. This reaction increases the membrane permeability and also stimulates chemotaxis of leukocytes, which release oxygen-derived free radicals and proteolytic enzymes when activated. Activated leukocytes release a variety of inflammatory mediators, including cytokines, neutrophil proteases, and reactive oxygen species. All of these products cause damage to adjacent endothelial cells, and they have been thought to play key roles in tissue injury15. Restoring blood flow to the extremities exposed to ischemia can cause the reperfusion injury by leading to formation of oxygen-derived free radicals that lead to more muscle necrosis than that caused by the ischemia itself16,17. Reperfusion injury develops on remote organs such as lungs, heart, liver, and kidneys that threatening life and makes progress acute renal and respiratory failure, cardiac dysfunction, and even death resulting from systemic toxic effects of reperfusion products known as myonephropathic-metabolic syndrome18-20.
Yassin et al.21 explained that the restoration of blood flow to an acutely ischemic lower limb in rats may, paradoxically, result in systemic complications and unexpected mortality. There was a significant increase in plasma concentrations of urea, creatinine, aspartate transaminase, alanine transaminase, and lactic dehydrogenase in reperfused animals compared with controls. In Teruya et al.3 experimental model of unilateral hindlimb ischemia was associated to significant increase of plasmatic urea (p<0.0399) and creatinine (p<0.0382) shown the function of renal impairment caused by the skeletal muscle ischemia/reperfusion injury.
N-acetylcysteine is a simple and inexpensive water-soluble molecule that contains a sulfhydryl residue. It has long been approved for the prevention of hepatic and renal damage following acetaminophen overdose22. This well documented effect traditionally has been attributed to the restoration of intracellular glutathione levels23,24, required for detoxification of an acetaminophen-derived toxic metabolite. N-acetylcysteine also may attenuate the course of hepatorenal syndrome, a renal vasoconstrictive response of indeterminate nature that develops during advanced liver failure. This effect, shown in experimental settings25 and in a preliminary clinical report26, could imply a better preservation of liver function. However, direct renal protective mechanisms may play a role also, considering the ubiquitous distribution of acylases that catalyze the deacetylation of N-acetylcysteine27. Indeed, recent studies suggest that N-acetylcysteine may increase intracellular glutathione and ameliorate renal ischemia-reflow injury28,29, after inferior vena cava-occlusion30 or kidney damage from cis-platinum31, cyclosporine32, and other nephrotoxic insults33,34. N-acetylcysteine has been reported recently to prevent radiocontrast nephropathy in high-risk patients35.
In the Nitescu et al.36 study, treatment with N-acetylcysteine ameliorated the decline in glomerular filtration rate on day one, and reduced plasma creatinine by~40% on days one and three, after renal ischemia/reperfusion. A similar reduction in plasma creatinine was demonstrated by DiMari et al.37 using high intravenous doses of N-acetylcysteine (1g/kg-1) immediately before and after, bilateral renal ischemia/reperfusion in rats. Teruya et al.3 reported that pentoxifylline has some protecting effect on remote kidney injury due to hindlimb ischemia/reperfusion injury only in the early phase of reperfusion. In our study plasma urea and creatinine concentrations were significantly decreases in group II that treated with N-acetylcysteine.
Teruya et al.3reported that the mesangial enlargement and the tubular cells necrosis were evidences of the remote ischemia/reperfusion renal injury. Nitescu et al.36have previously demonstrated that N-acetylcysteine treated animals with renal ischemia/reperfusion injury showed a significant reduction in renal interstitial inflammation seven days after the ischaemic insult. Our data demonstrate that N-acetylcysteine significantly decreases the severity of acute renal injury after skeletal muscle ischemia-reperfusion injury in rats.
Conclusions
The temporary occlusion of the femoral artery in rats leaded to severe histological tubule-interstitial changes in group I. However, administration of the N-acetylcysteine treatment significantly decreased renal injury induced by skeletal muscle ischemia-reperfusion according to our histological and biochemical findings. These results suggest the possibility of clinical application of N-acetylcysteine on renal injury induced by skeletal muscle ischemia-reperfusion. Different dosages, alternate time protocols and way of N-acetylcysteine administration should be investigated in future studies.
Received: April 18, 2012
Review: June 19, 2012
Accepted: July 20, 2012
Conflict of interest: none
Financial source: none
- 1. Takito AM, Silva JCCB, Bueno V, Franco M, Burihan E. Ischemic and reperfusion syndrome of hind limbs: functional and histological renal changes in rats. Medicina (Ribeirão Preto). 2005;38(3/4):294-300.
- 2. Ozcan AV, Sacar M, Aybek H, Bir F, Demir S, Onem G, Goksin I, Baltalarli A, Colakoglu N. The effects of iloprost and vitamin C on kidney as a remote organ after ischemia/reperfusion of lower extremities. J Surg Res. 2007;140:20-6.
- 3. Teruya R, Fagundes DJ, Oshima CTF, Brasileiro JL, Marks G, Ynouye CM, Simões MJ. The effects of pentoxifylline into the kidneys of rats in a model of unilateral hindlimb ischemia/reperfusion injury. Acta Cir Bras. 2008;23(1):29-35.
- 4. Alves MA, Guimarães SB, Dias DA, Vasconcelos PRC, Coelho VPM, Vasconcelos PRL. Effects of L-alanyl glutamine upon the blood and kidney biochemical parameters in the rat hind limb model of ischemia/reperfusion. Acta Cir Bras. 2005;20(6):445-9.
- 5. McCord JM, Fridovich I. The biology and pathology of oxygen radicals. Ann Intern Med. 1978;89:122-7.
- 6. Dimari J, Megyesi J, Udvarhelyei N, Price P, Davis R, Safirstein R. N-acetylcysteine ameliorates ischemic renal failure. Am J Physiol. 1997;272:292-8.
- 7. Tariq M, Morais C, Sobki S, Al Sulaiman M, Al Khader A. N-acetylcysteine attenuates cyclosporin-induced nephrotoxicity in rats. Nephrol Dial Transplant. 1999;14:923-9.
- 8. Mazzon E, Britti D, De Sarro A, Caputi AP, Cuzzocrea S. Effect of N-acetylcysteine on gentamicin-mediated nephropathy in rats. Eur J Pharmacol. 2001;424:75-83.
- 9. Conesa EL, Valero F, Nadal JC, Fenoy FJ, López B, Arregui B, Salom MG. N-acetyl-L-cysteine improves renal medullary hypoperfusion in acute renal failure. Am J Physiol Regul Integr Comp Physiol. 2001;281:730-7.
- 10. Birck R, Krzossok S, Markowetz F, Schnülle P, van der Woude FJ, Braun C. Acetylcysteine for prevention of contrast nephropathy: meta-analysis. Lancet. 2003;362:598-603.
- 11. Tepel M, Van der Giet M, Schawarzfeld C, Laufer U, Liermann D, Zidek W. Prevention of radiographic-contrast-agent-induced reductions in renal function by acetylcysteine. N Engl J Med. 2000;343:180-4.
- 12. Drager LF, Andrade L, Barros de Toledo JF, R M Laurindo F, César LAM, Seguro AC. Renal effects of N-acetylcysteine in patients at risk for contrast nephropathy: decrease in oxidant stress-mediated renal tubular injury. Nephrol Dial Transplant. 2004;19:1803-7.
- 13. Eberlin KR, McCormack MC, Nguyen JT, Tatlidede HS, Randolph MA, Austen Jr W. Ischemic preconditioning of skeletal muscle mitigates remote injury and mortality. J Surg Res. 2008;148:24-30.
- 14. Chatterjee PK, Patel NS, Kvale EO, Cuzzocrea S, Brown PA, Stewart KN, Mota-Filipe H, Thiemermann C. Inhibition of inducible nitric oxide synthase reduces renal ischemia/reperfusion injury. Kidney Int. 2002;61:862-71.
- 15. Kearns SR, Kelly CJ, Barry M, Abdih H, Condron C, Leahy A, Bouchier-Hayes D. Vitamin C reduces ischaemia-reperfusion-induced acute lung injury. Eur J Vasc Endovasc Surg. 1999;17:533-6.
- 16. Akar H, Sarac A, Konuralp C, Yildiz L, Kolbakir F. Comparison of histopathologic effects of carnitine and Vit C on reperfusion injury. Eur J Cardiothorac Surg. 2001;19:500-6.
- 17. Demir S, Inal-Erden M. Pentoxifylline and N-acetylcysteine in hepatic ischemia/reperfusion injury. Clin Chim Acta. 1998;275(2):127-35.
- 18. Parrino PE, Laubach VE, Gaughen JR, Shockey KS, Wattsman TA, King RC, Tribble CG, KronIL. Inhibition of inducible nitric oxide synthase after myocardial ischemia increases coronary flow. Ann Thorac Surg. 1998;66(3):733-9.
- 19. Koksel O, Ozdulger A, Aytacoglu B, Tamer L, Polat A, Sucu N, Yildirim C, Degirmenci U, Kanik A. The influence of iloprost on acute lung injury induced by hind limb ischemia-reperfusion in rats. Pulm Pharmacol Ther. 2005;18(4):235-41.
- 20. Harris K, Walker PM, Mickle DA, Harding R, Gatley R, Wilson GJ, Kuzon B, McKee N, Romaschin AD. Metabolic response of skeletal muscle to ischemia. Am J Physiol. 1986;250:213-20.
- 21. Yassin MM, Harkin DW, Barros D'Sa AA, Halliday MI, Rowlands BJ: Lower limb ischemia-reperfusion injury triggers a systemic inflammatory. World J Surg. 2002;26(1):115-21.
- 22. Smilkstein MJ, Knapp GL, Kulig KW, Rumack BH. Efficacy of oral N-acetylcysteine in the treatment of acetaminophen overdose. Analysis of the national multicenter study (1976 to 1985). N Engl J Med. 1988;319:1557-62.
- 23. Corcoran GB, Wong BK. Role of glutathione in prevention of acetaminophen-induced hepatotoxicity by N-acetyl-L-cysteine in vivo: studies with N-acetyl-D-cysteine in mice. J Pharmacol Exp Ther. 1986;238:54-61.
- 24. Grattagliano I, Vendemiale G, Deiss VB, Junker E, Lauterburg BH. Effect of oral and intravenous S,N-diacetylcysteine monoethyl ester on circulating and hepatic sulfhydryls in the rat. J Pharmacol Exp Ther. 2000;294:155-9.
- 25. Simko V, Michael S, Katz J, Oberstein E, Popescu A. Protective effect of oral acetyl- cysteine against the hepatorenal toxicity of carbon tetrachloride potentiated by ethyl alcohol. Alcohol Clin Exp Res. 1992;16:795-9.
- 26. Holt S, Goodier D, Marley R, Patch D, Burroughs A, Fernando B, Harry D, Moore K. Improvement in renal function in hepatorenal syndrome with N-acetylcysteine. Lancet. 1999;353:294-5.
- 27. Uttamsingh V, Baggs RB, Krenitsky DM, Anders MW. Immunohistochemical localization of the acylases that catalyze the deacetylation of N-acetyl-L-cysteine and haloalkene-derived mercapturates. Drug Metab Dispos. 2000;28:625-32.
- 28. Slusser SO, Grotyohann LW, Martin LF, Scaduto RC Jr. Glutathione catabolism by the ischemic rat kidney. Am J Physiol. 1990;258(6 Pt 2):1546-53.
- 29. Pincemail J, Defraigne JO, Detry O, Franssen C, Meurisse M, Limet R. Ischemia-reperfusion injury of rabbit kidney: Comparative effects of desferrioxamine and N-acetylcysteine as antioxidants. Transplant Proc. 2000;32:475-6.
- 30. Conesa EL, Valero F, Nadal JC, Fenoy FJ, López B, Arregui B, Salom MG. N-acetyl-L-cysteine im proves renal medullary hypoperfusion in acute renal failure. Am J Physiol Regul Integr Comp Physiol. 2001;281(3):R730-7.
- 31. Sheikh-Hamad D, Timmins K, Jalali Z. Cisplatin-induced renal possible reversal by N-acetylcysteine treatment. J Am Soc Nephrol. 1997;8:1640-4.
- 32. Tariq M, Morais C, Sobki S, Al Sulaiman M, Al Khader A. N-acetylcysteine attenuates cyclosporin-induced nephrotoxicity in rats. Nephrol Dial Transplant. 1999;14:923-9.
- 33. Wispriyono B, Matsuoka M, Igisu H, Matsuno K. Protection from cadmium cytotoxicity by N-acetylcysteine in LLC-PK1 cells. J Pharmacol Exp Ther. 1998;287:344-51.
- 34. Girardi G, Elias MM. Effectiveness of N-acetylcysteine in protecting against mercuric chloride-induced nephrotoxicity. Toxicology. 1991;67:155-64.
- 35. Tepel M, van der Giet M, Schwarzfeld C, Laufer U, Liermann D, Zidek W. Prevention of radiographic-contrast-agent-induced reductions in renal function by acetylcysteine. N Engl J Med. 2000;343:180-4.
- 36. Nitescu N, Ricksten SE, Marcussen N, Haraldsson B, Nilsson U, Basu S, Guron G. N-acetylcysteine attenuates kidney injury in rats subjected to renal ischaemia-reperfusion. Nephrol Dial Transplant. 2006;21:1240-7.
- 37. DiMari J, Megyesi J, Udvarhelyi N, Price P, Davis R, Safirstein R. N-acetyl cysteine ameliorates ischemic renal failure. Am J Physiol Renal Physiol. 1997;272:292-8.
Publication Dates
-
Publication in this collection
27 Aug 2012 -
Date of issue
Sept 2012
History
-
Received
18 Apr 2012 -
Accepted
20 July 2012 -
Reviewed
19 June 2012