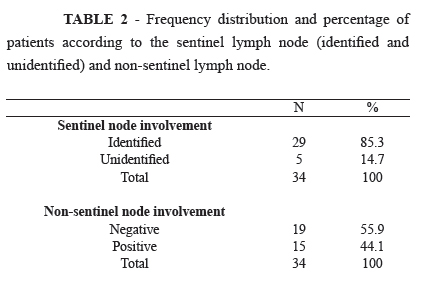
PURPOSE: To check the rate of sentinel lymph node (SLN) identification in patients with locally advanced breast cancer who underwent neoadjuvant chemotherapy comparing intraoperative contact cytology (imprint) and embedded in paraffin and validation of methods. METHODS: A cross-sectional validation of diagnostic test involving 34 patients from the outpatient clinic of the Maternity School Assis Chateaubriand. The patients had locally advanced breast cancer and were treated with neoadjuvant chemotherapy. Those with clinically negative axilla underwent SLN biopsy, studied by imprint and histopathology in paraffin. All patients underwent axillary dissection and its histopathological study. RESULTS: The SLN identification rate was 85.3% (29/34). The sensitivity of imprint associated with paraffin on detection of metastasis compared to histopathology of the axillary content was 84.62% and specificity of 100% with false-negative rate of 12.01% and an accuracy of 92.77%. CONCLUSION: The search for metastases in the SLN by imprint and histopathological analysis in paraffin compared to the gold standard (axillary dissection) had a low sensitivity with high rate of false negatives in our sample.
Sentinel Lymph Node Biopsy; Breast Neoplasm; Neoadjuvant Therapy