ABSTRACT
As pregnant women are susceptible to changes in iodine, which can cause miscarriage, goiter, thyroid nodules, hypothyroidism, in addition to fetal neurological impairment or development. The aim of this study was to verify the implications of the iodine alteration in each gestational trimester and its consequences of physiological justification. The review was based on PRISMA. Searching for articles that took place in March 2020 without delimiting data. As bases consulted were the Clinical Trials, Cochrane Library, Lilacs and Medline (PubMed). The descriptors were combined as follows: "pregnancy" AND "iodine deficiency". Articles that addressed iodine deficiency and its implications were included. The selection followed the steps of reading the titles, abstracts and full articles. To assess the methodological quality of the studies, the STROBE Instruction instrument was used. The research resulted in 1,266 studies and 11 were included. In assessing methodological quality, the lowest score was and the maximum 20. According to studies, the fourth most affected by iodine loss are the second and third, it is possible to increase the volume and pneumatic nodules, subclinical hypothyroidism, pre-eclampsia, among others. The damages caused by iodine deficiency in the first or second trimester are still reversible, therefore, they need to be diagnosed early, to guarantee an iodic homeostasis and prevent damage to the health of the mother-child binomial.
Keywords
Implications; iodine deficiency; pregnancy
INTRODUCTION
Gestation is a period of two great vulnerabilities: biological, because there is intense growth and fetal development and; nutritional, due to increased energy requirements (11. Ghassabian A, Graaff JS, Peeters RP, Ross HA, Jaddoe VW, Hofman A, et al. Maternal urinary iodine concentration in pregnancy and children's cognition: results from a population-based birth cohort in an iodine-sufficient area. BMJ Open. 2014;4:10-5.). Therefore, it is necessary to make pregnant women aware of the importance of adequate feeding, in order to prevent health problems caused by nutritional deficiencies (22. Xiao Y, Sun H, Li C, Li Y, Peng S, Fan C, et al. Effect of Iodine Nutrition on Pregnancy Outcomes in an Iodine-Sufficient Area in China. Biol Trace Elem Res. 2018;182(2):231-7.).
Iodine is an essential micronutrient for the regulation of metabolic processes and for the synthesis of thyroid hormones responsible for the development of the central nervous system in the embryonic period (11. Ghassabian A, Graaff JS, Peeters RP, Ross HA, Jaddoe VW, Hofman A, et al. Maternal urinary iodine concentration in pregnancy and children's cognition: results from a population-based birth cohort in an iodine-sufficient area. BMJ Open. 2014;4:10-5.). Pregnant women are more susceptible to the deficiency of this mineral due to the transfer of hormones to the fetus and increased glomerular filtration, leading to iodine loss in the urine (22. Xiao Y, Sun H, Li C, Li Y, Peng S, Fan C, et al. Effect of Iodine Nutrition on Pregnancy Outcomes in an Iodine-Sufficient Area in China. Biol Trace Elem Res. 2018;182(2):231-7.).
Iodine deficiency during pregnancy can lead to spontaneous abortion, goiter, thyroid nodules, hypothyroidism, and compromise fetal neurological development. Thus, there is an increase with expenses on health and education, generating social and economic losses for the countries (33. Torlinska B, Bath SC, Janjua A, Boelaert K, Chan S. Iodine Status during Pregnancy in a Region of Mild-to-Moderate Iodine Deficiency is not Associated with Adverse Obstetric Outcomes; Results from the Avon Longitudinal Study of Parents and Children. Nutrients. 2018;291:1-13.).
Studies have already evaluated the damage caused by insufficient iodine intake, but there is not yet a compiled study in the literature to highlight the implications that iodine deficiency can cause during pregnancy. This systematic review was based on PRISMA and sought to answer the guiding question “what are the implications of iodine deficiency in each gestational trimester?” Therefore, the objective of this review was to verify the implications of iodine deficiency in each gestational trimester and their respective physiological justifications.
METHODS
It is a systematic review, based on the recommendations of the Preferred Reporting Items for Systematic Reviews (PRISMA) (44. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009;6(7):e1000100.). The guiding question was “What are the implications of iodine deficiency in each gestational trimester?” To define this question, the PECOS criteria were used, as shown in Table 1.
We have registered the article at the International Prospective Register of Ongoing Systematic Reviews (PROSPERO) with identification: CRD42019129885.
The search occurred in March 2020 without date delimitation. The databases consulted were Clinical Trials, Cochrane Library Center, Latin American and Caribbean Literature in Health Sciences (Lilacs) and Publisher Medline (PubMed). The descriptors determined by the Health Science Descriptor system (DeCS) were combined as follows: “pregnancy AND “iodine deficiency” in English, Portuguese and Spanish, using the terms “human” and “women” as filters.
We have included articles that addressed iodine deficiency and implications during pregnancy. We have excluded systematic review papers, experimental papers, government documents, studies on other micronutrients that did not include iodine, iodine supplementation/fortification, salt iodization monitoring, thyroid function assessment (without considering the deficiency of iodine), other age groups and other subjects.
The selection of articles was conducted by two researchers, independently. In case of disagreement regarding the inclusion or not of a particular study, a third researcher was consulted. The titles, abstracts and full articles were read; if they did not meet the inclusion criteria, the studies were eliminated.
After the selection, we conducted a qualitative synthesis of the articles included in this review, systematizing the most relevant information, such as: authors, study design, main results and conclusions.
In order to assess the methodological quality of the articles, we used the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE Statement) instrument (55. Malta M, Cardoso LO, Bastos IF, Magnanini MMF, Silva CMFP. Iniciativa STROBE: subsídios para a comunicação de estudos observacionais STROBE initiative: guidelines on. Rev Saúde Pública. 2010;44(3):559-65.), which contains a list of 22 items to verify the information that should be present in the title, abstract, introduction, methodology, results and discussion of scientific articles. Responses were scored as “1” (when the criterion that characterized quality was present) or “0” (when the criterion was absent). The studies were classified in scores from zero (worst quality) to 22 (best quality).
RESULTS
The search resulted in 1,266 studies. After excluding the duplications by base and between the bases, 760 remained. Afterwards, we made the selection, through the steps of reading the titles, abstracts and full article. At the end, we included 11 articles (Figure 1).
Of the included studies, 63.6% were of longitudinal design, 27.3% were cross-sectional and 9.1% were control cases. The years ranged from 2009 (88. Cuellar-Rufino S, Navarro-Meza M, García-Solis P, Xochihua-Rosas I, Arroyo-Helguera O. Iodine levels are associated with oxidative stress and antioxidant status in pregnant women with hypertensive disease. Nutr Hosp. 2017;34(3):661-6.,99. Joshi K, Nair S, Khade C, Rajan MG. Early gestation screening of pregnant women for iodine deficiency disorders and iron deficiency in urban centre in Vadodara, Gujarat, India. J Dev Orig Health Dis. 2014;5(1):62-8.) to 2018 (33. Torlinska B, Bath SC, Janjua A, Boelaert K, Chan S. Iodine Status during Pregnancy in a Region of Mild-to-Moderate Iodine Deficiency is not Associated with Adverse Obstetric Outcomes; Results from the Avon Longitudinal Study of Parents and Children. Nutrients. 2018;291:1-13.) and the sample size from 57 (88. Cuellar-Rufino S, Navarro-Meza M, García-Solis P, Xochihua-Rosas I, Arroyo-Helguera O. Iodine levels are associated with oxidative stress and antioxidant status in pregnant women with hypertensive disease. Nutr Hosp. 2017;34(3):661-6.) to 5256 (99. Joshi K, Nair S, Khade C, Rajan MG. Early gestation screening of pregnant women for iodine deficiency disorders and iron deficiency in urban centre in Vadodara, Gujarat, India. J Dev Orig Health Dis. 2014;5(1):62-8.). The main results of the studies are described in Table 2.
The consequences of iodine deficiency differed according to the gestational trimester. Figure 2 presents, according to the included studies, the main damages identified when the pregnant woman has insufficient intake of iodine.
Summary of the implications caused by iodine deficiency in each gestational trimester observed in the articles included in the systematic review.
In the assessment of the methodological quality of the studies according to STROBE Statement, the lowest score was 15 (27.3%) and the highest 20 points (9%), indicating good methodological quality among the included studies and a satisfactory level of reliability for the presented results.
DISCUSSION
Iodine deficiency disorders (IDD) occur when the pregnant woman has insufficient iodine intake, which results in a lower production of thyroid hormones, negatively affecting the child's muscle, heart, liver, kidneys and brain development (33. Torlinska B, Bath SC, Janjua A, Boelaert K, Chan S. Iodine Status during Pregnancy in a Region of Mild-to-Moderate Iodine Deficiency is not Associated with Adverse Obstetric Outcomes; Results from the Avon Longitudinal Study of Parents and Children. Nutrients. 2018;291:1-13.).
The implications of iodine deficiency in pregnancy differ according to the degree of deficiency and gestational trimester. In light deficiency the process is continuous, while the others present a summation effect and independent unfolding (Figure 3). This division is essential to guide strategies to control and prevent these health implications.
Implications for the pregnant woman according to the degree of iodine deficiency (22. Xiao Y, Sun H, Li C, Li Y, Peng S, Fan C, et al. Effect of Iodine Nutrition on Pregnancy Outcomes in an Iodine-Sufficient Area in China. Biol Trace Elem Res. 2018;182(2):231-7.,1111. Vidal ZE, Rufino SC, Tlaxcalteco EH, Trejo CH, Campos RM, Meza MN, et al. Oxidative Stress Increased in Pregnant Women with Iodine Deficiency. Biol Trace Elem Res. 2014;157:211-7.,1313. Charoenratana C, Leelapat P, Traisrisilp K, Tongsong T. Maternal iodine insufficiency and adverse pregnancy outcomes. Matern Child Nutr. 2016;12:680-7.,1414. World Health Organization (WHO). Assessment of the iodine deficiency disorders and monitoring their elimination. WHO: Geneva; 2007. Available from: http://apps.who.int/iris/bitstream/10665/43781/1/9789241595827_eng.pdf.
http://apps.who.int/iris/bitstream/10665... ).
First trimester
In the first trimester of gestation, the blood flow and glomerular filtration increase resulting in loss of iodine in urine. The organism, as an adaptation mechanism, increases the secretion of the hormone human chorionic gonadotrophin and thyroid stimulating hormone (TSH), which induces the thyroid to increase the concentration of free thyroxine (T4), guaranteeing the production of thyroid hormones (1515. Glinoer D. Clinical and Biological Consequences of Iodine Deficiency during Pregnancy. Endocr Dev. 2007;10:62-85.).
The light iodine deficiency acted as a risk factor for diabetes mellitus and placental abruption in a study conducted in China. According to the authors, the lack of iodine in the organism induces the increase of TSH secretion in plasma, producing an antagonistic effect on insulin, generating hyperglycemia; which is associated with increased oxidative stress, which is a risk factor for placental abruption (22. Xiao Y, Sun H, Li C, Li Y, Peng S, Fan C, et al. Effect of Iodine Nutrition on Pregnancy Outcomes in an Iodine-Sufficient Area in China. Biol Trace Elem Res. 2018;182(2):231-7.).
These results corroborate a study conducted in Mexico, where oxidative stress was higher in pregnant women with iodine deficiency in the first trimester, with reduction of antioxidant activity and superoxide dismutase (SOD). Due to oxidative stress, there is an increase in the production of free radicals, which cause hypertension and endothelial dysfunction, which can lead to miscarriage and pre-eclampsia (1111. Vidal ZE, Rufino SC, Tlaxcalteco EH, Trejo CH, Campos RM, Meza MN, et al. Oxidative Stress Increased in Pregnant Women with Iodine Deficiency. Biol Trace Elem Res. 2014;157:211-7.).
Severe iodine deficiency, in addition to affecting the mother, also causes harm to her child. A longitudinal study conducted in England with pregnant women found the influence of iodine deficiency on the cognitive ability of their respective children. Children of descendants of women with severe disabilities presented results below the intelligence quotient (IQ) ideal, presenting speech difficulties at 8 and 9 years of age in reading accuracy and comprehension. The low socioeconomic level of the participants associated with insufficient iodine intake may have been determinant for these detected results (1010. Bath SC, Steer CD, Golding J, Emmett P, Rayman MP. Effect of inadequate iodine status in UK pregnant women on cognitive outcomes in their children: results from the Avon Longitudinal Study of Parents and Children (ALSPAC). Lancet. 2013;6736(13):1-7.).
Some authors suggest that iodine supplementation should ideally be performed in the first trimester, since it would be possible to maintain iodine reserves in the body, guaranteeing the production of thyroid hormones and avoiding damage to maternal and child health (22. Xiao Y, Sun H, Li C, Li Y, Peng S, Fan C, et al. Effect of Iodine Nutrition on Pregnancy Outcomes in an Iodine-Sufficient Area in China. Biol Trace Elem Res. 2018;182(2):231-7.,1010. Bath SC, Steer CD, Golding J, Emmett P, Rayman MP. Effect of inadequate iodine status in UK pregnant women on cognitive outcomes in their children: results from the Avon Longitudinal Study of Parents and Children (ALSPAC). Lancet. 2013;6736(13):1-7.,1111. Vidal ZE, Rufino SC, Tlaxcalteco EH, Trejo CH, Campos RM, Meza MN, et al. Oxidative Stress Increased in Pregnant Women with Iodine Deficiency. Biol Trace Elem Res. 2014;157:211-7.).
Second trimester
In the second trimester of pregnancy, TSH levels return to normal and fetal development becomes indirectly dependent on the maternal thyroid because the fetal thyroid, even when immature, also begins to produce hormones (1515. Glinoer D. Clinical and Biological Consequences of Iodine Deficiency during Pregnancy. Endocr Dev. 2007;10:62-85.).
In a study conducted in India, 16.4% of pregnant women with low iron intake showed an increase in corpuscular volume and glomerular filtration, being diagnosed with iron and iodine deficiency (99. Joshi K, Nair S, Khade C, Rajan MG. Early gestation screening of pregnant women for iodine deficiency disorders and iron deficiency in urban centre in Vadodara, Gujarat, India. J Dev Orig Health Dis. 2014;5(1):62-8.). In Bangladesh, 39.4% of anemic pregnant women had iodine deficiency and, due to the nutritional vulnerability caused by the association of these two deficiencies, pregnant women were more likely to have diarrhea/dysentery, pneumonia and ear infection (77. Harun-Or-Rashid M, Khatun UF, Yoshida Y, Morita S, Chowdhury N, Sakamoto J. Iron and iodine deficiencies among under-2 children, adolescent girls, and pregnant women of Bangladesh: association with common diseases. Nagoya J Med Sci. 2009;71(1-2):39-49.).
In Thailand, a study showed that iodine deficiency was higher in the second trimester, increasing the risk of pre-eclampsia, fetal growth restriction, low birth weight and prematurity (1313. Charoenratana C, Leelapat P, Traisrisilp K, Tongsong T. Maternal iodine insufficiency and adverse pregnancy outcomes. Matern Child Nutr. 2016;12:680-7.).
Ghassabian and cols. assessed the association between iodine deficiency during pregnancy and IQ in their 6-year-old children. The authors observed an association between insufficient iodine intake and sub-optimal nonverbal IQ in children. However, after adjusting for confounders, there was no association between non-verbal IQ and deficiency (11. Ghassabian A, Graaff JS, Peeters RP, Ross HA, Jaddoe VW, Hofman A, et al. Maternal urinary iodine concentration in pregnancy and children's cognition: results from a population-based birth cohort in an iodine-sufficient area. BMJ Open. 2014;4:10-5.).
According to the studies, mild iodine deficiency, if diagnosed in the second trimester of pregnancy, it is still possible to resolve the implications caused by the deficiency through iodine supplementation (11. Ghassabian A, Graaff JS, Peeters RP, Ross HA, Jaddoe VW, Hofman A, et al. Maternal urinary iodine concentration in pregnancy and children's cognition: results from a population-based birth cohort in an iodine-sufficient area. BMJ Open. 2014;4:10-5.,77. Harun-Or-Rashid M, Khatun UF, Yoshida Y, Morita S, Chowdhury N, Sakamoto J. Iron and iodine deficiencies among under-2 children, adolescent girls, and pregnant women of Bangladesh: association with common diseases. Nagoya J Med Sci. 2009;71(1-2):39-49.,99. Joshi K, Nair S, Khade C, Rajan MG. Early gestation screening of pregnant women for iodine deficiency disorders and iron deficiency in urban centre in Vadodara, Gujarat, India. J Dev Orig Health Dis. 2014;5(1):62-8.,1313. Charoenratana C, Leelapat P, Traisrisilp K, Tongsong T. Maternal iodine insufficiency and adverse pregnancy outcomes. Matern Child Nutr. 2016;12:680-7.).
Third trimester
In the third trimester of gestation, thyroid hormone-dependent neurogenesis is still underway and if iodine deficiency is present since the first trimester, the implications may be irreversible (88. Cuellar-Rufino S, Navarro-Meza M, García-Solis P, Xochihua-Rosas I, Arroyo-Helguera O. Iodine levels are associated with oxidative stress and antioxidant status in pregnant women with hypertensive disease. Nutr Hosp. 2017;34(3):661-6.).
A study conducted in Spain showed that pregnant women with iodine deficiency in the third trimester are more likely to have a low weight newborn and Small for Gestational Age (SGA), due to fetal growth restriction and low production of thyroid hormones (66. Alvarez-Pedrerol M, Guxens M, Mendez M, Canet Y, Martorell R, Espada M, et al. Iodine levels and thyroid hormones in healthy pregnant women and birth weight of their offspring. Eur J Endocrinol. 2009;160:423-9.).
Iodine has an important antioxidant function. Its deficiency can increase the levels of oxidative stress, causing the development of complications during pregnancy and hypertensive disorders. In a study conducted in Mexico, iodine deficiency during gestation was associated with hypertension, lower SOD activity and higher oxidative stress. According to the authors, these results are worrying because they can cause placental abruption, eclampsia and even hemorrhages (88. Cuellar-Rufino S, Navarro-Meza M, García-Solis P, Xochihua-Rosas I, Arroyo-Helguera O. Iodine levels are associated with oxidative stress and antioxidant status in pregnant women with hypertensive disease. Nutr Hosp. 2017;34(3):661-6.).
The main target of iodine deficiency in pregnancy is the thyroid. Due to the mechanism of autoregulation, there is an increase in the secretion of TSH that overestimates this gland, leading to an increase in thyroid volume and the formation of nodules (1212. Sahin SB, Ogullar S, Ural UM, Ilkkilic K, Metin Y, Ayaz T. Alterations of thyroid volume and nodular size during and after pregnancy in a severe iodine-deficient area. Clin Endocrinol (Oxf). 2014;81:762-8.).
A study conducted in Turkey, 50% of iodine deficient multiparous women had thyroid nodules that were increasing in volume during pregnancy, with the largest diameter detected in the third trimester; however, the quantitative of nodules remained the same. According to the authors, these results may be justified by the fact that pregnant women with nodules are older, have more children and have a worse socioeconomic status than those without nodules. Also, because of the compression that the placenta exerts under the bladder, the pregnant women increase the urinary frequency in the last trimester, leading to a negative balance of circulatory iodine, causing thyroid nodules (1212. Sahin SB, Ogullar S, Ural UM, Ilkkilic K, Metin Y, Ayaz T. Alterations of thyroid volume and nodular size during and after pregnancy in a severe iodine-deficient area. Clin Endocrinol (Oxf). 2014;81:762-8.).
On the other hand, a study in England that analyzed the impact of iodine deficiency in all trimesters of pregnancy found no difference between the incidence of pre-eclampsia, hypertension, gestational diabetes, glycosuria, anemia, preterm birth, SGA babies and hemorrhage postpartum among pregnant women with deficiency and adequacy of iodine status (33. Torlinska B, Bath SC, Janjua A, Boelaert K, Chan S. Iodine Status during Pregnancy in a Region of Mild-to-Moderate Iodine Deficiency is not Associated with Adverse Obstetric Outcomes; Results from the Avon Longitudinal Study of Parents and Children. Nutrients. 2018;291:1-13.).
Strengths and limitations
Strengths: the work was conducted in different regions, representativeness of the samples and the review was a compilation of information subdivided according to the trimesters, which will contribute to clinical practice and generation of evidence, which will be important for diagnosis and consequent decision making, thus avoiding future harm to the child.
The limitation was that only 63.6% of the included studies were of a longitudinal design, that is, the other studies did not allow to verify the causality, only to identify associations between iodine deficiency and the implication identified in that period of pregnancy.
Final remarks
The consequences caused by iodine deficiency vary according to the trimester of gestation. The most affected trimesters in the studies assessed were the second and third, probably due to the neurological development of the fetus that increase the nutritional need of the mother.
However, if such implications still occur in the first or second trimester, there is a possibility of reversal. Therefore, the early diagnosis of iodine deficiency during the gestational period is essential, since it provides guidance for adequate intake, either dietary or supplementation, guaranteeing iodine homeostasis and preventing damage to maternal and child health.
Acknowledgements:
we would like to thank the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) – Financing Code 001. Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), case 408295/2017-1. Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG), case APQ-03336-18.
REFERENCES
-
1Ghassabian A, Graaff JS, Peeters RP, Ross HA, Jaddoe VW, Hofman A, et al. Maternal urinary iodine concentration in pregnancy and children's cognition: results from a population-based birth cohort in an iodine-sufficient area. BMJ Open. 2014;4:10-5.
-
2Xiao Y, Sun H, Li C, Li Y, Peng S, Fan C, et al. Effect of Iodine Nutrition on Pregnancy Outcomes in an Iodine-Sufficient Area in China. Biol Trace Elem Res. 2018;182(2):231-7.
-
3Torlinska B, Bath SC, Janjua A, Boelaert K, Chan S. Iodine Status during Pregnancy in a Region of Mild-to-Moderate Iodine Deficiency is not Associated with Adverse Obstetric Outcomes; Results from the Avon Longitudinal Study of Parents and Children. Nutrients. 2018;291:1-13.
-
4Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009;6(7):e1000100.
-
5Malta M, Cardoso LO, Bastos IF, Magnanini MMF, Silva CMFP. Iniciativa STROBE: subsídios para a comunicação de estudos observacionais STROBE initiative: guidelines on. Rev Saúde Pública. 2010;44(3):559-65.
-
6Alvarez-Pedrerol M, Guxens M, Mendez M, Canet Y, Martorell R, Espada M, et al. Iodine levels and thyroid hormones in healthy pregnant women and birth weight of their offspring. Eur J Endocrinol. 2009;160:423-9.
-
7Harun-Or-Rashid M, Khatun UF, Yoshida Y, Morita S, Chowdhury N, Sakamoto J. Iron and iodine deficiencies among under-2 children, adolescent girls, and pregnant women of Bangladesh: association with common diseases. Nagoya J Med Sci. 2009;71(1-2):39-49.
-
8Cuellar-Rufino S, Navarro-Meza M, García-Solis P, Xochihua-Rosas I, Arroyo-Helguera O. Iodine levels are associated with oxidative stress and antioxidant status in pregnant women with hypertensive disease. Nutr Hosp. 2017;34(3):661-6.
-
9Joshi K, Nair S, Khade C, Rajan MG. Early gestation screening of pregnant women for iodine deficiency disorders and iron deficiency in urban centre in Vadodara, Gujarat, India. J Dev Orig Health Dis. 2014;5(1):62-8.
-
10Bath SC, Steer CD, Golding J, Emmett P, Rayman MP. Effect of inadequate iodine status in UK pregnant women on cognitive outcomes in their children: results from the Avon Longitudinal Study of Parents and Children (ALSPAC). Lancet. 2013;6736(13):1-7.
-
11Vidal ZE, Rufino SC, Tlaxcalteco EH, Trejo CH, Campos RM, Meza MN, et al. Oxidative Stress Increased in Pregnant Women with Iodine Deficiency. Biol Trace Elem Res. 2014;157:211-7.
-
12Sahin SB, Ogullar S, Ural UM, Ilkkilic K, Metin Y, Ayaz T. Alterations of thyroid volume and nodular size during and after pregnancy in a severe iodine-deficient area. Clin Endocrinol (Oxf). 2014;81:762-8.
-
13Charoenratana C, Leelapat P, Traisrisilp K, Tongsong T. Maternal iodine insufficiency and adverse pregnancy outcomes. Matern Child Nutr. 2016;12:680-7.
-
14World Health Organization (WHO). Assessment of the iodine deficiency disorders and monitoring their elimination. WHO: Geneva; 2007. Available from: http://apps.who.int/iris/bitstream/10665/43781/1/9789241595827_eng.pdf
» http://apps.who.int/iris/bitstream/10665/43781/1/9789241595827_eng.pdf -
15Glinoer D. Clinical and Biological Consequences of Iodine Deficiency during Pregnancy. Endocr Dev. 2007;10:62-85.
Publication Dates
-
Publication in this collection
28 Aug 2020 -
Date of issue
Sep-Oct 2020
History
-
Received
18 May 2020 -
Accepted
20 July 2020



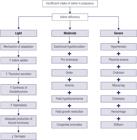

 Source: PRISMA (
Source: PRISMA (
