ABSTRACT.
Elasmopalpus lignosellus (Zeller, 1848) (Lepidoptera, Pyralidae) is an insect pest of 60 economically important crops, including sugarcane, wheat, soybean, rice, beans, sorghum, peanuts, and cotton. The aim of this work was to select and characterize Bacillus thuringiensis isolates with insecticidal activity against E. Lignosellus that could be used as an alternative method of control. Selective bioassays were done to evaluate the toxicity of 47 isolates against first instar larvae of E. lignosellus. For the most toxic bacterial strains, the lethal concentration (LC50) was estimated and morphological, biochemical and molecular methods were used to characterize the isolates. Among the 47 isolates tested, 12 caused mortality above 85% and showed LC50 values from 0.038E+8 to 0.855E+8 spores mL-1. Isolates BR83, BR145, BR09, BR78, S1534, and S1302 had the lowest LC50 values and did not differ from the standard HD-1 strain; the exception was BR83.The protein profiles produced bands with molecular masses of 60-130 kDa. The genes cry1, cry2, cry3, and cry11 were identified in the molecular characterization. The morphological analysis identified three different crystal inclusions: bipyramidal, spherical and cuboidal. Among the tested isolates, 12 isolates have potential for biotechnological control of E. Lignosellus by development of new biopesticides or genetically modified plants.
Keywords:
biological control; cry genes; entomopathogenic bacteria; lesser cornstalk borer
RESUMO.
Elasmopalpus lignosellus (Zeller, 1848) (Lepidoptera, Pyralidae) é considerada praga para mais de 60 espécies de plantas cultivadas como o milho, cana-de-açúcar, trigo, soja, arroz, feijão, sorgo, amendoim e algodão. O objetivo deste trabalho foi selecionar e caracterizar isolados de Bacillus thuringiensis virulentos a E. lignosellus atuando como método alternativo de controle. Assim, 47 isolados foram avaliados em bioensaios seletivos contra lagartas de 1º ínstar de E. lignosellus. Para os isolados mais tóxicos, a concentração letal (CL50) foi estimada e a sua caracterização foi realizada por métodos morfológicos, bioquímicos e moleculares. Dos 47 isolados testados, 12 causaram mortalidade acima 85% e apresentaram CL50 entre 0.038E+8 a 0.855E+8 esporos mL-1. Os menores valores de CL50 foram obtidos pelos isolados BR83, BR145, BR09, BR78, S1534 e S1302, os quais não diferiram da linhagem padrão HD-1, com exceção do BR83. O perfil proteico revelou proteínas Cry entre 60 e 130 kDa, e a caracterização molecular mostrou a presença dos genes cry1, cry2, cry3 e cry11. A análise morfológica identificou três diferentes inclusões cristalinas: bipiramidais, esféricas e cuboides. Entre os isolados avaliados, 12 apresentam potencial biotecnológico para controle de E. lignosellus via formulação de novos bioinseticida ou produção de plantas transgênicas.
Palavras-chave:
controle biológico; genes cry; bactéria entomopatogênica; broca-do-colo
Introduction
The lesser cornstalk borer Elasmopalpus lignosellus (Zeller, 1848) (Lepidoptera, Pyralidae) is a polyphagous pest, and larvae feed on more than 60 species of cultivated plants (Viana, 2004Viana, P. A. (2004). Lagarta-elasmo. In J. R. Salvadori, C. J. Ávila, M. T. B. Silva. (Ed.), Pragas de solo no Brasil (p. 379-408). Passo Fundo, RS: Embrapa Trigo; Dourados, MS: Embrapa Agropecuária Oeste; Cruz Alta, RS: Fundacep Fecotrigo.). These plants include crops of high economic value, such as corn, beans, wheat, soy, peanuts, and sugarcane, which suffer extensive losses by attack from this pest. The larvae damage newly germinated plants and reduces the number of seedlings per planting area. Larvae penetrate the stalk of a recently sprouted plant, make galleries toward the central core and then feed inside the stem causing new leaves to dry up and die, resulting in the so-called “dead heart” (Gallo et al., 2002Gallo, D., Nakano, O., Neto, S. S., Carvalho, R. P. L., Batista, G. C., Filho, E. B., ... Omoto, C. (2002). Entomologia agrícola. Piracicaba, SP: Fealq.).
The lesser cornstalk borer is a difficult pest to control because it can remain close to the plant stem, within the stem, or in silken web habitats and land shelters they build on the soil surface. In experiments conducted with different control methods, pest management using pheromone and light traps and soil cover with Crotalaria jucea resulted in a small reduction in the pest population (Jham, Silva, Lima, & Viana, 2007Jham, G. N., Silva, A. A., Lima, E. R., & Viana, P. A. (2007). Identification of acetates in Elasmopalpulus lignosellus pheromone glands using a newly created mass spectral database and Kóvats retention indices. Química Nova, 30(4), 916-919.; Gill, McSorley, Goyal, & Webb, 2010Gill, H. K., McSorley, R., Goyal, G., & Webb, S. E. (2010). Mulch as a potential management strategy for lesser cornstalk borer, Elasmopalpus lignosellus (Insecta: Lepidoptera: Pyralidae), in bush bean (Phaseolus vulgaris). Florida Entomologist, 93(2), 183-190.). Thus, preventive chemical control and seed treatment remain the most widely used methods to control E. lignosellus. However, when chemicals are indiscriminately applied, human contamination and environmental imbalance can result, leading to an increase in the pest population.
Entomopathogenic bacteria, such as Bacillus thuringiensis, are among the alternatives to reduce the use of insecticides for pest control. The insecticidal characteristics of these bacteria are caused by the formation of parasporal crystals in the early sporulation phase. These crystals are composed of Cry proteins, which are toxic to a variety of insects that attack crops of high economic value (Vilas-Bôas, Peruca, & Arantes, 2007Vilas-Bôas, G. T., Peruca, A. P. S., & Arantes, O. M. N. (2007). Biology and taxonomy of Bacillus cereus, Bacillus anthracis and Bacillus thuringiensis. Canadian Journal of Microbiology, 53(6), 673- 687.; Vidal-Quist, Castañera, & González-Cabrera, 2009Vidal-Quist, J. C., Castañera, P., & González-Cabrera, J. (2009). Diversity of Bacillus thuringiensis strains isolated from citrus orchards in Spain and evaluation of their insecticidal activity against Ceratitis capitata. Journal of Microbiology and Biotechnology, 19(8), 749-759. ).
The toxic activity of these proteins against insect pests led to the formulation of bioinsecticides and the selection of genes encoding insecticidal proteins to produce transgenic plants resistant to different species of insects. Several Bacillus thuringiensis isolates specific to insects of the orders Lepidoptera, Coleoptera, and Diptera have been investigated (Pardo-López, Soberón, & Bravo, 2013Pardo-López, L., Soberón, M., & Bravo, A. (2013). Bacillus thuringiensis insecticidal three-domain Cry toxins: mode of action, insect resistance and consequences for crop protection. FEMS Microbiology Reviews, 37(1), 3-22.). These isolates typically harbour one or more cry genes, and the isolates containing a wider range of genes are the most targeted. Thus, further studies are required to select these isolates, identify the cry genes, and assess isolate toxicity (Sun, Fu, Ding, & Xia, 2008Sun, Y., Fu, Z., Ding, X., & Xia, L. (2008). Evaluating the insecticidal genes and their expressed products in Bacillus thuringiensis strains by combining PCR with Mass Spectrometry. Appliedand Environmental Microbiology, 74(21), 6811-6813.).
Although the search for isolates of B. thuringiensis that are effective against E. lignosellus is of great significance for the management of this insect pest, studies remain limited. Therefore, the aim of this work was to select and characterize native isolates of B. thuringiensis toxic to E. lignosellus, with the goal to conduct further studies focused on new formulations of bioinsecticides and development of genetically modified plants.
Material and methods
Insect rearing
Larvae of E. lignosellus were reared on an artificial diet according to the methodology described by Greene, Leppla, and Dickerson (1976Greene, G. L., Leppla, N. C., & Dickerson, W. A. (1976). Velvetbean caterpillar: a rearing procedure and artificial medium. Journal of Economic Entomology, 69(4), 487-488.). The adults were maintained at 27 ± 2ºC and 60 ± 10% RH with a 14h photoperiod in plastic cages (10 cm diameter, 20 cm height) coated with filter paper and closed on the upper end with tissue and on the lower end with a petri dish (14.3 cm diameter) and fed a 10% aqueous honey solution. The eggs obtained were transferred to petri dishes at 25°C for incubation; the first instar larvae were used in bioassays.
Bacterial isolates
Forty-seven native isolates of B. thuringiensis were examined from the Collection of Entomopathogenic Microorganisms of Londrina State University (Universidade Estadual de Londrina, UEL) and the Brazilian Agricultural Research Corporation, Embrapa Genetic Resources and Biotechnology (Empresa Brasileira de Pesquisa Agropecuária - Embrapa Recursos Genéticos e Biotecnologia). The HD-1 strain of B. thuringiensis subsp. kurstaki was obtained from the Collection of B. thuringiensis at the Institut Pasteur, Paris, France.
Selective bioassay to choose the most toxic isolates
Suspensions of each B. thuringiensis isolate were prepared by adding 1.0 mg of lyophilized material to 1.0 mL of sterile distilled water. The artificial diet was prepared according to Greene et al. (1976Greene, G. L., Leppla, N. C., & Dickerson, W. A. (1976). Velvetbean caterpillar: a rearing procedure and artificial medium. Journal of Economic Entomology, 69(4), 487-488.) and distributed (3 mL) when still liquid into glass tubes (2 cm diameter x 3 cm height). After the diet solidified, 50 µL of a mixture of spores and crystals were applied on the diet surface. The glass tubes were kept in a laminar flow hood until the complete absorption of the suspension by the diet. Subsequently, five first-instar larvae were released inside each glass tube, which were sealed with a plastic lid. The bioassay consisted of three replicates with four glass tubes for each B. thuringiensis isolate. The standard strain B. thuringiensis subsp. kurstaki HD-1 (Btk) and water were used as positive and negative controls, respectively. The insects were maintained in an incubator (27 ± 2ºC, 60 ± 10% RH and a 14h photoperiod) for six days after which mortality was assessed. The corrected mortality was calculated using Abbott’s control adjusted mortality (Abbott, 1925Abbott, W. S. (1925). A method of computing the effectiveness of an insecticide. Journal of Economic Entomology, 18(2), 265-266.). The data were subjected to analysis of variance (ANOVA), and the means were compared using Tukey’s test at 5% probability. The most toxic isolates, those that caused a reliable mortality (above 85%) and therefore had potential for further testing, were used in dose-response bioassays and evaluated according to their molecular, protein and morphological profiles. The mortality rate was selected based on the minimum efficacy threshold (80% efficacy) required for pesticide registration in Brazil (MAPA, 1995Ministério da Agricultura, Pecuária e Abastecimento [MAPA]. (1995). Normas e exigências para execução de testes de produtos químicos para fins de registro no MAPA. Brasília, DF: Ministério da Agricultura e Reforma Agrária.).
Estimation of the lethal concentration (LC50) of B. thuringiensis isolates
Bioassays for dose estimation were performed with the 12 isolates that showed the greatest toxicity in the selective bioassays and with the HD-1 standard strain. Seven suspensions of spores and crystals of B. thuringiensis were prepared to estimate the concentration of each isolate that would cause 50% mortality in E. lignosellus larvae (LC50). The suspensions were prepared with 5.0 mg of lyophilized material that was diluted in 5 mL of sterile distilled water. Dilutions were performed using the initial suspension to obtain the seven concentrations used in the study (1.0, 0.2, 0.1, 0.05, 0.025, 0.008, and 0.0025 mg mL-1 in sterile distilled water), and the number of spores per mL of water in each dilution was counted using a Neubauer chamber. The bioassay was conducted in the same way as previously described. For each concentration evaluated, three replicates with four tubes were used, for a total of 20 larvae per replicate and 60 per concentration. The mortality data were subjected to Probit analysis (Finney, 1971Finney, D. J. (1971). Probit analysis (3rd ed.). Cambridge, UK: Cambridge University Press.) to determine the lethal concentration. The LC50 bioassay results were analysed by checking for the overlap of the 95% confidence intervals according to Probit analysis.
Protein and molecular characterization of B. thuringiensis isolates pathogenic to E. lignosellus
Genomic DNA samples of the B. thuringiensis strains were isolated according to the method described by Ricieto, Fazion, Carvalho Filho, Vilas-Boas, and Vilas-Bôas (2013Ricieto, A. P. S., Fazion, F. A. P., Carvalho Filho, C. D., Vilas-Boas, L. A., & Vilas-Bôas, G. T. (2013). Effect of vegetation on the presence and genetic diversity of Bacillus thuringiensis in soil. Canadian Journal of Microbiology, 59(1), 28-33.). The isolates were cultivated for 15h at 30°C on plates containing Luria-Bertani (LB) medium (Bertani, 1951Bertani, G. (1951). Studies on lysogenesis I. The mode of phage liberation by lysogenic Escherichia coli. Journal of Bacteriology, 62(3), 293-300.). With the aid of a sterile toothpick, a colony of approximately 2 mm in diameter was transferred to microtubes containing 200 µL of TE (10 mMTris; 1 mM EDTA; pH 8.0). The suspension was homogenized and incubated in boiling water for 10 min. Then, the suspension was centrifuged at 12,000 xg for 3 min, and the supernatant was transferred to a new tube and used as DNA template in the PCR reactions. The presence of the genes cry1, cry2, cry3, cry4A, cry4B, cry10, and cry11 was analysed using specific primers and amplification conditions (Céron, Ortí, Quintero, Güereca, & Bravo, 1995Céron, J., Ortíz, A., Quintero, R., Güereca, L., & Bravo, A. (1995). Specific PCR primers directed to identify cry1 and cry3 genes within a Bacillus thuringiensis strains collection. Applied and Environmental Microbiology, 61(11), 3826-3831., Bravo et al., 1998; Ibarra et al., 2003Ibarra, J. E., Rincón, M. C. D., Ordúz, S., Noriega, D., Benintende, G., Monnerat, R., ... Bravo, A. (2003). Diversity of Bacillus thuringiensis strains from Latin America with insecticidal activity against different mosquito species. Applied and Environmental Microbiology, 69(9), 5269-5274.; Vidal-Quist et al., 2009Vidal-Quist, J. C., Castañera, P., & González-Cabrera, J. (2009). Diversity of Bacillus thuringiensis strains isolated from citrus orchards in Spain and evaluation of their insecticidal activity against Ceratitis capitata. Journal of Microbiology and Biotechnology, 19(8), 749-759. ). DNA amplification was performed using an Endurance TC-412 thermocycler. For each amplification reaction, a total reaction volume of 20 µL was prepared that contained 1 U Taq DNA polymerase (Invitrogen, Brazil), 2.0 µL of Buffer 10 (200 mM Tris-HCl, pH 8.0, 500 mM KCl), 1.5 mM MgCl2, 0.25 mM dNTP, 0.5 µM each primer, 2 µL of DNA and sterile Milli-Q water. The same reaction was used for all the primers. The PCR products were visualized by electrophoresis on 1.2% agarose gel in TBE buffer (89 mM Tris Borate, 2 mM EDTA, pH 8.0) stained with Syber Safe (Invitrogen, UK) using a 100 bp DNA ladder (Invitrogen, UK). After electrophoresis, the gel images were captured using a Sony Cyber-shot 8.1 digital camera.
The B. thuringiensis isolates were characterized by the protein profile of their crystals using protein electrophoresis on 10% polyacrylamide gel (SDS-PAGE). Initially, the crystals were obtained according to the protocol described by Lecadet, Chaufaux, Ribier, and Lereclus. (1992Lecadet, M. M., Chaufaux, J., Ribier, J., & Lereclus, D. (1992). Construction of novel Bacillus thuringiensis strain with different insecticidal activities by transduction and transformation. Applied and Environmental Microbiology, 58(3), 840-849.). Each isolate was cultivated in NB medium (Downes & Ito, 2001Downes, F. P., & Ito, K. (2001). Compendium of methods for the microbiological examination of foods (4th ed.). Washington, DC: American Public Health Association.) at 30°C for 72h at 200 rpm, until complete sporulation. The B. thuringiensis subsp. kurstaki HD-1 standard strain was used as the reference.
Morphological characterization of B. thuringiensis isolates
The morphological characterization of the isolates was initially performed by optical microscopy using a microscope (Model CHS; Olympus Optical Co. Ltd., Tokyo, Japan) with a 100x phase contrast lens. For electron microscopy, the lyophilized material of each isolate used in the previous bioassays was directly deposited over metal supports and coated with gold for 180 s, using a 40 mA current under vacuum (10-1 mbar) in a BAL-TEC model SCD-050 Sputter Coater (Santos et al., 2009Santos, K., Neves, P. M. O. J., Meneguim, A. M., Santos, R. B., Santos, W. J., Vilas-Bôas, G. T., ... Monnerat, R. (2009). Selection and characterization of the Bacillus thuringiensis strains toxic to Spodoptera eridania (Cramer), Spodoptera cosmioides (Walker) and Spodoptera frugiperda (Smith) (Lepidoptera: Noctuidae). Biological Control, 50(2), 157-163.). Subsequently, the material was analysed using a scanning electron microscope Philips QUANTA 200 (FEI) in high vacuum under 20 kV tension with a working distance of 10.2 mm. The selected images were captured and stored for later analysis.
Results
Selective bioassay and determination of the Median Lethal Concentration (LC50) of Bacillus thuringiensis isolates
Among the 47 isolates of B. thuringiensis tested, 12 isolates (25.53%), in addition to B. thuringiensis subsp. kurstaki (Btk) HD-1 strain, caused mortality of E. lignosellus above 85%, for a total of 13 isolates, which were selected for all other conclusive tests. Dose-response bioassays were used to evaluate these 13 isolates. The X2 values related to the LC50 were no significant for 12 of the isolates, which indicated that the data were homogeneous for those strains and fit the Probit analysis model (Table 1) (Finney, 1971Finney, D. J. (1971). Probit analysis (3rd ed.). Cambridge, UK: Cambridge University Press.).
The LC50 values of the selected isolates varied from 0.038E+8 to 0.855E+8 spores mL-1. The lowest LC50 values were obtained for the group consisting of BR83, BR145, BR09, BR78, and S1534. The LC50 values within that group did not differ statistically according to the Probit analysis, as shown by the overlap of the 95% confidence intervals (Table 1). Only theLC50 value of isolate BR83 was significantly lower than that of the HD-1 standard strain, with a toxicity that was three-fold greater than that of the standard. Additionally, the BR 83 isolate was approximately 22-fold more toxic than the S1269 isolate, which had the highest LC50. Only the S1450 isolate presented a significant X2; thus, the LC50 could not be estimated (Table 1).
Protein and molecular characterization of B. thuringiensis isolates pathogenic to E. lignosellus
The PCR technique using total DNA of isolates and specific primers for the detection of cry1, cry2, cry3, cry4A, cry 4B, cry10, and cry11 resulted in the amplification of fragments of the expected sizes (Bravo et al., 1998Bravo, A., Sarabia, S., Lopez, L., Ontiveros, H., Abarca, C, Ortiz, A., ... Quintero, R. (1998). Characterization of cry genes in a Mexican Bacillus thuringiensis strain collection. Applied and Environmental Microbiology, 64(12), 4965-4972.; Céron et al., 1995Céron, J., Ortíz, A., Quintero, R., Güereca, L., & Bravo, A. (1995). Specific PCR primers directed to identify cry1 and cry3 genes within a Bacillus thuringiensis strains collection. Applied and Environmental Microbiology, 61(11), 3826-3831.; Ibarra et al., 2003Ibarra, J. E., Rincón, M. C. D., Ordúz, S., Noriega, D., Benintende, G., Monnerat, R., ... Bravo, A. (2003). Diversity of Bacillus thuringiensis strains from Latin America with insecticidal activity against different mosquito species. Applied and Environmental Microbiology, 69(9), 5269-5274.; Vidal-Quist et al., 2009Vidal-Quist, J. C., Castañera, P., & González-Cabrera, J. (2009). Diversity of Bacillus thuringiensis strains isolated from citrus orchards in Spain and evaluation of their insecticidal activity against Ceratitis capitata. Journal of Microbiology and Biotechnology, 19(8), 749-759. ) and consequently, the determination of which cry genes were in the isolates of B. thuringiensis.
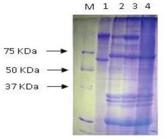
The amplicons produced with the greatest frequency corresponded to cry1, cry2, and cry3 genes. The cry1 gene was detected in all isolates, except BR52 and BR53, which also did not exhibit the cry2 gene. The expected fragment for the cry3 gene appeared in BR145, S1534, and S1302 isolates, whereas the fragment for the cry11 gene occurred only in the BR53 isolate. Only the BR52 isolate did not show a PCR product consistent with the selected primers. The fragments of the expected size for the other primers used were not observed in all isolates, indicating the absence of cry4A, cry4B, and cry10 genes in some isolates (Table 2). The protein profile analysis of the spore-crystal mixtures of the isolates from the selective bioassays revealed bands of 60, 65, 70, 80, and 130 kDa. The isolate used as the standard, B. thuringiensis subsp. kurstaki, had a protein profile of 65 and 130 kDa (Höfte & Whiteley, 1989Höfte, H., & Whiteley, H. R. (1989). Insecticidal crystal proteins of Bacillus thuringiensis. Microbiology and Molecular Biology Reviews, 53(2), 242-255.; Lereclus, Delécluse, & Lecadet, 1993Lereclus, D., Delécluse, A., & Lecadet, M. M. (1993). Diversity of Bacillus thuringiensis toxins and genes. In: P. F. Enwistle, J. Cory, M. Bailey, S, Higgs (Eds.), Bacillus thuringiensis, an environmental biopesticide: Theory and practice (p. 37-69). Chichester, UK: John Wiley & Son Ltd.). All data related to the protein and molecular characterization of B. thuringiensis isolates are presented in Table 2. An image of protein electrophoresis on 10% polyacrylamide gel (SDS-PAGE) illustrating the protein profile of some isolates is shown in Figure 1.
Morphological characterization of B. thuringiensis isolates
Morphological analysis using optical and scanning electron microscopy showed protein inclusions of different forms. The BR83, BR09, BR78, and S1450 isolates showed three different crystalline protein inclusions: bipyramidal, spherical, and cuboidal. Isolates BR145, S1534, S1302, and BR38 exhibited bipyramidal and spherical crystals, whereas isolates S545, S1269, BR52, and BR53 contained only bipyramidal crystals (Table 2).
Protein profile produced by isolates toxic to Elasmopalpus lignosellus (Zeller, 1848) (Lepidoptera, Pyralidae). M, Rainbow molecular weight marker (GE); 1 - BR09; 2 - BR38; 3 - BR83; 4 - BR52.
Discussion
The selective and dose bioassays revealed that B. thuringiensis isolates were active against E. lignosellus. Of the isolates tested, 25.53% caused mortality above 85%; therefore, these isolates can be further tested as a possible alternative method for the management of populations of E. lignosellus. Based on dose bioassays, isolate BR83 had the lowest lethal concentration. Furthermore, BR83 had superior activity compared with that of the standard B. thuringiensis subsp. kurstaki HD-1 strain, although the activity was not significantly different from that of the BR145, BR09, BR78, and S1534 isolates. Those four isolates carried cry1 and cry2 genes, which are toxic to insects of the order Lepidoptera (Ricieto et al., 2013Ricieto, A. P. S., Fazion, F. A. P., Carvalho Filho, C. D., Vilas-Boas, L. A., & Vilas-Bôas, G. T. (2013). Effect of vegetation on the presence and genetic diversity of Bacillus thuringiensis in soil. Canadian Journal of Microbiology, 59(1), 28-33.). In addition to these genes, BR145 and S1302 isolates also carried the cry3 gene, with reported toxicity to Lepidoptera and Coleoptera (Brizzard & Whitley, 1988Brizzard, B. L., & Whiteley, H. R. (1988). Nucleotide sequence of an additional crystal protein gene cloned from Bacillus thuringiensis subsp. thuringiensis. Nucleic Acids Research, 16(6), 2723-2724. ).
The cry1 and cry2 genes were found in almost all selected isolates, except for BR52, which did not yield an amplification product for any of the used primers, and BR53, which amplified only for the cry11 gene. The genes in the Cry1 subfamily are the most abundant and occur in approximately half of the isolates identified to date. The cry2 gene is also very common, particularly among isolates harbouring the cry1 gene (Porcar & Juárez-Perez, 2003Porcar, M., & Juárez-Pérez, V. (2003). PCR-based identification of Bacillus thuringiensis pesticidal crystal genes. FEMS Microbiology Reviews, 26(5), 419-432.; Arrieta, Hernández, & Espinoza, 2004Arrieta, G., Hernández, A., & Espinoza, A. M. (2004). Diversity of Bacillus thuringiensis strains isolated from coffee plantations infested with the coffee berry borer Hypothenemus hampei Ferrari. Revista de Biología Tropical, 52(3), 757-764.).
Isolates containing cry1 and/or cry2 genes were also the most abundant in the collection studied by Vidal-Quist et al. (2009Vidal-Quist, J. C., Castañera, P., & González-Cabrera, J. (2009). Diversity of Bacillus thuringiensis strains isolated from citrus orchards in Spain and evaluation of their insecticidal activity against Ceratitis capitata. Journal of Microbiology and Biotechnology, 19(8), 749-759. ), representing over 45% of the total. In studies with the BR37 isolate, Santos et al. (2009Santos, K., Neves, P. M. O. J., Meneguim, A. M., Santos, R. B., Santos, W. J., Vilas-Bôas, G. T., ... Monnerat, R. (2009). Selection and characterization of the Bacillus thuringiensis strains toxic to Spodoptera eridania (Cramer), Spodoptera cosmioides (Walker) and Spodoptera frugiperda (Smith) (Lepidoptera: Noctuidae). Biological Control, 50(2), 157-163.) identified eight genes of which seven were in the Cry1 group, showing the frequent occurrence of cry1 genes in isolates of B. thuringiensis (Bravo et al., 1998Bravo, A., Sarabia, S., Lopez, L., Ontiveros, H., Abarca, C, Ortiz, A., ... Quintero, R. (1998). Characterization of cry genes in a Mexican Bacillus thuringiensis strain collection. Applied and Environmental Microbiology, 64(12), 4965-4972.).
Genes in the Cry1 and Cry2 protein families have been reported in the isolates used for the control of E. lignosellus, and the effects of Cry1 proteins in transgenic peanut and soybeans plants have been examined under field conditions. In peanuts, various levels of resistance to the lesser cornstalk borer were provided by the introduction of the cry1Ac gene, from complete larval mortality to 66% reduction in larval weight (Singsit et al., 1997Singsit, C., Adang, M. J., Lynch, R. E., Anderson, W. F., Wang, A., Cardineau, G., & Ozias-Akins, P. (1997). Expression of a Bacillus thuringiensis cryA(c) gene in transgenic peanut and its efficacy against lesser cornstalk borer. Transgenic Research, 6(2), 169-176.). For soybean, plants expressing the cry1Ac gene had four-fold more resistance to E. lignosellus than that of the wild-type isolate (Walker, All, Mcpherson, Boerma, & Parrott, 2000Walker, D. R., All, J. N., Mcpherson, R. M., Boerma, H. R., & Parrott, W. A. (2000). Field evaluation of soybean engineered with a synthetic crylAc transgene for resistance to corn earworm, soybean looper, velvetbean caterpillar (Lepidoptera: Noctuidae), and lesser cornstalk borer (Lepidoptera: Pyralidae). Journal of Economic Entomology, 93(3), 613-622.).
Assessments of genetically modified corn crops revealed that hybrids containing cry9C, cry1F, and cry1Ab genes did not differ in resistance to E. lignosellus; however, transgenic plants were superior to non-transgenic hybrids (Vilella, Waquil, Vilela, Siegfried, & Foster, 2002). Additionally, in laboratory bioassays, isolates containing cry2A gene and the HD-1 standard isolate proved effective against E. lignosellus (Moar, Pusztai-Carey, & Mack, 1995Moar, W. J., Pusztai-Carey, M., & Mack, T. P. (1995). Toxicity of purified proteins and the HD-1 strain from Bacillus thuringiensis against lesser cornstalk borer (Lepidoptera: Pyralidae). Journal of Economic Entomology, 88(3), 606-609.).
These examples demonstrate the different methodologies used for the control of E. lignosellus, but the mortality rates are different between isolates harbouring many genes and those caused by genetically modified plants carrying only the primary gene responsible for the toxicity. However, to select new isolates that carry toxic genes to be tested individually against the pest, the bioassays used in this study must be conducted. Thereby, the selected gene, after many tests, can be inserted into plants for pest control and increase the possibilities for pest management.
The protein profiles of most of the tested isolates that amplified with the cry1 and cry2 genes (BR83, BR145, BR09, BR38, S1534, and S1450) showed bands of 130 and 65/70 kDa, which confirmed their specificity for Lepidoptera. Cry1 class proteins have a molecular weight of approximately 130-140 kDa (Höfte & Whiteley, 1989Höfte, H., & Whiteley, H. R. (1989). Insecticidal crystal proteins of Bacillus thuringiensis. Microbiology and Molecular Biology Reviews, 53(2), 242-255.), whereas proteins of the Cry2 and Cry3 groups, which are active against Lepidoptera and Coleoptera, have values of 65-70 kDa (Bravo et al., 2004Bravo, A., Gómez, I., Conde, J., Muñoz-Garay, C., Sánchez, J., Miranda, R., ... Soberón, M. (2004). Oligomerization triggers binding of a Bacillus thuringiensis Cry1Ab pore-forming toxin to aminopeptidase N receptor leading to insertion into membrane microdomains. Biochimica et Biophysica Acta, 1667(3), 38-46.).
Although the BR09 isolate did not differ from the most toxic isolate (BR83) or the HD-1 standard, the LC50 of the BR09 isolate was approximately 1.8-fold lower than that of HD-1. Santos et al. (2009Santos, K., Neves, P. M. O. J., Meneguim, A. M., Santos, R. B., Santos, W. J., Vilas-Bôas, G. T., ... Monnerat, R. (2009). Selection and characterization of the Bacillus thuringiensis strains toxic to Spodoptera eridania (Cramer), Spodoptera cosmioides (Walker) and Spodoptera frugiperda (Smith) (Lepidoptera: Noctuidae). Biological Control, 50(2), 157-163.) obtained a similar value when testing the same isolate against Spodoptera eridania. Nevertheless, LC50 values may vary among species; for example, in studies against Spodoptera cosmioides, the LC50 of the HD-1 standard strain was two-fold lower than that of the BR09 isolate (Santos et al., 2009). Moreover, in a selection performed by Constanski et al., 2015Constanski, K. C., Zorzetti, J., Vilas Bôas, G. T., Ricieto, A. P. S., Fazion, F. A. P., Vilas Boas, L. A., ... Neves, P. M. O. J.(2015). Seleção e caracterização molecular de isolados de Bacillus thuringiensis para o controle de Spodoptera spp. Pesquisa Agropecuária Brasileira, 50(8), 730-733., no differences were detected between the LC50 of the HD-1 standard strain and that of the other isolates tested against S. eridania and S. cosmioides.
The toxicological profile of the BR09 isolate that was effective against E. lignosellus can be explained by the expression of cry1 and cry2 genes, similar to the HD-1 strain, which has the cry1Aa, cry1Ab, cry1Ac, cry2A, and cry2B genes in its genome (Li et al., 2005Li, H., Oppert, B., Higgins, R. A., Huang, F., Buschman, L. L., & Zhu, K. Y. (2005). Susceptibility of Dipel-resistant and -susceptible Ostrinia nubilalis (Lepidoptera: Crambidae) to individual Bacillus thuringiensis protoxins. Journal of Economic Entomology, 98(4), 1333-1340.). Furthermore, SDS-PAGE protein analysis of the spore and crystal mixture revealed two polypeptides with approximate molecular weights of 70-130 kDa. Santos et al. (2009Santos, K., Neves, P. M. O. J., Meneguim, A. M., Santos, R. B., Santos, W. J., Vilas-Bôas, G. T., ... Monnerat, R. (2009). Selection and characterization of the Bacillus thuringiensis strains toxic to Spodoptera eridania (Cramer), Spodoptera cosmioides (Walker) and Spodoptera frugiperda (Smith) (Lepidoptera: Noctuidae). Biological Control, 50(2), 157-163.) also tested the BR09 isolate and showed a gene profile with cry1Aa, cry1Ab, cry1Ac, and cry2Aa genes.
Although the S1450 isolate caused mortality of E. lignosellus larvae above 85%, in the selective bioassays, the X2 value was significant, i.e., the data did not fit the Probit model. Thus, the LC50 could not be estimated for isolate S1450. This isolate is in the kurstaki serotype described in the literature as toxic to insects of the order Lepidoptera (Monnerat et al., 2007Monnerat, R. G., Batista, A. C., Medeiros, P. T., Martins, E., Melatti, V., Praça, L., Dumas, V., … Berry, C. (2007). Screening of Brazilian Bacilus thuringiensis isolates active against Spodoptera frugiperda, Plutella xylostella and Anticarsia gemmatalis. Biological Control, 41(3), 291-295.) and causing 100% mortality of Agrotis ipsilon (Lepidoptera: Noctuidae). Additionally, cry1Aa, cry1Ab, cry1Ac, cry1, and cry2 genes were revealed by the molecular analysis, which confirmed the activity of the isolate against Lepidoptera (Menezes, Fiuza, Martins, Praça, & Monnerat, 2010Menezes, R. S., Fiuza, V. D., Martins, E. S.,Praça, L. B., & Monnerat, R. G. (2010). Seleção e caracterização de estirpes de Bacillus thuringiensis tóxicas a Agrotisipsilon. Universitas Ciências da Saúde, 8(1), 1-13.).
The BR78 isolate, with an LC50 that did not differ from that of the most toxic isolates, also carried cry1 and cry2 genes. However, only two primary polypeptides of approximately 65 and 80 kDa were associated with this isolate, which are related only to the cry2 gene. The result was similar for the S1302 isolate, which despite amplifying with cry1, cry2, and cry3 genes, revealed only a band of 70 kDa. As a possible explanation, a poor performance or even the absence of the promoter led to low expression of the cry1 gene, which prevented the display of a 130 kDa band.
According to Alper et al. (2014Alper, M., Günes, H., Tatlipina, A., Çöl, B., Civelek, H. S., Özkan, C., & Poyraz, B. (2014). Distribution, occurrence of cry genes, and lepidopteran toxicity of native Bacillus thuringiensis isolated from fig tree environments in Aydın Province. Turkish Journal of Agriculture and Forestry, 38(6), 898-907.), some strains that harbour the same genes may not be as effective as other toxic strains that have those same genes, indicating that these genes could be poorly expressed because of a weak promoter in the strains. Thus, poor expression of the cry genes in the BR78 and S1302 isolates could explain the cry genes in the genetic profile but not in the protein profile.
Armengol, Escobar, Maldonado, and Orduz (2007Armengol, G., Escobar, M. C., Maldonado, M. E., & Orduz, S. (2007). Diversity of Colombian strains of Bacillus thuringiensis with insecticidal activity against dipteran and lepidopteran insects. Journal of Applied Microbiology, 102(1), 77-88.), identified isolates toxic to S. frugiperda containing cry1Aa, cry1Ab, cry1Ac, cry1B, and cry1D genes, with protein profiles that revealed bands only at 60 kDa. According to these authors, the correlation between the identified protein profiles and the cry genes cannot occur when the cry proteins are encoded by unknown genes or have not been amplified by the primers used. Additionally, the identified genes may encode proteins with low-level or inactive expression.
Although the LC50 did not differ from that of the HD-1 standard isolate, only the BR52 isolate failed to obtain amplification of the expected fragment sizes with the specific primers employed. Nevertheless, the optical and electron microscope observations revealed the production of protein inclusions, suggesting that the isolate might contain genes not covered by the set of primers used in the PCR analysis or cry genes not yet described. Additionally, the analysis of the protein profile showed a polypeptide of approximately 70 kDa that corresponded to the Cry2 class, possibly explaining the insecticidal activity of isolate BR52 against Lepidoptera.
Only isolate BR53 expressed the cry11 gene. However, this gene was most likely not the cause of the toxicity of this isolate against E. lignosellus, because the gene is usually associated with activity against larvae of Diptera, such as Simulium spp., Culex spp. and Aedes aegypti (Vidal-Quist et al., 2009Vidal-Quist, J. C., Castañera, P., & González-Cabrera, J. (2009). Diversity of Bacillus thuringiensis strains isolated from citrus orchards in Spain and evaluation of their insecticidal activity against Ceratitis capitata. Journal of Microbiology and Biotechnology, 19(8), 749-759. ). Thus, isolate BR53 might contain some other gene toxic to E. lignosellus not disclosed by the set of primers used, requiring further investigation to search for new cry genes.
Based on the different shapes of protein crystals detected by optical and electron microscopy, the types of Cry proteins that composed the crystal could be inferred, which provided information on the insecticidal activity of the isolates (Lereclus, Delécluse, & Lecadet, 1993Lereclus, D., Delécluse, A., & Lecadet, M. M. (1993). Diversity of Bacillus thuringiensis toxins and genes. In: P. F. Enwistle, J. Cory, M. Bailey, S, Higgs (Eds.), Bacillus thuringiensis, an environmental biopesticide: Theory and practice (p. 37-69). Chichester, UK: John Wiley & Son Ltd.; Vilas-Boas et al., 2007Vilas-Bôas, G. T., Peruca, A. P. S., & Arantes, O. M. N. (2007). Biology and taxonomy of Bacillus cereus, Bacillus anthracis and Bacillus thuringiensis. Canadian Journal of Microbiology, 53(6), 673- 687.).
The similarity between the morphological and molecular analyses was notable in this study. Isolates that expressed cry1 and cry2 genes contained primarily bipyramidal, cuboidal, and spherical shaped crystals. These three different shapes were observed in the BR09, BR78, and BR83 isolates, which contained the cry1 and cry2 genes, and in the S1450 isolate that harboured cry1, cry2, and cry3 genes. The BR38 isolate with cry1 and cry2 genes and the S1302, S1534, and BR145 isolates with cry1, cry2 and cry3 genes all contained bipyramidal and spherical crystals. The crystal protein composition was studied in some of these isolates previously, and those studies confirm the shapes observed in this study (Praça et. al., 2004Praça, L. B., Batista, A. C., Martins, E. S., Siqueira, C. B., Dias, D. G. S., Gomes, A. C. M. M., ... Monnerat, R. G. (2004). Estirpes de Bacillus thuringiensis efetivas contra insetos das ordens Lepidoptera, Coleoptera e Diptera. Pesquisa Agropecuária Brasileira, 39(1), 11-16.; Santos et al., 2009Santos, K., Neves, P. M. O. J., Meneguim, A. M., Santos, R. B., Santos, W. J., Vilas-Bôas, G. T., ... Monnerat, R. (2009). Selection and characterization of the Bacillus thuringiensis strains toxic to Spodoptera eridania (Cramer), Spodoptera cosmioides (Walker) and Spodoptera frugiperda (Smith) (Lepidoptera: Noctuidae). Biological Control, 50(2), 157-163.; Ricieto et al., 2013Ricieto, A. P. S., Fazion, F. A. P., Carvalho Filho, C. D., Vilas-Boas, L. A., & Vilas-Bôas, G. T. (2013). Effect of vegetation on the presence and genetic diversity of Bacillus thuringiensis in soil. Canadian Journal of Microbiology, 59(1), 28-33.). Only bipyramidal crystals were produced by the isolates S545 and S1269, which contained only the cry1 gene, and BR52 and BR53.
The selection of isolates containing Cry1 and Cry2 proteins and harbouring more than one Cry protein is essential in the prospecting for new virulent cry genes against lepidopterans such as E. lignosellus. Once identified, these are the isolates that can be used for pest management either through bioinsecticide formulations or as a source for transgenic plant manipulation.
Bacillus thuringiensisis the most successful pathogen agent used for insect control, representing almost 2% of the total insecticide market and up to 90% of bioinsecticide formulations. Currently, formulations based on B. thuringiensis are increasing, and this market is expected to continue to grow. Most of these products are based on spore-crystal preparations derived from a few strains such as B. thuringiensis var. kurstaki HD1. Thus, the detection of B. thuringiensis strains with new genes is extremely important to develop more efficient bioinsecticides (Bravo, Likitvivatanavong, Gill, & Soberón, 2011Bravo, A., Likitvivatanavong, S., Gill, S. S., & Soberόn, M. (2011). Bacillus thuringiensis: a story of a successful bioinsecticide. Insect Biochemistry and Molecular Biology, 41(7), 423-431.; Lemes et al., 2015Lemes, A. R. N., Marucci, S. C., Costa, J. R. V., Alves, E. C. C., Fernandes, O. A., Lemos, M. V. F., & Desidério, J. A. (2015). Selection of strains from B. thuringiensis genes containing effective in the control of Spodoptera frugiperda. Bt Research, 6(1), 1-8.).
For some products formulated with B. thuringiensis, their use in agriculture is limited, because Cry toxins are more specific for first larval instars, are sensitive to sun radiation and have limited activity against borer insects (Bravo et al., 2011Bravo, A., Likitvivatanavong, S., Gill, S. S., & Soberόn, M. (2011). Bacillus thuringiensis: a story of a successful bioinsecticide. Insect Biochemistry and Molecular Biology, 41(7), 423-431.). Therefore, the internal feeding behaviour of E. lignosellus inside stalks and stems makes management with pesticide and biologic product applications alone difficult. Thus, to more effectively E. lignosellus control, plants must be genetically modified with B. thuringiensis genes.
The discovery of these cry genes is of great interest for the production of new transgenic plants and also for genes tacking or pyramidalization. For example, insertion of different cry genes into genetically modified plants that use different receptors in the insect midgut membrane can help to extend the protection against more insect pests. Additionally, the beginning of resistance can be delayed or prevented, because more than one toxic protein would be acting against the same insect species (Sanahuja, Banakar, Twyman, Capell, & Christou, 2011Sanahuja, G., Banakar, R., Twyman, R. M., Capell, T., & Christou, P. (2011). Bacillus thuringiensis: a century of research, development and commercial applications. Plant Biotechnology Journal, 9(3), 283-300.; Hernández-Rodríguez, Hernández-Martínez, Van Rie, Escriche, & Ferré, 2013Hernández-Rodríguez, C. S., Hernández-Martínez, P., Van Rie, J., Escriche, B., & Ferré, J. (2013). Shared midgut binding sites for Cry1A, Cry1Aa, Cry1Ab, Cry1Ac and Cry1Fa proteins from Bacillus thuringiensis in two important corn pests, Ostrinian ubilalis and Spodoptera frugiperda. PLoS ONE, 8(7), e68164. ).
Researchers and commercial companies have recently used the technique of gene pyramidalization. As an example, the SmartStax® corn developed in cooperation between Monsanto and Dow Agro Sciences companies showed that the pyramidalization of cry1A.105, cry2Ab, cry3Bb1, cry34Ab1, cry35Ab1, and cry1Fa2 genes was essential for managing pesticide-resistant pests, in addition to providing effective control against a long list of pests in Coleoptera and Lepidoptera, including E. lignosellus (Marra, Piggott, & Goodwin, 2010Marra, M. C., Piggott, N. E., & Goodwin, B. K. (2010). The anticipated value of Smart Stax™ for US corn growers. AgBio Forum, 13(1), 1-12. ).
The isolates evaluated in this study have potential for biotechnological control of E. lignosellus. Additionally, the isolates can be a gene source for the production of new crops or the management of currently insect-resistant plant cultivars. The genome sequencing of the studied isolates ensured accurate quantification of cry genes for further selection and use against E. lignosellus through insertion into the genome of economically relevant crops attacked by this pest.
Conclusion
Among the 47 isolates studied, 12 caused mortality above 85%. Isolates BR145, BR09, BR78, S1534, and S1302 had the lowest LC50 values and did not differ from the standard HD-1. The protein profiles produced bands with molecular masses of 60-130 kDa. The molecular characterization showed the presence of cry1, cry2, cry3, and cry11 genes.
The morphological analysis identified three different crystal inclusions: bipyramidal, spherical and cuboidal. As a result of these characterizations, these isolates have potential for biotechnological control of E. lignosellus and should be important candidates for more studies and the development of new biopesticides or genetically modified plants.
Acknowledgements
The authors wish to thank the National Council for Scientific and Technological Development (CNPq) for financial support
References
- Abbott, W. S. (1925). A method of computing the effectiveness of an insecticide. Journal of Economic Entomology, 18(2), 265-266.
- Alper, M., Günes, H., Tatlipina, A., Çöl, B., Civelek, H. S., Özkan, C., & Poyraz, B. (2014). Distribution, occurrence of cry genes, and lepidopteran toxicity of native Bacillus thuringiensis isolated from fig tree environments in Aydın Province. Turkish Journal of Agriculture and Forestry, 38(6), 898-907.
- Armengol, G., Escobar, M. C., Maldonado, M. E., & Orduz, S. (2007). Diversity of Colombian strains of Bacillus thuringiensis with insecticidal activity against dipteran and lepidopteran insects. Journal of Applied Microbiology, 102(1), 77-88.
- Arrieta, G., Hernández, A., & Espinoza, A. M. (2004). Diversity of Bacillus thuringiensis strains isolated from coffee plantations infested with the coffee berry borer Hypothenemus hampei Ferrari. Revista de Biología Tropical, 52(3), 757-764.
- Bertani, G. (1951). Studies on lysogenesis I. The mode of phage liberation by lysogenic Escherichia coli Journal of Bacteriology, 62(3), 293-300.
- Bravo, A., Gómez, I., Conde, J., Muñoz-Garay, C., Sánchez, J., Miranda, R., ... Soberón, M. (2004). Oligomerization triggers binding of a Bacillus thuringiensis Cry1Ab pore-forming toxin to aminopeptidase N receptor leading to insertion into membrane microdomains. Biochimica et Biophysica Acta, 1667(3), 38-46.
- Bravo, A., Likitvivatanavong, S., Gill, S. S., & Soberόn, M. (2011). Bacillus thuringiensis: a story of a successful bioinsecticide. Insect Biochemistry and Molecular Biology, 41(7), 423-431.
- Bravo, A., Sarabia, S., Lopez, L., Ontiveros, H., Abarca, C, Ortiz, A., ... Quintero, R. (1998). Characterization of cry genes in a Mexican Bacillus thuringiensis strain collection. Applied and Environmental Microbiology, 64(12), 4965-4972.
- Brizzard, B. L., & Whiteley, H. R. (1988). Nucleotide sequence of an additional crystal protein gene cloned from Bacillus thuringiensis subsp. thuringiensis Nucleic Acids Research, 16(6), 2723-2724.
- Céron, J., Ortíz, A., Quintero, R., Güereca, L., & Bravo, A. (1995). Specific PCR primers directed to identify cry1 and cry3 genes within a Bacillus thuringiensis strains collection. Applied and Environmental Microbiology, 61(11), 3826-3831.
- Constanski, K. C., Zorzetti, J., Vilas Bôas, G. T., Ricieto, A. P. S., Fazion, F. A. P., Vilas Boas, L. A., ... Neves, P. M. O. J.(2015). Seleção e caracterização molecular de isolados de Bacillus thuringiensis para o controle de Spodoptera spp. Pesquisa Agropecuária Brasileira, 50(8), 730-733.
- Downes, F. P., & Ito, K. (2001). Compendium of methods for the microbiological examination of foods (4th ed.). Washington, DC: American Public Health Association.
- Finney, D. J. (1971). Probit analysis (3rd ed.). Cambridge, UK: Cambridge University Press.
- Gallo, D., Nakano, O., Neto, S. S., Carvalho, R. P. L., Batista, G. C., Filho, E. B., ... Omoto, C. (2002). Entomologia agrícola Piracicaba, SP: Fealq.
- Gill, H. K., McSorley, R., Goyal, G., & Webb, S. E. (2010). Mulch as a potential management strategy for lesser cornstalk borer, Elasmopalpus lignosellus (Insecta: Lepidoptera: Pyralidae), in bush bean (Phaseolus vulgaris). Florida Entomologist, 93(2), 183-190.
- Greene, G. L., Leppla, N. C., & Dickerson, W. A. (1976). Velvetbean caterpillar: a rearing procedure and artificial medium. Journal of Economic Entomology, 69(4), 487-488.
- Hernández-Rodríguez, C. S., Hernández-Martínez, P., Van Rie, J., Escriche, B., & Ferré, J. (2013). Shared midgut binding sites for Cry1A, Cry1Aa, Cry1Ab, Cry1Ac and Cry1Fa proteins from Bacillus thuringiensis in two important corn pests, Ostrinian ubilalis and Spodoptera frugiperda PLoS ONE, 8(7), e68164.
- Höfte, H., & Whiteley, H. R. (1989). Insecticidal crystal proteins of Bacillus thuringiensis Microbiology and Molecular Biology Reviews, 53(2), 242-255.
- Ibarra, J. E., Rincón, M. C. D., Ordúz, S., Noriega, D., Benintende, G., Monnerat, R., ... Bravo, A. (2003). Diversity of Bacillus thuringiensis strains from Latin America with insecticidal activity against different mosquito species. Applied and Environmental Microbiology, 69(9), 5269-5274.
- Jham, G. N., Silva, A. A., Lima, E. R., & Viana, P. A. (2007). Identification of acetates in Elasmopalpulus lignosellus pheromone glands using a newly created mass spectral database and Kóvats retention indices. Química Nova, 30(4), 916-919.
- Lecadet, M. M., Chaufaux, J., Ribier, J., & Lereclus, D. (1992). Construction of novel Bacillus thuringiensis strain with different insecticidal activities by transduction and transformation. Applied and Environmental Microbiology, 58(3), 840-849.
- Lemes, A. R. N., Marucci, S. C., Costa, J. R. V., Alves, E. C. C., Fernandes, O. A., Lemos, M. V. F., & Desidério, J. A. (2015). Selection of strains from B. thuringiensis genes containing effective in the control of Spodoptera frugiperda Bt Research, 6(1), 1-8.
- Lereclus, D., Delécluse, A., & Lecadet, M. M. (1993). Diversity of Bacillus thuringiensis toxins and genes. In: P. F. Enwistle, J. Cory, M. Bailey, S, Higgs (Eds.), Bacillus thuringiensis, an environmental biopesticide: Theory and practice (p. 37-69). Chichester, UK: John Wiley & Son Ltd.
- Li, H., Oppert, B., Higgins, R. A., Huang, F., Buschman, L. L., & Zhu, K. Y. (2005). Susceptibility of Dipel-resistant and -susceptible Ostrinia nubilalis (Lepidoptera: Crambidae) to individual Bacillus thuringiensis protoxins. Journal of Economic Entomology, 98(4), 1333-1340.
- Ministério da Agricultura, Pecuária e Abastecimento [MAPA]. (1995). Normas e exigências para execução de testes de produtos químicos para fins de registro no MAPA Brasília, DF: Ministério da Agricultura e Reforma Agrária.
- Marra, M. C., Piggott, N. E., & Goodwin, B. K. (2010). The anticipated value of Smart Stax™ for US corn growers. AgBio Forum, 13(1), 1-12.
- Menezes, R. S., Fiuza, V. D., Martins, E. S.,Praça, L. B., & Monnerat, R. G. (2010). Seleção e caracterização de estirpes de Bacillus thuringiensis tóxicas a Agrotisipsilon Universitas Ciências da Saúde, 8(1), 1-13.
- Moar, W. J., Pusztai-Carey, M., & Mack, T. P. (1995). Toxicity of purified proteins and the HD-1 strain from Bacillus thuringiensis against lesser cornstalk borer (Lepidoptera: Pyralidae). Journal of Economic Entomology, 88(3), 606-609.
- Monnerat, R. G., Batista, A. C., Medeiros, P. T., Martins, E., Melatti, V., Praça, L., Dumas, V., … Berry, C. (2007). Screening of Brazilian Bacilus thuringiensis isolates active against Spodoptera frugiperda, Plutella xylostella and Anticarsia gemmatalis Biological Control, 41(3), 291-295.
- Pardo-López, L., Soberón, M., & Bravo, A. (2013). Bacillus thuringiensis insecticidal three-domain Cry toxins: mode of action, insect resistance and consequences for crop protection. FEMS Microbiology Reviews, 37(1), 3-22.
- Porcar, M., & Juárez-Pérez, V. (2003). PCR-based identification of Bacillus thuringiensis pesticidal crystal genes. FEMS Microbiology Reviews, 26(5), 419-432.
- Praça, L. B., Batista, A. C., Martins, E. S., Siqueira, C. B., Dias, D. G. S., Gomes, A. C. M. M., ... Monnerat, R. G. (2004). Estirpes de Bacillus thuringiensis efetivas contra insetos das ordens Lepidoptera, Coleoptera e Diptera. Pesquisa Agropecuária Brasileira, 39(1), 11-16.
- Ricieto, A. P. S., Fazion, F. A. P., Carvalho Filho, C. D., Vilas-Boas, L. A., & Vilas-Bôas, G. T. (2013). Effect of vegetation on the presence and genetic diversity of Bacillus thuringiensis in soil. Canadian Journal of Microbiology, 59(1), 28-33.
- Sanahuja, G., Banakar, R., Twyman, R. M., Capell, T., & Christou, P. (2011). Bacillus thuringiensis: a century of research, development and commercial applications. Plant Biotechnology Journal, 9(3), 283-300.
- Santos, K., Neves, P. M. O. J., Meneguim, A. M., Santos, R. B., Santos, W. J., Vilas-Bôas, G. T., ... Monnerat, R. (2009). Selection and characterization of the Bacillus thuringiensis strains toxic to Spodoptera eridania (Cramer), Spodoptera cosmioides (Walker) and Spodoptera frugiperda (Smith) (Lepidoptera: Noctuidae). Biological Control, 50(2), 157-163.
- Singsit, C., Adang, M. J., Lynch, R. E., Anderson, W. F., Wang, A., Cardineau, G., & Ozias-Akins, P. (1997). Expression of a Bacillus thuringiensis cryA(c) gene in transgenic peanut and its efficacy against lesser cornstalk borer. Transgenic Research, 6(2), 169-176.
- Sun, Y., Fu, Z., Ding, X., & Xia, L. (2008). Evaluating the insecticidal genes and their expressed products in Bacillus thuringiensis strains by combining PCR with Mass Spectrometry. Appliedand Environmental Microbiology, 74(21), 6811-6813.
- Viana, P. A. (2004). Lagarta-elasmo. In J. R. Salvadori, C. J. Ávila, M. T. B. Silva. (Ed.), Pragas de solo no Brasil (p. 379-408). Passo Fundo, RS: Embrapa Trigo; Dourados, MS: Embrapa Agropecuária Oeste; Cruz Alta, RS: Fundacep Fecotrigo.
- Vidal-Quist, J. C., Castañera, P., & González-Cabrera, J. (2009). Diversity of Bacillus thuringiensis strains isolated from citrus orchards in Spain and evaluation of their insecticidal activity against Ceratitis capitata Journal of Microbiology and Biotechnology, 19(8), 749-759.
- Vilas-Bôas, G. T., Peruca, A. P. S., & Arantes, O. M. N. (2007). Biology and taxonomy of Bacillus cereus, Bacillus anthracis and Bacillus thuringiensis Canadian Journal of Microbiology, 53(6), 673- 687.
- Vilella, F. M. F., Waquil, J. M., Vilela, E. F., Siegfried, B. D., & Foster, J. E. (2002). Selection of the fall armyworm, Spodoptera frugiperda (J. E. Smith) (Lepidoptera: Noctuidae) for survival on Cry 1A(b)Bt toxin. Revista Brasileira de Milho e Sorgo, 1(1), 12-17.
- Walker, D. R., All, J. N., Mcpherson, R. M., Boerma, H. R., & Parrott, W. A. (2000). Field evaluation of soybean engineered with a synthetic crylAc transgene for resistance to corn earworm, soybean looper, velvetbean caterpillar (Lepidoptera: Noctuidae), and lesser cornstalk borer (Lepidoptera: Pyralidae). Journal of Economic Entomology, 93(3), 613-622.
Publication Dates
-
Publication in this collection
Oct 2017
History
-
Received
13 July 2016 -
Accepted
09 Dec 2016