ABSTRACT.
This study investigated the thermodynamic properties of water sorption in soybean using the DM 68I69 Ipro variety from Campo Verde, Mato Grosso, Brazil. Grains with initial moisture contents of 21.95% (w.b.) and 3.50% (w.b.) were used for desorption and adsorption analyses, respectively. The static-gravimetric method determined equilibrium moisture content at various temperatures (10, 20, 30, 40, and 50°C) and relative humidity (between 0.11 and 0.92 ± 2%). The Modified Halsey model quantified the hygroscopicity of soybean. Key findings include: (a) desorption equilibrium moisture content exceeded adsorption values, demonstrating hysteresis; (b) decreasing equilibrium moisture content increased the energy required for water removal and its release during adsorption; (c) differential entropy of desorption and adsorption increased with decreasing equilibrium moisture content; (d) Gibbs free energy decreased with increasing temperature for both desorption and adsorption. The enthalpy-entropy compensation theory effectively described the observed phenomena.
Keywords:
gibbs free energy; differential enthalpy; isosteric heat; differential entropy
Introduction
Understanding the thermodynamic properties of soybean water sorption is crucial for optimizing post-harvest processes, given their intricated relation with food quality (Arslan-Tontul, 2020). They can be calculated through sorption isotherms (Hassini et al., 2015), providing a deeper understanding of the interaction between water molecules and a product. This calculation also assists in determining the energy related to heat and mass transfer in biological systems (Teixeira et al., 2018). Additionally, knowledge of these properties is crucial for predicting the drying limit to obtain a storable product over extended periods (Resende et al., 2017).
The integral isosteric heat of sorption (Qst) indicates the strength of the binding force between the solid and water. It serves as an indicator of the state in which water is found within the biological material, based on the type of force involved in the molecular interconnection of water vapor with sorption sites (Corrêa et al., 2010). The state of sorbed water is indicative of the physical, chemical, and microbiological stability of stored food products. Understanding the magnitude of integral isosteric heat helps design dryers capable of providing heat above the latent heat of pure water vaporization for drying materials at low moisture content levels.
The liquid isosteric heat of sorption (∆hst), or differential enthalpy, is defined as the difference between the integral isosteric heat of sorption and the latent heat of vaporization of pure water at a given temperature. It originates from the Clausius-Clapeyron equation, assuming that the latent heat of vaporization, liquid heat of sorption, and equilibrium humidity remain constant with temperature variation (Basu et al., 2013).
The value of differential entropy (∆S) is proportional to the number of sorption sites available at a specific energy level, revealing the degree of disorder in a system. Higher disorder corresponds to higher entropy associated with the process (Lago et al., 2013). This information is useful for interpreting phenomena such as dissolution, crystallization, and hydration that typically occur during water sorption in food products.
Gibbs free energy (∆G) is a thermodynamic state function that represents the maximum energy released in a process occurring at constant temperature and pressure. It allows us to assess the affinity between food constituents and water molecules during desorption and adsorption processes (Montanuci et al., 2013). This criterion determines whether water sorption is a spontaneous process (with a negative ∆G value) or non-spontaneous (with a positive ∆G value) (Sousa et al., 2015).
A linear relationship often exists between enthalpy and differential entropy during the water sorption process in certain foods. Because of this correlation, the compensation theory is assumed to be valid for the water sorption process (Hassini et al., 2015). The theory of enthalpy-entropy compensation is a valuable tool for differentiating the mechanisms of water sorption under varying conditions and identifying whether they are governed by enthalpy or entropy.
To confirm the existence of compensation, the isokinetic temperature (TB) should be compared with the harmonic mean of temperatures (Thm) used for determining sorption isotherms (Krug et al., 1976a and b). TB represents the temperature at which all reactions occur at the same rate. If TB is greater than Thm, enthalpy governs the process; otherwise, entropy governs it (Spada et al., 2013).
Given the importance of understanding the energy requirements in the water sorption process, the objective of this study is to evaluate the thermodynamic properties of soybean water sorption.
Material and methods
A mathematical model describing desorption and adsorption isotherms of soybean was utilized to calculate the thermodynamic properties. The model was selected based on data from a previously published study (Zeymer et al., 2022), which evaluated sorption isotherms and hysteresis effect. Water activity values were used to calculate the integral isosteric heat of sorption and differential entropy of sorption.
According to Naveen Kumar and Das (2015), to quantify the total energy used during desorption and adsorption, liquid isosteric heat of sorption should be estimated. This parameter was determined using Equation 1, which corresponds to the Clausius-Clapeyron equation.
(1)
where: aw - water activity (decimal); Ta - absolute temperature (K); ∆Hst - liquid isosteric heat of sorption (kJ kg-1); R - universal constant of gases (8,314 kJ kmol-1 K-1).
By integrating Equation 1 and assuming that the liquid isosteric heat of sorption is independent of temperature, we calculated the liquid isosteric heat of sorption for each equilibrium moisture content, as shown in Equation 2.
(2)
where: C1 - model constant (dimensionless).
The integral isosteric heat of sorption was obtained by summing the liquid isosteric heat of sorption and the latent heat of vaporization of free water, defined by Equation 3.
(3)
where: Qst - integral isosteric heat of sorption (kJ kg-1); ∆Hst - liquid isosteric heat of sorption (kJ kg-1); Xe - equilibrium moisture content (% d.b.); A and B - model’s coefficients.
The latent heat of vaporization of free water, necessary for calculating the integral isosteric heat of sorption, is determined as a function of the mean temperature of the range under study (30°C), as described in Equation 4.
(4)
where: L - latent heat of vaporization (kJ kg-1); T- - mean temperature (K).
The latent heat of vaporization of free water represents the energy required to change a unit of mass from the liquid phase to the vapor phase at a given temperature. A negative enthalpy variation signifies an exothermic transformation, indicating heat release, while a positive enthalpy suggests heat absorption, characterizing an endothermic process.
Equation 5, known as the Gibbs-Helmholtz equation, describes changes in differential entropy.
(5)
where: ∆S - differential entropy of sorption (kJ kg-1 K-1); ∆G - Gibbs free energy (kJ kg-1 mol-1); Ta - absolute temperature (K).
Gibbs free energy, in KJ kg-1 mol-1, can be calculated using Equation 6. The signs "+" and "-" in the equation indicate the direction of heat transfer, corresponding to the spontaneity of the studied process. In the present study, a positive sign indicates desorption processes, whereas a negative sign is associated with adsorption processes.
(6)
where: ∆G - Gibbs free energy (kJ kg-1 mol-1); R - universal gas constant (8.314 kJ kmol-1 K-1); Ta - absolute temperature (K); aw - water activity (decimal).
Changes in water sorption affecting Gibbs free energy are often accompanied by variations in integral isosteric heat values. By substituting Equation 5 into Equation 6 and rearranging, we obtain Equation 7.
(7)
The liquid isosteric heat of sorption and differential entropy can be calculated using Equation 7 by plotting the values of the natural logarithm of water activity against the reciprocal of temperature for the respective equilibrium moisture content of soybean.
The linear relationship between enthalpy and entropy was verified by correlating the calculated values of differential sorption enthalpy (∆hst) and entropy (∆S), as shown in Equation 8:
(8)
where: TB - isokinetic temperature (K); ∆GB - Gibbs free energy at isokinetic temperature (KJ kg-1).
Isokinetic temperature is a characteristic property of materials' surface, described by the angular coefficient of the linear relationship between enthalpy and entropy. It represents the temperature at which all reactions occur in series at the same rate. Since enthalpy and entropy are highly correlated, the compensation theory is assumed to be valid for sorption.
To confirm the existence of compensation, we compared the isokinetic temperature with the harmonic mean (Equation 9) of the temperatures used to determine the sorption isotherms (Krug et al., 1976a and b).
(9)
where: Thm - harmonic mean of temperature (K); n - number of temperatures used; Ti - temperature of the i-th isotherm (K).
According to Krug et al. (1976a and b), linear chemical compensation or the compensation theory exists only if the isokinetic temperature (TB) differs from the harmonic mean of temperature (Thm). When TB > Thm, the process is governed by enthalpy; otherwise, the process is controlled by entropy. The isokinetic temperature was calculated using Equation 10.
(10)
The variables T B and V ar (T B ) were defined following equations 11 and 12, respectively.
(11)
(12)
where: m - number of enthalpy and entropy data pairs; (H- - mean enthalpy (kJ kg-1); (S- - mean entropy (kJ kg-1 K-1).
If the harmonic mean of the temperature Thm falls within the calculated range of the isokinetic temperature TB, any relationship between the values of enthalpy and differential entropy of sorption primarily reflects experimental errors rather than the influence of chemical and physical factors governing the compensation theory.
Results and discussion
According to the results reported by Zeymer et al. (2022), the Modified Halsey model (Equation 13) demonstrated superior suitability in describing the desorption and adsorption phenomena in soybean. Employing this model and the associated mathematical coefficients, we derived water activity values (expressed as decimals) for both desorption and adsorption, as delineated in Equations 14 and 15, correspondingly.
(13)
(14)
(15)
**Significant at 5% probability by the ''t" test.
where: Xe - equilibrium moisture content (% d.b.); a w - Water activity (decimals); T - Temperature (°C).
Water activity was estimated using the Modified Halsey model at temperatures of 10, 20, 30, 40, and 50°C. Equilibrium moisture ranged from 3.80 to 26.70% (d.b.) for desorption and 3.50 to 26.50% (d.b.) for adsorption (data not presented). These datasets were then employed to calculate the differential enthalpy and differential entropy during both desorption and adsorption in soybean. It is worth noting that as equilibrium moisture content and/or temperature increased, water activity also rose, indicating enhanced water availability within the product. Similar findings have been reported by Teixeira et al. (2018) for pumpkin seeds over a temperature range of 20 to 70°C, by Zeymer et al. (2018a) for lettuce seeds spanning temperatures from 10 to 50°C, and by Campos et al. (2019) for sunflower seeds exposed to temperatures between 10 and 55°C.
Using the water activity data, the liquid isosteric heat of sorption was calculated based on the angular coefficients obtained from the curves plotting ln (aw) against 1/T (K-1). This calculation was performed for various equilibrium moisture contents during both desorption and adsorption processes, as outlined in Equation 1.
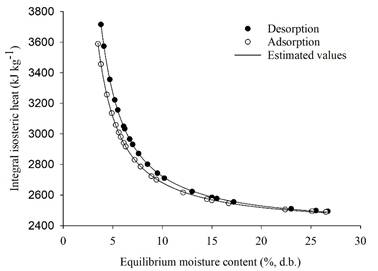
The isosteric heat values for soybean were subsequently determined by extracting the angular coefficients through linear regression applied to Equation 1. In accordance with Equation 4, the mean empirical temperature employed for calculating the latent heat of vaporization of free water was 30°C, yielding a value of 2,430.50 kJ kg-1. The outcomes of these calculations are depicted in Figure 1, illustrating both the observed and estimated values of the integral isosteric heat for both desorption and adsorption in relation to the equilibrium moisture content.
Observed and estimated values of integral isosteric heat of soybean desorption and adsorption.
Figure 1 reveals a notable exponential increase in the integral isosteric heat for both desorption and adsorption as the equilibrium moisture content decreases. This trend underscores a critical observation: in products characterized by higher moisture content, the binding force between water molecules and dry matter significantly diminishes. At lower water levels, specific sorption sites exhibit elevated interaction energy, causing water molecules to concentrate and form a monomolecular layer. As water chemically binds to these highly active sorption sites, it becomes sorbed at locations characterized by lower interaction energy (associated with higher water levels), resulting in a correspondingly reduced isosteric heat of sorption.
Figure 1 also illustrates that the integral isosteric heat values for desorption exceeded those for adsorption. Nevertheless, these values tend to approach similarity and remain relatively constant at elevated levels of equilibrium moisture content. This pattern suggests that the desorption process demands less energy for its occurrence when compared to adsorption. Notably, similar findings have been supported by previous studies, such as those conducted by Bonner and Kenney (2013) on sorghum grains, Corrêa et al. (2017) on bean grains, and Bustos-Vanegas et al. (2018) on quinoa seeds.
Understanding the magnitude of integral isosteric heat of sorption for a specific moisture content offers valuable insights into the state of water sorbed within a product. Furthermore, it serves as an indicator of the physical, chemical, and biological stability of food under given storage conditions. For soybean, the values ranged from 3,715.71 to 2,493.76 kJ kg-1 over a moisture content range of 3.80 to 26.70% (d.b.) for desorption. Meanwhile, for adsorption, these values spanned from 3,588.88 to 2,489.02 kJ kg-1 within a moisture content range of 3.50 to 26.50% (d.b.). These results align with the findings of Goneli et al. (2016), who studied castor beans and reported values between 3,324 to 2,486 kJ kg-1 for desorption, covering a moisture content range of 4.77 to 18.91% (d.b.). For adsorption, the range was 3,139 to 2,479 kJ kg-1 within a moisture content range of 4.34 to 11.82% (d.b.).
Table 1 displays the equations adjusted to the experimental data of integral isosteric heat for both desorption and adsorption in soybean grains. These equations demonstrate excellent fit, as indicated by their high coefficients of determination (R²) and statistically significant coefficients.
Figure 2 depicts the observed and estimated values of differential entropy for desorption and adsorption in soybean, measured in kJ kg⁻¹ K⁻¹, with respect to the equilibrium moisture content (%, d.b.).
Observed and estimated values of differential entropy of soybean desorption and adsorption.
A notable trend emerges, in which as the interaction between water molecules and the product's constituents intensifies (characterized by lower equilibrium moisture content), the energy required for these molecules to transition from the solid surface of the product to the vapor state increases. Simultaneously, the mobility of water molecules within the system rises. This phenomenon aligns with observations made by Rosa et al. (2013) for orange seeds and by Silva and Pena (2018) for pumpkin seeds.
Furthermore, it is worth noting that the differential entropy exhibits a pattern similar to that of the integral isosteric heat of sorption. This alignment was anticipated, given that entropy generation is inherently linked to heat transfer between two systems, with its magnitude consistently proportional to the heat exchanged at identical temperatures (Zeymer et al., 2018b). Consequently, due to the greater heat transfer during desorption compared to adsorption, the magnitudes of differential entropy are consistently higher. Similar findings have been reported by Aviara et al. (2016) and Silva et al. (2018) in their assessments of the thermodynamic properties during the desorption and adsorption of corn grains and pepper seeds, respectively.
Still analyzing Figure 2, it is evident that the magnitude of the differential entropy values for desorption exceeds those for adsorption. This disparity in differential entropy is directly proportional to the number of available sorption sites at a specific energy level, reflecting the varying mobility of water molecules within the product. Consequently, our findings suggest that water molecules exhibit greater mobility during desorption compared to adsorption. Notably, for higher moisture contents, there is a decline in differential entropy values, with a tendency to stabilize, as the active sites tend to saturate. In this context, we posit that the maximum stability of soybean corresponds to the region of minimum differential entropy, signifying well-organized water molecules with limited participation in deterioration reactions (Souza et al., 2015).
The differential entropy of desorption and adsorption strongly correlates with the moisture content of soybean, with values ranging from 2.95 to 0.15 kJ kg-1 K-1 for desorption within the moisture range of 3.80 to 26.70 (%, d.b.), and from 2.58 to 0.13 kJ kg-1 K-1 for adsorption within the moisture range of 3.50 to 26.50 (%, d.b.). These values represent the upper and lower limits of differential entropy, highlighting the observable alterations in the arrangement of water molecules as moisture content increases. It is plausible that sorption isotherms exert an influence on grain properties, as water, serving as a solvent, appears to enhance the mobility of the product's chemical constituents through dissolution, consequently impacting the entropy magnitude within the process.
Table 2 provides further support for our observations, revealing that, similar to the integral isosteric heat of sorption, the equations linking differential entropy for desorption and adsorption yield satisfactory coefficients of determination (R²). This implies a strong alignment between the observed data and the estimates derived from our proposed equations.
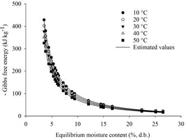
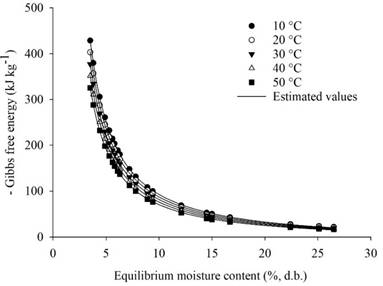
Figures 3 and 4 depict the observed and estimated Gibbs free energy values (in kJ kg-1) in relation to equilibrium moisture content (expressed as % d.b.) for rice grains in husk, with data collected during desorption and adsorption, respectively. From a thermodynamic standpoint, the Gibbs free energy of a product serves as a critical parameter indicating the affinity between the product and water. It offers insights into the spontaneity of the process, with positive Gibbs free energy values indicative of non-spontaneous processes. In such cases, entropy is negative, and enthalpy is positive. Conversely, spontaneous processes exhibit positive entropy and negative enthalpy, resulting in negative values for Gibbs free energy.
Analyzing Figures 3 and 4, we observed that Gibbs free energy values were positive during desorption and negative during adsorption. This indicates that desorption is endothermic and non-spontaneous, while adsorption is exothermic and spontaneous. These findings align with those of Zeymer et al. (2018b) in their evaluation of the thermodynamic properties of rice grains in husk. Changes in Gibbs free energy during water exchange between the product and surrounding medium represent the energy required to transfer water molecules between the gaseous and solid states, or vice versa. This value quantifies the work performed by the system during the desorption or adsorption process, with equilibrium attained when the Gibbs free energy gradient equals zero.
Figures 3 and 4 also indicate that Gibbs free energy decreased with increasing moisture content during both desorption and adsorption processes, eventually stabilizing at higher equilibrium moisture levels. This suggests that less energy was required to make sorption sites available at lower Gibbs free energy values across all temperatures, a trend supported by findings from Sousa et al. (2015) for mango peel, Zeymer et al. (2018b) for rice grains, Bustos-Vanegas et al. (2018) for quinoa seeds, and Campos et al. (2019) for sunflower seeds.
The adjusted equations for Gibbs free energy (kJ kg-1 mol-1) as a function of equilibrium moisture content (%, d.b.) at various temperatures are provided in Table 3 for both desorption and adsorption. These equations demonstrate a strong correspondence between the observed and estimated data, as indicated by the coefficient of determination (R2) and level of significance.
Figure 5 illustrates the relationship between the values of differential enthalpy and differential entropy for both desorption and adsorption processes. These values were computed using Equation 7 for each corresponding equilibrium moisture content. It is important to note that, for a given moisture content, differential enthalpy and differential entropy remain constant across different temperatures.
As described by Velázquez-Gutiérrez et al. (2015), the theory of enthalpy-entropy compensation serves as a valuable tool for assessing physical and chemical phenomena within food, particularly sorption reactions. This theory allows us to examine whether increased molecular interactions or connections between molecules, stemming from reduced freedom or the binding of water molecules, lead to greater organization (associated with enthalpy) or disorganization (associated with entropy) within the system (Spada et al., 2013).
Figure 5 provides evidence of linear relationships for both desorption and adsorption processes. The high degree of linearity observed between differential enthalpy and differential entropy for sorption, in both desorption and adsorption, supports the validity of the enthalpy-entropy compensation theory, or the isokinetic theory, for the phenomenon of water sorption in soybean.
Table 4 presents the calculated isokinetic temperatures, harmonic mean temperatures, and Gibbs free energy values at the isokinetic temperature for both desorption and adsorption of soybean, along with their 99% confidence intervals. To substantiate the existence of the theory of linear chemical compensation, the isokinetic temperature should be compared to the harmonic mean temperature (Krug et al., 1976a; 1976b). The data in Table 4 show that the isokinetic temperatures for the desorption and adsorption processes of soybean are 432.68 ± 0.42 and 448.95 ± 0.02, respectively.
The harmonic mean temperature calculated was 302.50 K, a value significantly distinct from the aforementioned isokinetic temperature values. This distinction confirms the presence of enthalpy-entropy compensation in the sorption of water in soybean. Furthermore, the Gibbs free energy exhibited positive values for desorption and negative values for adsorption. This observation implies that, in the current study, the desorption process is non-spontaneous, while the adsorption process is considered spontaneous (Slavutsky & Bertyzzi, 2015).
The water sorption in agricultural products can be influenced by either enthalpy or entropy. According to Leffler (1955), when TB>Thm, the process is primarily governed by enthalpy, whereas if TB<Thm, entropy takes precedence. In our study, we have observed that the first condition holds true, indicating that the sorption mechanism of water in soybean is predominantly controlled by enthalpy. This finding aligns with the conclusions of numerous researchers who have also established that water sorption is predominantly driven by enthalpy (Koua et al., 2014; Sousa et al., 2015; Simón et al., 2016; Resende et al., 2017; Silva & Pena, 2018; Silva et al., 2018; Bustos-Vanegas et al., 2018; Zeymer et al., 2018b; Campos et al., 2019).
Conclusion
The following conclusions can be drawn from our findings: The equilibrium moisture content obtained through desorption was higher than that obtained through adsorption, indicating the presence of hysteresis within the studied temperature range. Decreasing the equilibrium moisture content resulted in an increased energy requirement for water removal from the product, as indicated by the integral isosteric heat values for desorption. Conversely, it led to higher energy release during water adsorption, reflected in the integral isosteric heat values for adsorption. A reduction in equilibrium moisture content corresponded to an increase in the differential entropy values for both desorption and adsorption. Gibbs free energy decreased as temperature increased for both processes. Gibbs free energy values were positive for desorption, indicating an endothermic process, and negative for adsorption, indicating an exothermic process. The theory of enthalpy-entropy compensation, or isokinetic theory, was successfully applied to describe the water sorption phenomenon in soybean grains, with both desorption and adsorption processes primarily controlled by enthalpy.
Acknowledgements
The authors thank the Brazilian National Council for Scientific and Technological Development (CNPq) for the doctoral scholarship granted
References
-
Arslan-Tontul, S. (2020). Moisture sorption isotherm, isosteric heat and adsorption surface area of whole chia seeds. LWT, 119, e108859. https://doi.org/10.1016/j.lwt.2019.108859
» https://doi.org/https://doi.org/10.1016/j.lwt.2019.108859 - Aviara, N. A., Ojediran, J. O., Marwan, S. I. U., & Raji, A. O. (2016). Effect of moisture sorption hysteresis on thermodynamic properties of two millet varieties. Agricultural Engineering International: CIGR Journal, 18(1), 363-383.
-
Basu, S., Shivhare, U. S., & Muley, S. (2013). Moisture adsorption isotherms and glass transition temperature of pectin. Journal of Food Science and Technology, 50, 585-589. https://doi.org/10.1007/s13197-011-0327-y.
» https://doi.org/https://doi.org/10.1007/s13197-011-0327-y -
Bonner, I. J., & Kenney, K. L. (2013). Moisture sorption characteristics and modeling of energy sorghum (Sorghum bicolor (L) Moench). Journal of Stored Products Research, 52, 128-136. https://doi.org/10.1016/j.jspr.2012.11.002
» https://doi.org/https://doi.org/10.1016/j.jspr.2012.11.002 -
Bustos-Vanegas, J. D., Corrêa, P. C., Zeymer, J. S., Baptestini, F. M., & Campos, R. C. (2018). Moisture sorption isotherms of quinoa seeds: Thermodynamic analysis. Engenharia Agrícola, 38(6), 941-950. https://doi.org/10.1590/1809-4430-Eng.Agric.v38n6p941-950/2018
» https://doi.org/https://doi.org/10.1590/1809-4430-Eng.Agric.v38n6p941-950/2018 -
Campos, R. C., Corrêa, P. C., Zaidan, I. R., Zaidan, U. R., & Leite, R. A. (2019). Moisture sorption isotherms of sunflower seeds: Thermodynamic analysis. Ciencia & Agrotecnologia, 43, 1-12. https://doi.org/10.1590/1413-7054201943011619
» https://doi.org/https://doi.org/10.1590/1413-7054201943011619 -
Corrêa, P. C., Oliveira, G. H. H., Botelho, F. M., Goneli, A. L. D., & Carvalho, F. M. (2010). Modelagem matemática e determinação das propriedades termodinâmicas do café (Coffea arabica L.) durante o processo de secagem. Revista Ceres, 57(5), 595-601. https://doi.org/10.1590/S0034-737X2010000500005
» https://doi.org/https://doi.org/10.1590/S0034-737X2010000500005 -
Corrêa, P. C., Baptestini, F. M., Vanegas, J. D. B., Leite, R., Botelho, F. M., & Oliveira, G. H. H. (2017). Kinetics of water sorption of damaged bean grains: Thermodynamic properties. Revista Brasileira de Engenharia Agrícola e Ambiental, 21(8), 556-561. https://doi.org/10.1590/1807-1929/agriambi.v21n8p556-561
» https://doi.org/https://doi.org/10.1590/1807-1929/agriambi.v21n8p556-561 -
Goneli, A. L. D., Corrêa, P. C., Oliveira, G. H. H., Oliveira, A. P. L. R., & Orlando, R. C. (2016). Moisture sorption isotherms of castor beans. Part 2: Thermodynamic properties. Revista Brasileira de Engenharia Agrícola e Ambiental, 20(8), 757-762. https://doi.org/10.1590/1807-1929/agriambi.v20n8p757-762
» https://doi.org/https://doi.org/10.1590/1807-1929/agriambi.v20n8p757-762 -
Hassini, L., Bettaieb, E., Desmorieux, H., Torres, S. S., & Touil, A. (2015). Desorption isotherms and thermodynamic properties of prickly pear seeds. Industrial Crops and Products, 67, 457-465. https://doi.org/10.1016/j.indcrop.2015.01.078
» https://doi.org/https://doi.org/10.1016/j.indcrop.2015.01.078 -
Koua, B. K., Koffi, P. M. E., Gbaha, P., & Toure, S. (2014). Thermodynamic analysis of sorption isotherms of cassava (Manihot esculenta). Journal of Food Science and Technology, 51, 1711-1723. https://doi.org/10.1007/s13197-012-0687-y
» https://doi.org/https://doi.org/10.1007/s13197-012-0687-y -
Krug, R. R., Hunter, W. G., & Grieger, R. A. (1976a). Enthalpy-entropy compensation. 1 - Some fundamental statistical problems associated with the analysis of Van’t Hoff and Arrhenius data. Journal of Physical Chemistry, 80(21), 2335-2341. https://doi.org/10.1021/j100562a006
» https://doi.org/https://doi.org/10.1021/j100562a006 -
Krug, R. R., Hunter, W. G., & Grieger, R. A. (1976b). Enthalpy-entropy compensation. 2 - Separation of the chemical from the statistical effect. Journal of Physical Chemistry, 80(21), 2341-2351. https://doi.org/10.1021/j100562a007
» https://doi.org/https://doi.org/10.1021/j100562a007 -
Lago, C. C., Liendo-Cárdenas, M., & Noreña, C. P. Z. (2013). Thermodynamic sorption properties of potato and sweet potato flakes. Food and Bioproducts Processing, 91(4), 389-395. https://doi.org/10.1016/j.fbp.2013.02.005
» https://doi.org/https://doi.org/10.1016/j.fbp.2013.02.005 -
Leffler, J. E. (1955). The enthalpy-entropy relationship and its implications for organic chemistry. The Journal of Organic Chemistry, 20(9), 1202-1231. https://doi.org/10.1021/jo01126a009
» https://doi.org/https://doi.org/10.1021/jo01126a009 -
Montanuci, F. D., Jorge, L. M. M., & Jorge, R. M. M. (2013). Kinetic, thermodynamic properties, and optimization of barley hydration. Food Science and Technology, 33(4), 690-698. https://doi.org/10.1590/S0101-20612013000400014
» https://doi.org/https://doi.org/10.1590/S0101-20612013000400014 -
Naveen Kumar, M., & Das, S. K. (2015). Moisture sorption isotherms of preconditioned pressure parboiled brown rice. Journal of Food Processing & Technology, 6(12), 1-9. https://doi.org/10.4172/2157-7110.1000519
» https://doi.org/https://doi.org/10.4172/2157-7110.1000519 -
Resende, O., Oliveira, D. E. C., Costa, L. M., & Ferreira Júnior, W. N. (2017). Thermodynamic properties of baru fruits (Dipteryx alata Vogel). Engenharia Agrícola, 37(4), 739-749. https://doi.org/10.1590/1809-4430-Eng.Agric.v37n4p739-749/2017
» https://doi.org/https://doi.org/10.1590/1809-4430-Eng.Agric.v37n4p739-749/2017 -
Rosa, D. P., Villa-Vélez, H. A., & Telis-Romero, J. (2013). Study of the enthalpy-entropy mechanism from water sorption of orange seeds (C. sinensis cv. Brazilian) for the use of agro-industrial residues as a possible source of vegetable oil production. Food Science and Technology, 33(suppl. 1), 95-101. https://doi.org/10.1590/S0101-20612013000500015
» https://doi.org/https://doi.org/10.1590/S0101-20612013000500015 -
Silva, D. A., & Pena, R. S. (2018). Thermodynamic properties of Buriti (Mauritia flexuosa) tree gum. Food Science and Technology, 38(3), 390-398. https://doi.org/10.1590/fst.02917
» https://doi.org/https://doi.org/10.1590/fst.02917 -
Silva, H. W., Rodovalho, R. S., & Silva, I. L. (2018). Hysteresis and thermodynamic properties of water sorption of ‘MalagXeta’ pepper seeds. Revista Brasileira de Engenharia Agrícola e Ambiental, 22(9), 658-663. https://doi.org/10.1590/1807-1929/agriambi.v22n9p658-663
» https://doi.org/https://doi.org/10.1590/1807-1929/agriambi.v22n9p658-663 -
Simón, C., Esteban, L. G., Palacios, P., Fernández, F. G., & García-Iruela, A. (2016). Thermodynamic properties of the water sorption isotherms of wood of limba (Terminalia superba Engl. & Diels), obeche (Triplochiton scleroxylon K. Schum.), radiate pine (Pinus radiate D. Don) and chestnut (Castanea sativa Mill.). Industrial Crops and Products, 94, 122-131. https://doi.org/10.1016/j.indcrop.2016.08.008
» https://doi.org/https://doi.org/10.1016/j.indcrop.2016.08.008 -
Slavutsky, A. M., & Bertuzzi, M. A. (2015). Thermodynamic study of water sorption and water barrier properties of nanocomposite films based on brea gum. Applied Clay Science, 108, 144-148. https://doi.org/10.1016/j.clay.2015.02.011
» https://doi.org/https://doi.org/10.1016/j.clay.2015.02.011 -
Sousa, K. A., Resende, O., Goneli, A. L. D., Smaniotto, T. A. S., & Oliveira, D. E. C. (2015). Thermodynamic properties of water desorption of forage turnip seeds. Acta Scientiarum. Agronomy, 37(1), 11-19. https://doi.org/10.4025/actasciagron.v37i1.19333
» https://doi.org/https://doi.org/10.4025/actasciagron.v37i1.19333 -
Souza, S. J. F., Alves, A. I., Vieira, E. N. R., Vieira, J. A. G., Ramos, A. M., & Telis-Romero, J. (2015). Study of thermodynamic water properties and moisture sorption hysteresis of mango skin. Food Science and Technology, 35(1), 157-166. https://doi.org/10.1590/1678-457X.6557
» https://doi.org/https://doi.org/10.1590/1678-457X.6557 -
Spada, J. C., Noreña, C. P. A., Marczak, L. D. F., & Tessaro, I. C. (2013). Water adsorption isotherms of microcapsules with hydrolyzed pinhão (Araucaria angustifolia seeds) starch as wall material. Journal of Food Engineering, 114(1), 64-69. https://doi.org/10.1016/j.jfoodeng.2012.07.019
» https://doi.org/https://doi.org/10.1016/j.jfoodeng.2012.07.019 -
Teixeira, L. P., Andrade, E. T., & Devilla, I. A. (2018). Isosteric heat, entropy and gibbs free energy of pumpkin seeds (Cucurbita moschata). Engenharia Agrícola, 38(1), 97-102. https://doi.org/10.1590/1809-4430-Eng.Agric.v38n1p97-102/2018
» https://doi.org/https://doi.org/10.1590/1809-4430-Eng.Agric.v38n1p97-102/2018 -
Velázquez-Gutiérrez, S. K., Figueira, A. C., Rodríguez-Huezo, M. E., Román-Guerrero, A., Carrillo-Navas, H., & Pérez-Alonso, C. (2015). Sorption isotherms, thermodynamic properties and glass transition temperature of mucilage extracted from chia seeds (Salvia hispanica L.). Carbohydrate Polymers, 121, 411-419. https://doi.org/10.1016/j.carbpol.2014.11.068
» https://doi.org/https://doi.org/10.1016/j.carbpol.2014.11.068 -
Zeymer, J. S., Correa, P. C., Oliveira, G. H. H., Araújo, M. E. V., Guzzo, F., & Baptestini, F. M. (2022). Moisture sorption isotherms and hysteresis of soybean grains. Acta Scientiarum. Agronomy, 45(1), 1-11. https://doi.org/10.4025/actasciagron.v45i1.56615
» https://doi.org/https://doi.org/10.4025/actasciagron.v45i1.56615 -
Zeymer, J. S., Corrêa, P. C., Oliveira, G. H. H., & Baptestini, F. M. (2018a). Thermodynamic properties of water desorption in lettuce seeds. Semina: Ciências Agrárias, 39(3), 921-932. https://doi.org/10.5433/1679-0359.2018v39n3p921
» https://doi.org/https://doi.org/10.5433/1679-0359.2018v39n3p921 -
Zeymer, J. S., Corrêa, P. C., Oliveira, G. H. H., Baptestini, F. M., & Faria, I. L. (2018b). Thermodynamic properties of sorption of rice in the husk. Engenharia Agrícola, 38(3), 369-375. https://doi.org/10.1590/1809-4430-Eng.Agric.v38n3p369-375/2018
» https://doi.org/https://doi.org/10.1590/1809-4430-Eng.Agric.v38n3p369-375/2018
Publication Dates
-
Publication in this collection
02 June 2025 -
Date of issue
2025
History
-
Received
27 Oct 2023 -
Accepted
30 Jan 2024

 Thermodynamic properties of moisture sorption of soybean (Glycine max L.) grains
Thermodynamic properties of moisture sorption of soybean (Glycine max L.) grains