Abstract
This study examined the effect of three sessions of cryotherapy (three sessions of 30 minutes applied each 2 h) and muscle compression in the regenerating skeletal muscle of the rats. The middle belly of tibialis anterior muscle was injured by a frozen iron bar and received one of the following intervention: injury + cryotherapy (treated with cryotherapy); injury + placebo (sand pack), and injury (I).The enzymatic activities of citrate synthase (CS) and lactate dehydrogenase (LDH) were measured in the presence of 1mM or 10mM pyruvate. The ANOVA and Tukey's test (p<0.05) were performed for the statistical analysis. In summary, the intermittent sessions of cryotherapy, associated to muscle compression and applied immediately after the primary muscle injury minimized the CS and LDH activity at 4h30 and 24h periods post-lesion, which could be related to the reduction in the secondary muscle injury inherent to cryotherapy treatment.
cryotherapy; muscle injury; enzymatic analysis; hypothermia; tibialis anterior; rehabilitation
HUMAN AND ANIMAL HEALTH
The effect of intermittent cryotherapy on the activities of citrate synthase and lactate dehydrogenase in regenerating skeletal muscle
Nuno Miguel Lopes de OliveiraI, II, * * Author for correspondence: nuno@fisioterapia.uftm.edu.br ; João Luiz Quagliotti DuriganI, III; Flávia Simone MuninIV; Maria Luiza Barcelos SchwantesIV; Tania de Fátima SalviniI
ILaboratório de Plasticidade Muscular; Departamento de Fisioterapia; Universidade Federal de São Carlos
IIDepartamento de Fisioterapia; Universidade Federal do Triângulo Mineiro; Uberaba - MG - Brasil
IIIDepartamento de Fisioterapia; Universidade de Brasília; Brasília - DF - Brasil
IVLaboratório de Evolução Molecular; Departamento de Genética e Evolução; Universidade Federal de São Carlos; São Carlos - SP - Brasil
ABSTRACT
This study examined the effect of three sessions of cryotherapy (three sessions of 30 minutes applied each 2 h) and muscle compression in the regenerating skeletal muscle of the rats. The middle belly of tibialis anterior muscle was injured by a frozen iron bar and received one of the following intervention: injury + cryotherapy (treated with cryotherapy); injury + placebo (sand pack), and injury (I).The enzymatic activities of citrate synthase (CS) and lactate dehydrogenase (LDH) were measured in the presence of 1mM or 10mM pyruvate. The ANOVA and Tukey's test (p<0.05) were performed for the statistical analysis. In summary, the intermittent sessions of cryotherapy, associated to muscle compression and applied immediately after the primary muscle injury minimized the CS and LDH activity at 4h30 and 24h periods post-lesion, which could be related to the reduction in the secondary muscle injury inherent to cryotherapy treatment.
Key words: cryotherapy, muscle injury, enzymatic analysis, hypothermia, tibialis anterior, rehabilitation
INTRODUCTION
Cryotherapy consists in locally applying the cold to remove heat from an injured area. The efficiency of cryotherapy in treating the acute muscle injuries results from the direct relationship between the cellular metabolism and temperature, i.e., to decrease the temperature as oxygen is consumed (Rubley 2002; Schaser et al. 2007), which could reduce secondary hypoxic injury (Knight 1995; Oliveira et al. 2006; Oliveira et al. 2007). The main cryotherapy objective in the short-term phase after muscle injury is to minimize the adverse effects related to the damage process, i.e., pain, edema, hemorrhage and muscle spasm, but mainly to reduce the secondary injury area caused by the ischemia induced by the primary injury (Jarvinen et al. 2005). The treatment of acute injury is supported by the hypothesis that the cold coud reduce the metabolic rate of these hypoxic tissues, enabling them to survive after the initial trauma (Merrick et al. 1999; Merrick 2002; Schaser et al. 2007).
Several studies have used the enzymes that are indicative of oxidative potential, such as citrate synthase (CS, mitochondrial Krebs's cycle) (Powers et al. 1997; Leek et al. 2001), or glycolytic potential, such as lactate dehydrogenase (LDH, terminal enzyme of the anaerobic glycolysis) (Powers et al. 1997). Although LDH is not related as an enzyme able to reduce the glycolysis rate, its activity has been demonstrated to be higher in the tissues with high glycolytic activity (Newsholme and Leech, 1988). Therefore, LDH/CS activity ratio presents low levels in the muscles with high oxidative capacity, while high levels are found in the muscles with low oxidative capacity (Driedzic and Almeida-Val 1996).
The recovery of metabolic pathways following the muscle injury and its relationship with physical therapy intervention, such as cryotherapy, remains to be investigated (Tonkonogi et al. 1997; Siu et al. 2003). Merrick et al. (1999) showed that the application of cryotherapy for five hours after crushing injury reduced the injured area of sural triceps of the rats. Another interesting study demonstrated that the prolonged superficial local cryotherapy (continuous for 6 h) applied in the tibialis anterior reduced the skeletal muscle secondary damage mediated by a decrease in the posttraumatic capillary dysfunction and leukocyte-endothelial cell interaction (Schaser et al. 2007). It was also concluded that this protocol was a valuable therapeutic approach to improve the nutritive tissue perfusion, preserve the cellular viability, attenuate the leukocyte-mediated tissue destruction, and to reduce the risk for evolving the compartment syndrome, thereby preventing further irreversible aggravation (Schaser et al. 2007). Nevertheless, the uninterrupted application of cryotherapy in humans is uncommon and could cause skin burns (Knight 1995).
Intermittent application of cryotherapy, for 30-minute sessions of ice-bag with 1-2 hour intervals has been recommended for treating the muscle injuries in humans (Jarvinen et al. 2005). Previous studies have demonstrated that three intermittent sessions of cryotherapy applied immediately after the muscle damage reduced the secondary muscle injury (Oliveira et al. 2006; Oliveira et al. 2007). However, the effects of cryotherapy after the skeletal muscle injury on CS and LDH responses are unknown. Therefore, the aim of this study was to investigate the effect of intermittent cryotherapy on the activities of CS and LDH in response to muscle injury induced in the rats. Based on the previous findings, the hypothesis that the cryotherapy could decrease or maintain the CS and LDH activity after TA lesion was tested. This could be related to the reduced in the secondary muscle injury inherent to cryotherapy treatment.
MATERIAL AND METHODS
Animal Care
Twenty-four Wistar rats (312 ± 20g) were randomly divided into four groups of six animals each one. They were housed in plastic cages in a room with controlled environmental conditions and had free access to water and standard chow (Socil, Paulínia - São Paulo, Brazil). The study was conducted in accordance with the University approval for the care and use of laboratory animals and was developed in compliance with the national guidelines of the Guide for care and use of laboratory animals (National Research Council 1996). The animals were anaesthetized by an intraperitoneal injection of xylazine (12 mg/kg) and ketamine (95 mg/kg) during the induction of tibialis anterior (TA) muscle injury, application of cryotherapy and compression on the muscle, and for the muscle remotion. Afterwards, they were euthanized by an overdose of the anaesthetic.
Freezing muscle injury
TA muscle was chosen because almost all fibers cross the middle belly of the muscle and are distributed from tendon to tendon. Additionally, as a superficial muscle, it was easily to perform the surgery (Oliveira et al. 2006; Durigan et al. 2008). To induce the muscle injury on the middle belly of the right TA, the skin around the muscle was trichotomized and cleaned. Then, a transversal skin incision (about 1 cm) over the muscle middle belly was carried out, exposing the TA muscle. A rectangular iron bar (40 x 20 mm2) was frozen in fluid nitrogen and then kept for 10s on the muscle belly. The same procedure was repeated two consecutive times with interval of 30s. After that, the skin was sutured. Injury by freezing (cryoinjury) is a common procedure used to induce muscle damage (Oliveira et al. 2006; Durigan et al. 2008).
Cryotherapy
Immediately after the induced muscle injury, the animals were maintained in horizontal position on a plastic table and the ankle of right hindlimb was maintained by tape for the exposition of TA muscle skin. The sessions of cryotherapy (three sessions of 30 minutes applied each 2 h) consisted of the application of a plastic pack filled with crushed ice, maintained by the tape directly on the skin of the right TA muscle. The ice pack covered all the extension of the TA muscle. As the ice pack also produced a muscle compression, which could affect the extension of the secondary muscle injury, an additional group of animals was evaluated using the same experimental conditions, but the ice pack was switched by a sand pack with the same weight used in the ice pack (30g) (Oliveira et al. 2006).
Animal groups
The middle belly of the right TA (RTA) muscle of three groups of the animals (n = 18) was injured and each group was submitted to one of the following procedures: a) three sessions of cryotherapy (ice pack, n = 6), as previously described; b) three sessions of compression (sand pack, n = 6); c) not treated (n = 6). One group of animals was not injured, but also received three sessions of cryotherapy (n = 6). This group was included to evaluate the possible presence of muscle injury induced by the sessions of muscle cooling. Immediately after the last cryotherapy or compression sessions, e.g., 4 ½ hours after induced muscle injury, both, right (injured side) and left (uninjured side) TA muscles of all the animals group were carefully dissected, avoiding mechanical injuries and removed. Afterwards, they were individually weighed (Denver Instruments Company, Model 100a, USA) and frozen in isopentane, previously frozen in liquid nitrogen and stored at -80°C (Forma Scientific, USA).
CS and LDH enzymatic activities
Two transverse incisions were made with a scalpel to delimit the damage area in the lesion area site of the RTA. The distance between the incisions was 1 cm, measured with the caliper, magnifying glass and microscope. The incision was performed in the entire length of the muscle belly in order to remove a single fragment containing the superficial and deep regions of the TA.
Subsequently, the superficial and deep regions of this fragment were separated using the caliper to determine the characteristics of the muscle region. Each fragment of the superficial and deep muscles of RTA was weighed and divided into two parts. One of these parts, covering approximately 33% of the original mass, was used to analyze to the CS kinetics, while the other part was used to determine the LDH levels. These fragments were immediately frozen by immersion in liquid nitrogen and stored in a freezer at -80 C. The same extraction procedure was performed in the LTA muscle of control group, which was determined by random choice of six muscles between the different experimental groups and was used as a parameter to identify the activity of these enzymes in a state of integrity tissue. To obtain the extracts, the surface and deep fractions of each RTA of the three experimental groups were weighed and mixed together (pool) in a phosphate buffered saline buffer. The homogenates were centrifuged in refrigerated centrifuge (Sorvall RC-5B) at 27,000 x g at 4 ºC for 15 minutes. After centrifugation, the supernatants of each fraction were used to measure the activities of LDH and CS.
Spectrophotometric determinations of enzyme activity were performed through the use of techniques described by Driedzic and Almeida-Val (1996). All enzyme assays were conducted in a final volume of 1.0 ml at 25 °C performed triplicate for each sample (pool). The samples of all the muscles were tested on the same day in order to reduce the variations assessment. For LDH, the reactions were initiated by the addition of substrate, followed by the oxidation of NADH at 340nm (mill molar extinction coefficient = 6.22). Regarding CS, the reaction was monitored according to the production of DTNB (5,5'-Dithiobis; 2-nitrobenzoic acid) reduced to 412 nm (mil imolar extinction coefficient = 13.6). The results were expressed in IU / g of fresh tissue, and UI unit represents enzyme activity and was defined as the amount of enzyme that converted 1 µmol of substrate to product per minute (moles of substrate µg-1 fresh tissue. min-1) at 25°C.
Statistical analysis
Levene's test was applied first to evaluate the homogeneity of the results. Multiple comparisons of mean values were performed using the analysis of variance (ANOVA) and a post hoc Tukey test to compare mean values when appropriate. Student paired t-test was used for the comparison between the TA muscles of the same animals group. For all the tests, the significance level was set at 5% (P < 0.05).
RESULTS AND DISCUSSION
Groups assessed 4h30 after injury
All the groups presented elevated values in the CS activity compared to control (p<0.05; Table 1). While I group presented the highest value compared to all groups, I+C group showed the lower values compared to I+P and I groups (Table 1).The LDH activity in the presence of 1mm pyruvate was highest in the control group compared to all the experimental groups (p<0.05; Table 1), without differences among the groups (p>0,05) In the presence of 10mM of pyruvate, the LDH activity was lower compared to 1 mM (Table 1). Similar results were found in the presence of 10mM of pyruvate, where the LDH presented higher values in the control group when compared to I+C+, I+P and I, without differences among the groups (p>0.05) (Table 1).
Low/high ratios (LDH activity in low/high substrate concentrations) were higher than 1, suggesting predominance of subunit B in the superficial region (Table 2). The LDH/CS ratios indicated the predominance of anaerobic metabolism in the four analyzed groups in the superficial region, In the superficial region of the RTA muscles, the LDH/CS ratio ranged from 1.2 (I+P Group) to 2 (I+C), indicating a decrease in the anaerobic metabolism in relation to control (Table 2).
Groups assessed 24h after injury
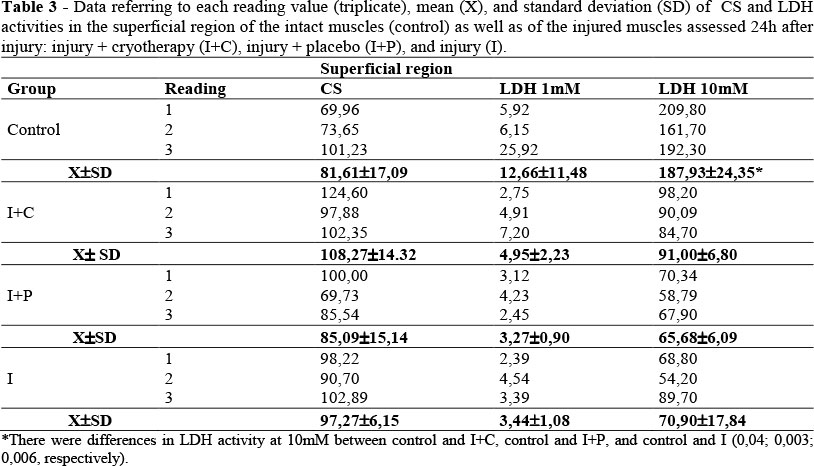
No significant differences were observed among the groups regarding CS activity, as well as in the LDH activity in the presence of 1 mM piruvate (p>0,05; Table 3). The LDH activities were higher in the presence of 10 mM pyruvate when compared with 1mM. The control group values were significantly higher than the I+C, I+P and I (p<0.05; Table 3).
The Low/high ratios values were lower than 1, which suggested the predominance of subunit A in the superficial region (Table 4). The LDH/CS ratio ranged from 0.03 (I, I+C Group) to 0.15 (control) indicating the predominance of aerobic metabolism in the four groups analyzed in the superficial region (Table 4).
Effect of cryolesion in the enzymatic activity of injured rats
Apparently no studies have been done on the effects of clinical-like cryotherapy after the skeletal muscle lesion on the regulation of CS and LDH activity. The present results clearly showed that the intermittent sessions of cryotherapy, associated to muscle compression and applied immediately after primary muscle injury minimized the accumulation of CS and LDH activity at 4h30 and 24h post-lesion. It could explain why cryotherapy treatment reduced the secondary muscle injury, as previously demonstrated (Oliveira et al. 2006). These findings showed cryotherapy as a scientific evidence-based practice.
At low temperatures around 0 to 5 ºC, many proteins such as LDH are inactivated (Zondag 1963). The cold inactivation is accompanied by dissociation into subunits (such as LDH, an oligomeric protein), which determines the loss of their quaternary structure. This occurs due the loss of hydrophobic interactions that maintain the quaternary structure of proteins at cold condition (Alexandrov 1977). As expected, the activity of LDH was decreased by cryolesion in both the periods, at 4h30 and 24h post-lesion. The low levels of activity were observed at 24h, in which there was a decrease of secondary injury, according to previous results (Oliveira et al. 2006). Concomitantly, a decrease of subunits B was observed (means of reasons B / A), in the superficial injury area at 4h30. According to Simoneau and Pette (1989), the LDH modification isoenzymes was an adaptation that reduced the conversion of pyruvate to lactate in the skeletal muscle.
It is widely accepted that the B4 isoforms of LDH have low Km (the amount of substrate that produces the half of the maximum velocity of the enzyme) for pyruvate and NADH, which are sensitive to inhibition by high concentrations of pyruvate and / or lactate, and the isoforms have a high affinity for lactate. Then, they are kinetically well adjusted to function in an aerobic metabolism, since it does not compete for pyruvate (which is metabolized for the CK). However, they compete for lactate and as a result it is involved their oxidation. The A4 isoforms of LDH have higher Km values for pyruvate and NADH and are insensitive to inhibition by pyruvate or lactate, and have low affinity for lactate. Therefore, they work well as pyruvate reductase in anaerobic glycolysis (Powers 1997).
Classical studies have shown that the LDH of air-breathing fish heart possess an important functional characteristic inherent to high sensitivity to inhibition by pyruvate (Hochachka and Storey 1975; Hochachka and Murphy 1979), which could justify the present results on the reasons for B / A greater than 1, for LDH activity at 4h30 after the injury. The results suggested that cryolesion induced the use of lactate oxidase function of LDH, once a high concentrations of epinephrine and glucagon was observed in the first response phase to tissue damage, which stimulated the glycogenolysis (breakdown of glycogen) and gluconeogenesis (glucose synthesis) (Baynes and Dominiczak 2000). Subsequently, the metabolic rate increased and the energy was supplied primarily by the oxidation of fatty acids and the proteins.
The metabolic response to muscle lesion is characterized by the suppression of anabolic pathways (glycogen synthesis and lipogenesis), the increase in catabolic pathways (glycogenolysis, lipolysis and proteolysis), as well as the increase glucose incorporation and insulin resistance. Here, the cryolesion increased the type A subunits (ratios B / A less than 1) in the superficial region (where they were most abundant), which could be related to the use of the function of pyruvate reductase of LDH, activated by decreased intracellular pH (lactate formation).
In this study, the activity of citrate synthase enzyme (CK) was used as a marker of oxidative metabolism, which was elevated in cryolesion groups at 4h30 and 24h after lesion. By the contrary, Merrick and colleagues (1999) demonstrated increase in the CS activity (cytochrome oxidase activity) after crush injury (5h post-lesion) of triceps surae muscle. This difference could be related to different lesion models assessed, since in the present study cryolesion was used, whereas Merrick et al. (1999) evaluated a crush injury model. In addition, these diverges results could be attributed to the type of muscle evaluated, since in this work tibialis anterior was evaluated, while other authors used triceps surae muscle. It would be important to emphasized that the cryolesion model (with liquid nitrogen) could increase the CS activity, since the quaternary structure of this molecule was stabilized by the weak interactions such as Van der Waals forces, hydrogen bonds or ionic bonds (electrostatic), which were formed with negative entropy changes (Newsholme and Leech 1988).
Another important point of the present results was related with the rations of LDH/CS, which showed that the predominant metabolism was anaerobic (values greater than 1) at 4h30, and after 24h post- lesion, it was switched by aerobic metabolism (values lower than 1). This modification in skeletal muscle metabolism could be an adaptation to preserve the tissue cell in a stress condition. According to Baynes and Domiczak (2000), in the first stage of the stress, a vasoconstriction response occurs that reduces the blood loss after the injury, as well as a mobilization of glucose from all the available sources. High concentrations of epinephrine and glucagon stimulate the glycogenolysis and gluconeogenesis. Subsequently, the metabolic rate increases and the energy are supplied primarily by the oxidation of fatty acids and proteins. Thus, the muscle metabolic response is characterized by the suppression of anabolic pathways (glycogen synthesis and lipogenesis), increase in the catabolic pathways (glycogenolysis, lipolysis and proteolysis), as well as the increase in peripheral glucose incorporation insulin-dependent and insulin resistance.
What intermittent cryotherapy triggered in the enzymatic activity of injured rats?
Some studies have suggested that cryotherapy in acute injuries has beneficial effects, due to metabolism reduction than to circulatory changes (Hocutt 1982; Knight 1985; Schaser et al. 2007). Previous works have shown that three intermittent sessions of cryotherapy, associated to muscle compression and applied immediately after primary muscle injury, were effective to reduce the area of secondary injury. Contrarily, although sessions consisted only of muscle compression were effective to avoid the increase of muscle weight, they were not able to avoid a significant increase in the area of secondary muscle injury (Oliveira et al. 2006). The results of the present study confirmed this, since cryotherapy activated the LDH and CS, without altering the predominance of the A subunits at 24 h post-lesion. Finally, cryotherapy increased the aerobic metabolism in the surface region of TA post-lesion.
There was no increase in the aerobic metabolism detected at 4h30; only at 24 h post-lesion in contrast with Merrick et al. (1999) results, which demonstrated increased aerobic metabolism. It was shown that hypoxia led to mitochondria damaged and could results in diminished enzyme activity. A mitochondrial function assay performed by reducing the triphenyltetrazolium chloride (TTC) to trifenilformazan (red formazan) has been used to examine the mitochondrial damage inherent to secondary hypoxia (Belkin et al. 1988). In mitochondria, cytochrome oxidase usually reduces O2 to H2O. In the Merrick et al. (1999) study, cytochrome oxidase reduced the TTC trifenilformazan (red dye), causing a measurable change in color. The inability to reduce the TTC was indicative of mitochondrial disruption and depletion of the oxidative phosphorylation enzymes, or both (Klein et al. 1981).
It was expected that the damage caused by the criolesion in this study was greater than that caused by the crushing injury promote by Merrick et al. (1999) study, since the low temperature could alter the quaternary structure of enzymes (Alexandrov 1977). However, cryotherapy caused an increase in anaerobic metabolism only at 4h30 groups. The increase in the aerobic metabolism observed in 24 h groups suggested a temporal effectiveness of cryotherapy, which could be associate to the recovery of the metabolic and circulatory condition during the regenerating skeletal muscle.
It is important to emphasize that the studies on the response of these enzymes to muscle injury treated with the cryotherapy are scarce in the literature. It was very difficult to discuss these results for comparative purposes. Although it is difficult to undertake such a study in humans for ethical reasons, future investigations will be necessary to clarify these findings.
In conclusion, the intermittent sessions of cryotherapy, associated to muscle compression applied immediately after the primary muscle injury minimized the accumulation of CS and LDH activity at 4h30 and 24h post-lesion. The results confirmed the initial hypothesis that cryotherapy could maintain the enzymatic activity pattern similar to the control muscle, which could be related to the reduction in the secondary muscle injury inherent to cryotherapy treatment. This study provided new information about the effects of cryotherapy on the enzymatic activity in the muscle regeneration. It could have clinical relevance and indicate the importance of cryotherapy therapeutic interventions in the acute phase muscle damage with the attempt to improve the muscle regeneration process.
REFERENCES
Alexandrov K. Effects of inducers and inhibitors of the benzo(a)pyrene hydroxylase of isolated rat liver nuclei and nuclear envelopes on the binding of benzo(a)pyrene to DNA. Eur. J. Cancer. 1977; 13(8): 847-853.
Baynes J, Dominiczak MH. Bioquímica médica, São Paulo: Manole; 2000.
Järvinen TA, Järvinen TL, Kääriäinen M, Kalimom H, Järvinen M. Muscle injuries: biology and treatment. Am J Sports Med. 2005; 33(5): 745-764.
Belkin M, Brown RD, Wright JG, LaMorte WW, Hobson RW. A new quantitative spectrophotometric assay of ischemia-reperfusion injury in skeletal muscle. Am J Surg. 1988; 156: 83-86.
Driedzic WR. Almeida Val. Enzymes of cardiac energy metabolism in Amazonian teleosts and the fresh-water stingray (Potamotrygon hystrix). J Exp Zool. 1996; 274: 327-333.
Durigan JL, Peviani SM, Russo TL, Delfino GB, Ribeiro JU, Cominetti MR, Selistre-de-Araujo HS, Salvini TF. Effects of alternagin-C from Bothrops alternatus on gene expression and activity of metalloproteinases in regenerating skeletal muscle. Toxicon. 2008; 52(6): 687-94.
Hochachka PW, Storey KB. Metabolic consequences of diving in animals and man. Science. 1975; 187: 613-621.
Hochachka PW, Murphy B. Metabolic status during diving and recovery in marine mammals. Int Rev Physiol. 1979; 20: 253-287.
Hocutt JE, Jaffe R, Rylander CR, Bebbe JK. Cryotherapy in ankle spairs. Am J Sports Med. 1982; 10: 316-319.
Klein HH, Puschmann S, Schaper J, Schaper, W. The mechanism of tetrazolium reaction in identifying experimental myocardial infarction. Virch Arch. 1981; 393: 287-291.
Knight KL. Cryotherapy in Sport Injury Management, Publisher: Champaign, IL: Human Kinetics, 1995. Knight K L, Bryan KS, Halvorsen JM. Circulatory changes in the forearm in 1, 5, 10 and 15° C water. Int J Sports Med. 1985; 4: 281.
Leek BT, Mudaliar SR, Henry R, Mathieu-Costello O, Richardson RS. Effect of acute exercise on citrate synthase activity in untrained and trained human skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2001; 280: 441-447.
Merrick MA, Rankin JM, Andres FA, Hinman CL. A preliminary examination of cryotherapy and secondary injury in skeletal muscle. Med Sci Sports Exerc. 1999; 31: 1516-1521.
Merrick MA. Secondary injury after musculoskeletal trauma: a review and update. J Athl Train. 2002; 37(2): 209-217.
National Research Council. Guide for the Care and Use of Laboratory Animals. Washington: National Academy Press; 1996.
Newsholme E, Leech A. Biochemistry for the Medical Sciences. New York: Wiley and Sons; 1988.
Oliveira NML, Rainero EP, Salvini TF. Three intermittent sessions of cryotherapy reduce the secondary muscle injury in skeletal muscle of rat. J Sports Sci Med. 2006; 5:228-34.
Oliveira NML, Gaba AD, Salvini TF. The effect of intermittent cryotherapy and compression on muscle injuries in rats: a morphometric analysis. Rev bras Fisiot. 2007; 11(5): 403-409.
Powers SK, Demirel HA, Coombes JS, Fletcher L, Calliaud C, Vrabas I, Prezant D. Myosin phenotype and bioenergetic characteristics of rat respiratory muscles. Med Sci Sports Exerc. 1997; 29(12): 1573-1579.
Rubley MD. Cold steerage: harnessing the healing power of cold. Biomechanics. (2002); 12(2): 1-5.
Schaser KD, Disch AC, Stover JF, Lauffer A, Bail HJ, Mittlmeier T. Prolonged superficial local cryotherapy attenuates microcirculatory impairment, regional inflammation, and muscle necrosis after closed soft tissue injury in rats. Am J Sports Med. 2007; 35(1): 93-102.
Simoneau JÁ, Pette D. Species-specific responses of muscle LDH isoenzymes to increased contractile activity. Pflugers Arch. 1989; 413: 679-81.
Siu PM, Donley DA, Bryner RW. Alway SE. Citrate synthase expression and enzyme activity after endurance training in cardiac and skeletal muscles. J Appl Physiol. 2003; 94: 555-560.
Tonkonogi M, Harris B, Sahlin K. Increased activity of citrate synthase in human skeletal muscle after a single bout of prolonged exercise. Acta Physiol Scand. (1997); 161: 435-436.
Zondag HA. Lactate dehydrogenase isozymes: lability at low temperature. Scienc. 1963; 15(142): 965-967.
Received: October 09, 2011
Revised: April 16, 2012
Accepted: October 25, 2012
- Alexandrov K. Effects of inducers and inhibitors of the benzo(a)pyrene hydroxylase of isolated rat liver nuclei and nuclear envelopes on the binding of benzo(a)pyrene to DNA. Eur. J. Cancer. 1977; 13(8): 847-853.
- Baynes J, Dominiczak MH. Bioquímica médica, São Paulo: Manole; 2000.
- Järvinen TA, Järvinen TL, Kääriäinen M, Kalimom H, Järvinen M. Muscle injuries: biology and treatment. Am J Sports Med. 2005; 33(5): 745-764.
- Belkin M, Brown RD, Wright JG, LaMorte WW, Hobson RW. A new quantitative spectrophotometric assay of ischemia-reperfusion injury in skeletal muscle. Am J Surg 1988; 156: 83-86.
- Driedzic WR. Almeida Val. Enzymes of cardiac energy metabolism in Amazonian teleosts and the fresh-water stingray (Potamotrygon hystrix). J Exp Zool. 1996; 274: 327-333.
- Durigan JL, Peviani SM, Russo TL, Delfino GB, Ribeiro JU, Cominetti MR, Selistre-de-Araujo HS, Salvini TF. Effects of alternagin-C from Bothrops alternatus on gene expression and activity of metalloproteinases in regenerating skeletal muscle. Toxicon 2008; 52(6): 687-94.
- Hochachka PW, Storey KB. Metabolic consequences of diving in animals and man. Science. 1975; 187: 613-621.
- Hochachka PW, Murphy B. Metabolic status during diving and recovery in marine mammals. Int Rev Physiol. 1979; 20: 253-287.
- Hocutt JE, Jaffe R, Rylander CR, Bebbe JK. Cryotherapy in ankle spairs. Am J Sports Med. 1982; 10: 316-319.
- Klein HH, Puschmann S, Schaper J, Schaper, W. The mechanism of tetrazolium reaction in identifying experimental myocardial infarction. Virch Arch. 1981; 393: 287-291.
- Knight KL. Cryotherapy in Sport Injury Management, Publisher: Champaign, IL: Human Kinetics, 1995.
- Knight K L, Bryan KS, Halvorsen JM. Circulatory changes in the forearm in 1, 5, 10 and 15° C water. Int J Sports Med. 1985; 4: 281.
- Leek BT, Mudaliar SR, Henry R, Mathieu-Costello O, Richardson RS. Effect of acute exercise on citrate synthase activity in untrained and trained human skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2001; 280: 441-447.
- Merrick MA, Rankin JM, Andres FA, Hinman CL. A preliminary examination of cryotherapy and secondary injury in skeletal muscle. Med Sci Sports Exerc. 1999; 31: 1516-1521.
- Merrick MA. Secondary injury after musculoskeletal trauma: a review and update. J Athl Train 2002; 37(2): 209-217.
- National Research Council. Guide for the Care and Use of Laboratory Animals. Washington: National Academy Press; 1996.
- Newsholme E, Leech A. Biochemistry for the Medical Sciences New York: Wiley and Sons; 1988.
- Oliveira NML, Rainero EP, Salvini TF. Three intermittent sessions of cryotherapy reduce the secondary muscle injury in skeletal muscle of rat. J Sports Sci Med. 2006; 5:228-34.
- Oliveira NML, Gaba AD, Salvini TF. The effect of intermittent cryotherapy and compression on muscle injuries in rats: a morphometric analysis. Rev bras Fisiot. 2007; 11(5): 403-409.
- Powers SK, Demirel HA, Coombes JS, Fletcher L, Calliaud C, Vrabas I, Prezant D. Myosin phenotype and bioenergetic characteristics of rat respiratory muscles. Med Sci Sports Exerc. 1997; 29(12): 1573-1579.
- Rubley MD. Cold steerage: harnessing the healing power of cold. Biomechanics. (2002); 12(2): 1-5.
- Schaser KD, Disch AC, Stover JF, Lauffer A, Bail HJ, Mittlmeier T. Prolonged superficial local cryotherapy attenuates microcirculatory impairment, regional inflammation, and muscle necrosis after closed soft tissue injury in rats. Am J Sports Med. 2007; 35(1): 93-102.
- Simoneau JÁ, Pette D. Species-specific responses of muscle LDH isoenzymes to increased contractile activity. Pflugers Arch. 1989; 413: 679-81.
- Siu PM, Donley DA, Bryner RW. Alway SE. Citrate synthase expression and enzyme activity after endurance training in cardiac and skeletal muscles. J Appl Physiol. 2003; 94: 555-560.
- Tonkonogi M, Harris B, Sahlin K. Increased activity of citrate synthase in human skeletal muscle after a single bout of prolonged exercise. Acta Physiol Scand (1997); 161: 435-436.
- Zondag HA. Lactate dehydrogenase isozymes: lability at low temperature. Scienc. 1963; 15(142): 965-967.
Publication Dates
-
Publication in this collection
09 Apr 2013 -
Date of issue
Feb 2013
History
-
Received
09 Oct 2011 -
Accepted
25 Oct 2012 -
Reviewed
16 Apr 2012