ABSTRACT
It is known that, there are many ways for increasing the yield at the crops specially cereal products. Genetic manipulations and physiological interventions are the primary studies that aim to obtain products at high quality and amounts. It is known that, the usage of synthetic chemicals for physiological inverventions negatively affects organism. But using natural plant products instead of these chemicals is a subject that draws attention of today scientific environment. In this study, this situation was taken into consideration and the essential oil obtained from Satureja hortensis (SEO) plant were applied to bean seeds. The genotoxic and physiological effects of SEO at four different dosages were detected against to Phaseolus vulgaris seedlings. According to obtained data, essential oil that were applied at different doses decreased the genomic stability of the bean seeds up to 30.77% in accordance with the increased dosage. The essential oil applied in the same way lead to stress on enzyme activities of seedlings. Among the antioxidant enzymes, while, significant changeswere observed at Superoxide dismutase and Peroxidase enzymes according to the control, no significant change was seen at Ascorbate peroxidase level.
Keywords:
Antioxidant Enzymes; Biyopesticide; Essential Oil; GTS
INTRODUCTION
Cereal products and legumes have an important place in the food sector. These products are used as an important nutritional source in every country and especially in the developing countries. Therefore, agricultural techniques in increasing the quality and yield ratio of the cereal products carry a big importance.Along with the developing technology, it is know that there are various ways of increasing the yield at the crop products. Genetic manipulations and physiological interventions are the primary studies that aim to obtain products at high quality and amounts. But it is a reality that the products are developed by adding genetic codes and making interventions to the chromosome numbers for the producing desired product, make the community anxious. On the other side, it is known that synthetic chemical improvers generally are used as devoted to increase the harvest amount. The pesticides that eliminate other organisms except for the plant desired to be produced are the exogenic substances that blocks many cellular metabolic pathway and therefore physiologically affects the organism. It was observed in various studies that these chemicals protects the plant from harmful pests and at the same time gives harms to the plant itself. Previous studies states that annually 2,5 million ton pesticide is used in the cereal production in the world and annual damage amounts that these pesticide lead is 100 milliard dollars (). Today it is known that harmful effects of the synthetic chemicals do not remain limited to the metabolism and in the same way it affects that genome which is a universal effect. In the previous studies, it was stated that synthetic chemicals expands to the environment especially at the usage stage (). For example; detection of mercury (Hg) and dicloro difenil tricloroethan (DDT) residuals even at the poles where human activity is limited put forth dimensions of these chemicals expansions (). While most of afore-mentioned chemicals are abolished from the market due to their harmful effects, day by day new chemical substances are synthesized. While these synthesized chemicals disturb the ecological balance, on the other side they reach to toxic dosages at high organisms by increasing at the every stages of the food chain.
Knowing that synthetic chemicals have harmful effect directs the human towards natural plant components. Because plant components are products of a natural struggle and their crack up after fulfilling their function is easier than synthetic products. If these specifications of the natural plant components are taken into consideration, the problems seen at production of important food crops such as cereals and legumes may be precluded.
Some of the plant components that may be used at the natural plant struggle are the essential oil (EO) that obtained from aromatic plants. The essential oil (natural oils, etheric oils) and aromatic extracts; it is widely used in fragrance and taste industries, at composition of perfume, food additives, cleaning products, cosmetic and drugs, source of aroma chemicals or starting substance for synthesis of the aroma nature identical or semi-synthetic beneficial aroma chemical().
Lamiceae family is one of the plant family which volatiles content is available at higher amounts. Important results were obtained from the studies that were done with this family. For example; it was observed that essential oil obtained from the plant of Thuja orientalis decreased the germination of most of wild seeds depending on the increased dosage (). It was observed that Origanum dubium L. essential oil lead to important decreases at germination ratios and root lengths of the seeds belonging to Rumexcrispus L., Amaranthus retroflexus L., Sinapis arvensis L. Physalisangulata L. species().
Although they are natural, the plant components may lead to problems, acute disorders or toxic effects at the livings (). It is recorded that they may even lead to fatal effects (). Also allelopathic interactions of aforementioned plant components were examined and found to prevent the development of weeds (). In a similar manner, it was detected that root and body developments of the weeds such as Lomatium rigidum and Phalaris brachystachys at which extracts obtained from Satureja hortensis species were negatively affected (). In this case, determination of applicable dosage has importance.
Satureja hortensis is belonging to Lamiaceae plant family is the one of the plants that have the relevant volatiles at high amount. As a matter of course, it has some specifications that limit usage of essential oil at the bio-control. For example; these may be deemed as disadvantages that they are volatile, insoluable in water and not present at the plants at high amounts (). But these are non-negligibly important that they are fast metabolized, natural and very effective at low concentrations. Therefore, it is required that possible toxic and physiologic effects of essential oil will be determined before their usage as bio-control agents. By starting from these information, toxic effect of essential oil obtained from Satureja hortensis againist Phaselous vulgaris seedlings were determined by Randomly Amplified Polymorphic DNA (RAPD) and physiological effect by measuring antioxidant enzyme levels.
MATERIALS AND METHODS
Satureja hortensis L. plants were collected from Bingol city of Turkey at 1100-1200 latitude during the flowering stage in July 2013. The identification of plant materials was confirmed by a plant taxonomist, Prof. Dr. Yusuf KAYA from Ataturk University, Erzurum, Turkey.
Commercial seeds of Phaseolus vulgaris were used for EO treatment. Equally sized seeds were chosen and sterilized according to Bozari et. al., (). After the surface sterilization with NaOCl, seeds were washed with double-distilled water and dried with the sterile filter paper. Fifteen seeds were germinated in 15cm diameter Petri dishes on two layers of sterile Whatman No. 1 filter paper. The seeds were exposed to four different (0.1, 0.2, 0.4, 0.8µl/ml) concentrations of EO. Tween 20 was added to the distilled water to dissolve the EO. Only double distilled water with Tween 20 was used for the control group. Three replicates were made for each concentration ().
Genomic DNA was extracted by using the before reported procedure (). 15 RAPD primers were selected and eight of them gave polymorphic bands and performed for the study. The primer sequences were (5'→3') TGCTCTGCCC (OPB-6), GTCCACACGG (OPB-8), TGGGGGACTC (OPB-9), CTGCTGGGAC (OPB-10), GTAGACCCGT (OPB-11), CAGCACCACA (OPA-16), CTGGACGTGA (OPW-19) and AAGGCTCACC (OPY-24). RAPD technique was performed as Aksakal et. al,.(). Genomic template stability (GTS, %) calculated with the following formula GTS= where "a" is the total polymorphic bands counted in each treated sample and "n" is the total bands in the control. Polymorphic bands were existed from missed bands and/or appearance of new bands against to control.
The root and stem samples of the germinated seeds were used to evaluate the antioxidant enzymes. Superoxide dismutase (SOD) was evaluated by using the technique performed by Agarval and Pandey (); peroxidase (POX) by Ye et.al., () and ascorbate peroxidase (APX) by Nakano and Asada ().
Homogenized leaf tissues (0.5 g) were ground in a mortar with liquid nitrogen and extracted in 500 μL of 10 mmol L−1 potassium phosphate buffer (pH 7.0) containing 4% (w/v) polyvinyl pyrrolidone and 1 mmol L−1 ethylenediaminetetraacetic acid (EDTA) followed by centrifugation at 12,000 × g for 15 min at 4°C. The supernatant was used as an enzymes source (SOD, POX and APX) ().
The statistical analyses were carried out using the package software SPSS 22.0 for windows.
RESULTS AND DISCUSSION
The plants come across so many types of biotic and abiotic stress factors at the natural environment. These exogen related substances may lead to so many physiological problems at the livings as well as genetic changes. The pesticides that used at the plant control are one of these substances. Pesticides are the mixtures that are formed from the substance(s) and used to prevent or decrease the harmful organisims and keep these organisims under control during production of agricultural products.
It has been documented that synthetically produced chemicals lead oxidative stress change the genetic structure of the plants, thus damage the genomic stability in plants (). The increase in amount of reactive oxygen species (ROS) due to stress factors, may lead to lose of cell membranes functions such as mitochondria, chloroplast and paroxysm and therefore necrosis (, ). ROS can induce several damages in plants by degradation of proteins, inactivation of enzymes, DNA alterations, and interfere in various pathways of metabolic importance. Plants improve different protection systems as antioxidant enzymes to scavenge these ROS (). Plants have a protective system that is consisted of membrane bounded antioxidants, water soluble antioxidant such as glutathione and ascorbate and enzymatic antioxidants such as Superoxide dismutase (SOD), Ascorbate peroxidases (APX), catalase (CAT) and peroxidase (POX) inorder to struggle with destructive effects of reactive oxygen species cause (, , ). In case of an increase in the amount of ROS, they are systematically transformed into harmless forms by enzymatic antioxidant system at the apoplastic region where cellular damages starts (, ).
It is known that superoxide radicals are very reactive and they may also lead oxidation of many biochemical components in the cell.. It was stated that lipidperoxidation of this radicals lead to membrane damage, cellular toxicity and single chain break at DNA (). Superoxide radicals may produce hydroxyl radical which are more toxic molecule by reacting with the hydrogen peroxide (, ). While, SOD catalyzing transformation of relevant radical to hydrogen peroxide and molecular oxygen, APX enzymes detoxify peroxides by using ascorbate as a substrate and POX break down hydrogen peroxide (, ).
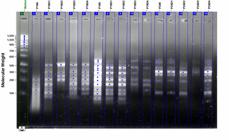
According to the results obtained from RAPD in this study, polymorphism ratio of bean seedlings were generally high. Number of the polymorphic bands at all dosages were determined as total 4 units as 2 new formed and 2 descending band. Polymorphic bands at high ratios were observed at OPY-24, OPW- 19 and OPA-16 primers among applied primers. The GTS values were 58.97%, 56.42%, 30.77% and 38.46% for 1, 2, 4 and 8 µl/ml, respectively (Table 1).It seems the essential oil have changed the genomic stability of the seedlings in a dose dependent manner.
It was also observed that RAPD band densities significantly changed (Figure 1).
RAPD profiles of Essential oils treated and non-treated (control) Phaseolus vulgaris seedlings with different primers (P: Phaseolus, 16: Primer 16, 19: Primer 19, 24: Primer 24, K: Control, D1: first dose (0.1µl/ml), D2: second dose (0.2µl/ml), D3: third dose (0.4µl/ml), D4: fourth dose (0.8µl/ml) ).
On the other hand, as seen from Table 2, it was detected that applicatoin of EO significantly increased the superoxide dismutase enzyme amount in the samples obtained from germinated parts of bean seeds at the first concentration (1 µl/ml), but this increase gradually decreased towards control at increased concentrations. It was detected that the ascorbate peroxidase enzyme increased at first two concentrations but they were available only at lower levels at 4 and 8 µl/ml concentrations compared to the control. But it was detected that the difference between control and application concentrations were not significant. On the other hand, it was detected that the peroxidase enzyme level was independently different than the applied concentrations. The POX level increased at 1 µl/ml concentration compared to the control and at 2 µl/ml concentration a significant decrease was observed compared to the control. At 4 and 8 µl/ml concentrations, it was detected that the level of POX were again increased and these values were important according to the control.
According to the data obtained from this study, it was observed that, the essential oil applied to bean seeds affected SOD enzyme level. Although there was a significant change at the first concentration compared to the control, the difference in the level of SOD at higher concentrations was not significant. In this case, increased concentration of the essential oil changed the genetic straucture of the plants and reduced the synthesis of enzyme. This case may be explained with the increase of polymorphism ratio (Table 1). The other scenario is that; SOD that provides the peroxidation of lipids may also oxidise the lipid derivative essential oilat an increased level, therefore, limits the activity of essential oil. But this case is inadequate for solely explaining decrease of genomic stability at increased concentrations.
On the other side, POX is an enzyme available at the fruits and vegetables. It protects the cell from oxidative stress that hydrogen peroxide causes. This enzyme catalyzes degeneration of many aromatic components such as phenols and hydroquinols by using H2O2(). It is known that peroxidase activity increases at the plants under the stress (). Peroxidase regulates level of harmful oxygen radicals produced under unfavorable environmental factors and protects the plants in this way (, ). According to the data gained from this study, it was observed that peroxidase amounts changed independently from the concentration. Germinated seeds get stressed even at the lowest concentration of the applied essential oil and triggered the antioxidant defense system at a level that may be deemed to be significant compared to the control. Although, the highest peroxidase level was observed at the lowest EO concentration, the mentioned increase in peroxidase levels were deacreased at 2, 4 and 8 µl/ml EO concentrations, but still it showes that EO gave important damages at higher concentrations. Such that, it may be thought that natural plant components triggered DNA damage or physiologically blocks enzyme production ways.
APX Ascorbate peroxidase (APX) is a scavenging enzyme against H2O2 and hydroxyl radicals behave in a similar way as in chloroplast and other cell components (). It was observed in this study that this enzyme providing protection of the organelles such as chloroplast did not show a significant decrease or increase compared to the control. This case was attributed to the inmature chloroplast organelles that available at the germinated seeds.
CONCLUSION
The problems that pesticides may cause in terms of environment, health and economy are very well known in the developed countries. Therefore, agricultural products are consuming continuously inspected in terms of environment and human in all developed countries especially in EU (). But, nowadays for the obtaining of the maximum yield at unit area in a short time, application of pesticide has been still a widely used control way to preventing the food deficiency for the increased human population. On the other side, various ways are tried to be applied for reliable food production. One of these ways is known as bio-control. Natural plant components that are easy to be decompose, has no toxicity or low toxicity are amoung the products that will be used at the control of agricultural processes. A similar natural substance was used in our study. Even if usage of natural components have lower genotoxicity than chemical pesticides (, ) more studies should be performed in order to confirm the reliability of these natural components.
ACKNOWLEDGEMENTS
I would like to thanks to Birsen ÇAKMAK and Havva KURT for their help in laboratory works.
REFERENCES
-
1Koul O, Walia S, Dhaliwal G. Essential oils as green pesticides: potential and constraints. Biopestic Int. 2008;4(1):63-84.
-
2Ayas D, Kalay M, Sangün MK. Mersin Körfezi'nden örneklenen yüzey suyu ve Patella türlerindeki (Patella caerulea, Patella rustica) Cr, Cd ve Pb düzeylerinin belirlenmesi. Ekoloji. 2009;18(70):32-7.
-
3Gordeev V. Pollution of the Arctic. Regional Environmental Change. 2002;3(1-3):88-98.
-
4Fakılı O, Özgüven M. Inventory of Researches on Thyme (Thymus vulgaris L.) in Turkey. ÇÜ Fen ve Mühendislik Bilimleri Dergisi 2012;27(3):54-66.
-
5Ismail A, Mohsen H, Bassem J, Lamia H. Chemical composition of Thuja orientalis L. essential oils and study of their allelopathic potential on germination and seedling growth of weeds. Archives of Phytopathology and Plant Protection. 2015;48(1):18-27.
-
6Aydin O, Tursun N. Bitkisel Kökenli Bazı Uçucu Yağların Bazı Yabancı Ot Tohumlarının Çimlenme ve Çıkışına Olan Etkilerinin Araştırılması. 2010.
-
7Khater HF. Prospects of botanical biopesticides in insect pest management. Pharmacologia. 2012;3(12):641-56.
-
8Rosell G, Quero C, Coll J, Guerrero A. Biorational insecticides in pest management. Journal of Pesticide Science. 2008;33(2):103-21.
-
9Martins N, Barros L, Santos-Buelga C, Henriques M, Silva S, Ferreira IC. Evaluation of bioactive properties and phenolic compounds in different extracts prepared from Salvia officinalis L. Food chemistry. 2015;170:378-85.
-
10Perera M, Karunaratne M, editors. Olax Zeylanica: An Environmentally Safe Bio-Pesticide For The Control of The Maize Weevil Sitophilus zeamais Mots.(Curculionidae). Proceedings of International Forestry and Environment Symposium; 2012.
-
11Araniti F, Sorgonà A, Lupini A, Abenavoli M. Screening of Mediterranean wild plant species for allelopathic activity and their use as bio-herbicides. Allelopathy J. 2012;29(1):107-24.
-
12Gitsopoulos T, Chatzopoulou P, Georgoulas I. Herbicidal effects of Satureja hortensis L. and Melissa officinalis L. essential oils on germination and root length of Lollium rigidum L. and Phalaris brachystachys L. grass weeds. Hellenic Plant Protection Journal. 2013;6(2):49-54.
-
13Tripathi AK, Upadhyay S, Bhuiyan M, Bhattacharya P. A review on prospects of essential oils as biopesticide in insect-pest management. Journal of Pharmacognosy and Phytotherapy. 2009;1(5):052-63.
-
14Bozari S, Agar G, Aksakal O, Erturk FA, Yanmis D. Determination of chemical composition and genotoxic effects of essential oil obtained from Nepeta nuda on Zea mays seedlings. Toxicology and industrial health. 2012:339-48
-
15Bozari S, Agar G, Yanmis D. Chemical Content, and Toxic Effects of Essential Oil of Origanum vulgare L. ssp vulgare Against to Zea mays Seedlings. Journal of Essential Oil Bearing Plants. 2014 2014;17(1):67-77.
-
16Aksakal O, Erturk FA, Sunar S, Bozari S, Agar G. Assessment of genotoxic effects of 2,4-dichlorophenoxyacetic acid on maize by using RAPD analysis. Industrial Crops and Products. 2013 Mar;42:552-7.
-
17Agarwal S, Pandey V. Antioxidant enzyme responses to NaCl stress in Cassia angustifolia Biologia Plantarum. 2004;48(4):555-60.
-
18Ye Y, Tam NF, Wong Y, Lu C. Growth and physiological responses of two mangrove species (Bruguiera gymnorrhiza and Kandelia candel) to waterlogging. Environmental and Experimental Botany. 2003;49(3):209-21.
-
19Nakano Y, Asada K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant and cell physiology. 1981;22(5):867-80.
-
20Erdal S, Genisel M, Turk H, Dumlupinar R, Demir Y. Modulation of alternative oxidase to enhance tolerance against cold stress of chickpea by chemical treatments. Journal of plant physiology. 2015;175:95-101.
-
21Aksakal O, Erturk FA, Sunar S, Bozari S, Agar G. Assessment of genotoxic effects of 2, 4-dichlorophenoxyacetic acid on maize by using RAPD analysis. Industrial Crops and Products. 2013;42:552-7.
- 22. Bozari S, Aksakal O. Application of random amplified polymorphic DNA (RAPD) to detect genotoxic effect of trifluralin on maize (Zea mays). Drug and Chemical Toxicology. 2013 Apr;36(2):163-9.
-
23Cenkci S, Yıldız M, Ciğerci İH, Konuk M, Bozdağ A. Toxic chemicals-induced genotoxicity detected by random amplified polymorphic DNA (RAPD) in bean (Phaseolus vulgaris L.) seedlings. Chemosphere. 2009;76(7):900-6.
-
24Esim N. Nitrik oksidin mısırda (Zea mays) düşük sıcaklık stresi toleransı üzerine etkisi [pHD]. Erzurum: Atatürk Üniversitesi; 2011.
-
25Mittler R. Abiotic stress, the field environment and stress combination. Trends in plant science. 2006;11(1):15-9.
-
26Terzi R, Kadioglu A, Kalaycioglu E, Saglam A. Hydrogen peroxide pretreatment induces osmotic stress tolerance by influencing osmolyte and abscisic acid levels in maize leaves. Journal of Plant Interactions. 2014;9(1):559-65.
-
27Jung JE, Lee J, Ha J, Kim SS, Cho YH, Baik HH, et al. 5-Aminoimidazole-4-carboxamide-ribonucleoside enhances oxidative stress-induced apoptosis through activation of nuclear factor-κB in mouse Neuro 2a neuroblastoma cells. Neuroscience letters. 2004;354(3):197-200.
-
28Pinheiro HA, DaMatta FM, Chaves AR, Fontes EP, Loureiro ME. Drought tolerance in relation to protection against oxidative stress in clones of Coffea canephora subjected to long-term drought. Plant science. 2004;167(6):1307-14.
-
29Hernandez J, Jimenez A, Mullineaux P, Sevilia F. Tolerance of pea (Pisum sativum L.) to long‐term salt stress is associated with induction of antioxidant defences. Plant, Cell & Environment. 2000;23(8):853-62.
-
30Ahmad P, Sarwat M, Sharma S. Reactive oxygen species, antioxidants and signaling in plants. Journal of Plant Biology. 2008;51(3):167-73.
-
31Halliwell B. Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant physiology. 2006;141(2):312-22.
-
32Fridovich I. Superoxide radical and superoxide dismutases. Annual review of biochemistry. 1995;64(1):97-112.
-
33Desikan R, Griffiths R, Hancock J, Neill S. A new role for an old enzyme: nitrate reductase-mediated nitric oxide generation is required for abscisic acid-induced stomatal closure in Arabidopsis thaliana Proceedings of the National Academy of Sciences. 2002;99(25):16314-8.
-
34Asada K. Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant physiology. 2006;141(2):391-6.
-
35Elstner EF. Oxygen activation and oxygen toxicity. Annual review of plant physiology. 1982;33(1):73-96.
-
36Halliwell B, Gutteridge J. Free radicals in biology and medicine. NY: Oxford University Press-1999-968 р. 2011.
-
37Eyidoğan FI, Öktem HA, Yücel M. Superoxide dismutase activity in salt stressed wheat seedlings. Acta physiologiae plantarum. 2003;25(3):263-9.
-
38Minibayeva F, Mika A, Lüthje S. Salicylic acid changes the properties of extracellular peroxidase activity secreted from wounded wheat (Triticum aestivum L.) roots. Protoplasma. 2003;221(1-2):67-72.
-
39Bergmeyer H-U. Methods of enzymatic analysis: Elsevier; 2012.
-
40Banci L. Structural properties of peroxidases. Journal of Biotechnology. 1997;53(2):253-63.
-
41Kim K-Y, Kwon H-K, Kwon S-Y, Lee H-S, Hur Y, Bang J-W, et al. Differential expression of four sweet potato peroxidase genes in response to abscisic acid and ethephon. Phytochemistry. 2000;54(1):19-22.
-
42Dehon L, Macheix J, Durand M. Involvement of peroxidases in the formation of the brown coloration of heartwood in Juglans nigra. Journal of experimental botany. 2002;53(367):303-11.
-
43Asada K. Ascorbate peroxidase-a hydrogen peroxide‐scavenging enzyme in plants. Physiologia Plantarum. 1992;85(2):235-41.
-
44Delen N, Durmuşoğlu E, Güncan A, Güngör N, Turgut C, Burçak A. Türkiye'de Pestisit Kullanimi, Kalinti ve Organizmalarda Duyarlilik Azalişi Sorunlari. Türkiye Ziraat Mühendisliği 6. Teknik Kongresi; 2005.
-
FUNDING This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Publication Dates
-
Publication in this collection
2016
History
-
Received
03 Feb 2016 -
Accepted
14 July 2016