Abstracts
The purpose of this study was to investigate the antimicrobial effect of a Punica granatum Linn (pomegranate) phytotherapeutic gel and miconazole (Daktarin® oral gel) against three standard streptococci strains (mutans ATCC 25175, sanguis ATCC 10577 and mitis ATCC 9811), S. mutans clinically isolated and Candida albicans either alone or in association. The effect of minimum inhibitory concentrations of the gels on the adherence of these microorganisms to glass was assessed in the presence of 5% sucrose, using increasing and doubled concentrations of the diluted solution of the gels ranging from 1:1 to 1:1024. The minimum inhibitory concentrations of adherence of Punica granatum L. gel against the test organisms were: 1:16 for S. mutans (ATCC), S. mutans (CI) and S. sanguis; 1:128 for S. mitis and 1:64 for C. albicans. The minimum inhibitory concentrations of adherence of miconazole against the same organisms were: 1:512, 1:64, 1:4, 1:128 and 1:16, respectively. In experiments with three and four associated microorganisms, the Punica granatum L. gel had greater efficiency in inhibiting microbial adherence than the miconazole. The results of this study suggest that this phytotherapeutic agent might be used in the control of adherence of different microorganisms in the oral cavity.
Punica granatum Linn; Streptococcus mutans; Candida albicans
O propósito deste estudo foi investigar a concentração inibitória mínima de aderência (CIMA) de três linhagens de estreptococos (mutans ATCC 25175, sanguis ATCC 10557 e mitis ATCC 9811), S. mutans isolado clinicamente e de cepas de Candida albicans, separadamente ou associadas, frente a um gel fitoterápico obtido da Punica granatum Linn. (romã) e ao agente antifúngico miconazol (Daktarin® gel oral). A concentração inibitória mínima de aderência das bactérias ao vidro foi determinada na presença de sacarose a 5%, usando-se concentrações crescentes e dobradas da solução diluída do gel variando de 1:1 a 1:1024. Os valores de inibição do gel fitoterápico foram de 1:16 para S mutans (ATCC), S. mutans (IC) e S. sanguis; 1:128 para S. mitis e 1:64 para C. albicans. Sobre as mesmas linhagens, as concentrações inibitórias mínimas de aderência do miconazol foram: 1:512, 1:64, 1:4, 1:128, 1:16 respectivamente. O gel da romã apresentou maior eficácia sobre associações de três e quatro microrganismos do que o gel do miconazol. Os achados deste estudo sugerem o emprego desse agente fitoterápico pode ser uma opção no controle da aderência dos microrganismos testados na cavidade bucal.
Minimum inhibitory concentration of adherence of Punica granatum Linn (pomegranate) gel against S. mutans, S. mitis and C. albicans
Laurylene César de Souza VasconcelosI; Fábio Correia SampaioI; Maria Carmélia Correia SampaioI; Maria do Socorro Vieira PereiraII; Jane Sheila HiginoIII; Maria Helena Pereira PeixotoII
IDepartament of Clinical and Social Dentistry, Federal University of Paraíba, João Pessoa, PB, Brazil
IIDepartament of Molecular Biology, Federal University of Paraíba, João Pessoa, PB, Brazil
IIIDepartament of Pharmaceutical Sciences, Federal University of Pernambuco, Recife, PE, Brazil
Correspondence Correspondence: Profa. Dra. Laurylene César de Souza Vasconcelos Departamento de Clínica e Odontologia Social, Universidade Federal da Paraíba Rua Maria Helena Rocha, 113, ap. 1301 B, 58036-670 João Pessoa, PB, Brasil Tel: +55-83-3246-4203. Fax: +55-83-3216-7094 e-mail: laurylene@uol.com.br
ABSTRACT
The purpose of this study was to investigate the antimicrobial effect of a Punica granatum Linn (pomegranate) phytotherapeutic gel and miconazole (Daktarin® oral gel) against three standard streptococci strains (mutans ATCC 25175, sanguis ATCC 10577 and mitis ATCC 9811), S. mutans clinically isolated and Candida albicans either alone or in association. The effect of minimum inhibitory concentrations of the gels on the adherence of these microorganisms to glass was assessed in the presence of 5% sucrose, using increasing and doubled concentrations of the diluted solution of the gels ranging from 1:1 to 1:1024. The minimum inhibitory concentrations of adherence of Punica granatum L. gel against the test organisms were: 1:16 for S. mutans (ATCC), S. mutans (CI) and S. sanguis; 1:128 for S. mitis and 1:64 for C. albicans. The minimum inhibitory concentrations of adherence of miconazole against the same organisms were: 1:512, 1:64, 1:4, 1:128 and 1:16, respectively. In experiments with three and four associated microorganisms, the Punica granatum L. gel had greater efficiency in inhibiting microbial adherence than the miconazole. The results of this study suggest that this phytotherapeutic agent might be used in the control of adherence of different microorganisms in the oral cavity.
Key words:Punica granatum Linn,Streptococcus mutans, Candida albicans.
RESUMO
O propósito deste estudo foi investigar a concentração inibitória mínima de aderência (CIMA) de três linhagens de estreptococos (mutans ATCC 25175, sanguis ATCC 10557 e mitis ATCC 9811), S. mutans isolado clinicamente e de cepas de Candida albicans, separadamente ou associadas, frente a um gel fitoterápico obtido da Punica granatum Linn. (romã) e ao agente antifúngico miconazol (Daktarin® gel oral). A concentração inibitória mínima de aderência das bactérias ao vidro foi determinada na presença de sacarose a 5%, usando-se concentrações crescentes e dobradas da solução diluída do gel variando de 1:1 a 1:1024. Os valores de inibição do gel fitoterápico foram de 1:16 para S mutans (ATCC), S. mutans (IC) e S. sanguis; 1:128 para S. mitis e 1:64 para C. albicans. Sobre as mesmas linhagens, as concentrações inibitórias mínimas de aderência do miconazol foram: 1:512, 1:64, 1:4, 1:128, 1:16 respectivamente. O gel da romã apresentou maior eficácia sobre associações de três e quatro microrganismos do que o gel do miconazol. Os achados deste estudo sugerem o emprego desse agente fitoterápico pode ser uma opção no controle da aderência dos microrganismos testados na cavidade bucal.
INTRODUCTION
Homeostasis is a characteristic of the oral microbiota in healthy individuals. The microorganisms are capable of cohabiting in saprophytism in the different sites of the mouth, depending directly on pH, availability of nutrients and mucous surface (1). Factors related to adhesion mechanisms can, however, modify this homeostasis and lead to microbial colonization and biofilm formation, which constitute the primary etiologic agents of oral diseases (2).
The establishment and maintenance of oral microbiota is related not only to interbacterial coaggregations but also to interactions of these bacteria with yeasts such as Candida albicans (3). Fungi are frequently isolated in several oral sites, including the tongue, jugal mucosa, palate, dental biofilm, subgingival microbiota, carious lesions and prosthetic appliances (4). Studies (5,6) have suggested a possible relation between C. albicans and periodontal disease, dentin and/or root caries. These studies showed that C. albicans has similar capacity of colonizing hydroxyapatite as that of S. mutans, however using different mechanisms.
Enamel and dentin demineralization produced by fungal organic acids, as well as the presence of cells with C. albicans hyphas invading dentinal tubules, prove this ability of fungi to invade and destroy organic and inorganic dental tissues (7). This microorganism adheres to hydroxyapatite, especially through electrostatic interactions and at smaller numbers. Candida albicans has also the ability to dissolve hydroxyapatite at a larger rate when compared to Streptococcus mutans (6).
Nostro et al. (8) investigated whether sublethal concentrations of Helichrysum italicum extract would affect the cariogenic properties of S. mutans. The antibacterial activity of the ethanolic extract against oral streptococci (S. mutans, S. salivarius and S. sanguis) was evaluated in vitro to determine whether this would influence cell surface hydrophobicity regarding glass surface adherence and S. mutans aggregation. All streptococci were sensitive to the minimum inhibitory concentrations of the extract. Concentrations of minimum inhibitory subconcentrations of H. italicium reduced the hydrophobicity and adherence (approximately 90%) of S. mutans to glass surface.
The antimicrobial activity of Punica granatum Linn has been widely investigated (9,10). The findings of several studies, including some relating to inhibition of adherence, suggest that the phytotherapeutic use of this plant might be a viable option in controlling different microbial species. The largest components of the Punica granatum L. fruit extract are tannin and polyphenolics (11).
There is a growing interest in using tannins as antimicrobial agents in caries prevention (12). The action of tannins against bacteria and yeasts can be established by a relation between their molecular structure and their toxicity, astringent properties or other mechanisms. The effect of tannins on microbial metabolism can be measured by their action on membranes. They can cross the cell wall, composed of several polysaccharides and proteins, and bind to its surface. This adhesion can also help determining minimum inhibitory concentrations for yeasts and bacteria.
The purpose of this study was to investigate the antimicrobial effect of a Punica granatum Linn. (pomegranate) phytotherapic gel and Miconazole (Daktarin® oral gel) against three standard streptococci strains (mutans ATCC 25175, sanguis ATCC 10577 and mitis ATCC 9811), S. mutans clinically isolated and Candida albicans either alone or associated with other microorganisms. The effect of minimum inhibitory concentrations of the gels on the adherence of these microorganisms to glass was assessed.
MATERIAL AND METHODS
The Punica grantum L. fruits used in this study were obtained at a public market in the city of João Pessoa, PB, Brazil. Botanical identification was conducted at the Pharmaceutical Technology Laboratory of the Federal University of Pernambuco. After washing, the peel was separated from the mesocarp, dried in an incubator at 33ºC for 7 days. The material was thereafter ground in an electric grinder to produce a powder. The active principles were isolated and a concentrated extract was obtained. At this stage, a basic gel consisting of carbopol, water and triethanolamine was prepared. Next, 0.5 mL of the brute extract, equivalent to 540 mg of the plant powder, was incorporated, thus resulting in the Punica granatum L. gel.
Standard Streptococcus mutans (ATCC 25175), Streptococcus sanguis (ATCC 10557), Streptococcus mitis (ATCC 9811) strains were used. These species were obtained from "André Tozello" Tropical Research and Technology Foundation (Campinas, SP, Brazil). Clinical isolates of S. mutans obtained from a patient and Candida albicans strains were also used. The latter were further reactivated at the Microorganism Genetics Laboratory of the Department of Molecular Biology, CLEN, Federal University of Paraíba, Brazil).
The minimum inhibitory concentration of adherence of the organisms to glass was determined in the presence of 5% sucrose, using increasing and doubled concentrations of the diluted gel ranging from 1:1 up to 1:1024. After overnight growth, the strains were subcultured (30 mL of growth) in 30 mL of sucrose-containing Mueller-Hinton Broth (Difco Laboratories, Detroit, MI, USA) at 37ºC for 1 h to obtain an inoculate. A gel scale was then prepared by solubilization of 5 g of the pomegranate gel in every 5 mL of sterile distilled water. Distribution was made of 1.6 mL saline and 0.2 mL of the subculture in hemolysis tubes, where 0.2 mL of gel scale was further added. Incubation was done at 37ºC for 24 h in microaerophilia, with the tubes inclined at a 30º angle. Reading of the results was done by visual inspection of microbial adherence to tube walls after shaking. Minimum inhibitory concentration of adherence was defined as the smallest concentration of the agent in sucrose that prevented adherence to the glass.
The minimum inhibitory concentration of adherence of the microorganisms in pairs or groups was also determined. S. mutans (ATCC), S. sanguis, S. mitis and Candida albicans were used. The microorganisms were placed in hemolysis tubes with 1.4 mL of the sucrose-containing solution in addition to 0.2 mL of the bacterial subculture and 0.2 mL of the pomegranate gel scale. Incubation and reading of the results were then performed in the same way as described above.
To compare the results, the experiment was also performed using a scale prepared with pure pomegranate extract and another with miconazole (Daktarin® gel oral, Janssen Farmacêutica Ltda, São Paulo, SP, Brazil).
RESULTS
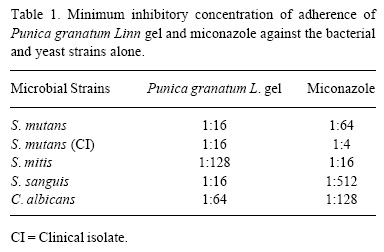
Punica granatum L. gel was effective in inhibiting the adherence of the bacterial strains and C. albicans, in the presence of sucrose. The minimum inhibitory concentrations of adherence of this phytotherapeutic agent are shown in Table 1. The antifungal agent (miconazole) also showed a significant adherence inhibition effect against the tested strains (Table 1).
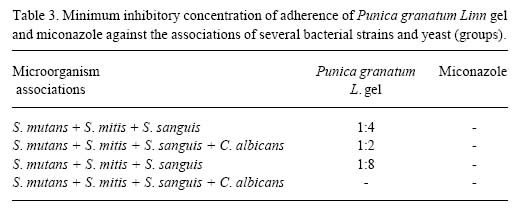
The etiology of oral diseases is related to various local factors including the simultaneous participation of bacteria and yeasts. In this study, the minimum inhibitory concentrations of the Punica granatum L. gel were also evaluated against microbial pools. There was an increase in microbial adherence when different bacteria were associated, or when bacteria were associated with C. albicans (Table 2). In this case, the minimum inhibitory concentrations of miconazole were lower than those of Punica granatum L. gel.
In the assays with three and four associated organisms, the Punica granatum L. gel had greater efficiency than miconazole. The Punica granatum L. gel was effective in inhibiting the adherence of the following associations: S. mutans (ATCC) + S. mitis + S. sanguis; S. mutans (ATCC) + S. mitis + S. sanguis + C. albicans and S. mutans (IC) + S. mitis + S. sanguis. Miconazole did not show inhibitory activity against these associations (Table 3). This suggests that phytotherapy seems to have a greater astringent strength than antifungal allopathy.
Table 3 also shows that 10% Punica granatum L. gel (1:2 concentration) inhibited glass adherence in the associations of C. albicans and S. mitis, S. sanguis and S. mutans strains. However, the phytotherapic gel was not able to inhibit glass adherence of the association of C. albicans with S. mitis, S. sanguis and S. mutans (CI). This indicates a possible strengthening of adherence promoted by clinically isolated microorganisms.
DISCUSSION
S. mutans are directly involved in the etiopathogenesis of caries and periodontal diseases, denture-associated stomatitis and other infections because they can contribute to alter the equilibrium of oral microbiota by creating favorable conditions to adherence of opportunistic organisms, such as fungi, to the surfaces of teeth, oral tissues and prosthetic appliances (6,13,14,16).
This study did not have the aim of investigating the action mechanisms by which streptococci increase the adherence of yeasts. It is believed that the quantification of involved species, the association of different organisms and the possible relations with clinically observed characteristics are of great diagnostic and therapeutic importance for several oral infections.
Few studies have addressed interactions between commensal bacteria and yeasts (17). However, the effect of these bacteria interacting with Candida albicans can be seen with the use of antibiotic therapy and in the increase of candidosis (18).
C. albicans is a fungus frequently found on dental biofilm and its ability to secrete organic acids and collagenolytic enzymes can determine its role in the onset of caries disease (13). Demineralization produced by fungal organic acids, as well as the presence of cells with C. albicans hyphas invading dentinal tubules, would prove the capacity of this yeast to invade and destroy organic and inorganic dental tissues.
As the participation of oral bacteria in fungal adhesion to prosthetic surfaces has been demonstrated (16), it may be speculated that denture wearers are not only susceptible to candidiasis, but also at higher risks of caries and periodontal disease progression due to the presence of cariogenic bacteria like S. mutans. The clinical implications of these fungal-bacterial interactions emphasize the importance of patient oral hygiene in controlling denture-associated stomatits. Supposing that the microbial adherence to glass observed in this study is similar to that occurring on prosthetic surfaces, the use of prosthetic plate samples as test specimens in future experiments would be of interest.
In vivo studies have demonstrated the antibacterial (9) and antifungal (10) effects of phytotherapeutic agents derived from Punica granatum Linn extract. This study investigated in vitro the therapeutic potential of this agent against bacteria and yeasts either alone or pairs and groups. In vitro studies using biofilm models are those that comes closer to a clinical situation and more precisely reflect in vivo conditions (17).
According to Cotter and Kavanagh (18) therapeutic agents that do not exhibit fungicidal or fungistatic activity can inhibit yeast adherence by other mechanisms and it is possible that these agents represent a new perspective in the combat and control of superficial fungal infection. It is believed that the use of a product containing a component that specifically reduces or inhibit the adherence capacity of C. albicans can be used in association with already existing antifungal agents.
As far as bacterial adherence inhibition is concerned, the findings of this study were consistent with those of Pereira (9), who assessed the minimum inhibitory concentrations of adherence of Punica granatum Linn extract against S. mitis (1:512), S. mutans (1:256), S. sanguis (1:128) and C. albicans (1:64). The antifungal action of this agent was tested in its brute state and diluted at different concentrations.
Recently, natural products have proved to be an alternative to synthetic chemical substances. Nostro et al. (8) demonstrated that the Helichrysum italicum extract interfered in the cariogenic properties of S. mutans through reductions of superficial hydrophobicity, inhibiting adherence of cell growth to glass in 90 to 93%. Those authors believe that the capacity of this extract to prevent bacterial adherence could be due to the effect of its flavonoid components, which have anti-glycosyltransferase activity. While assessing the minimum inhibitory concentrations of adherence of different vegetable dyes and propolis, Gebara et al. (19) observed that the inhibition of S. mutans and S. sobrinus adherence was a result of the inhibition of glucan synthesis by these substances. The present study found a similar effect on the inhibition of adherence to glass of S. mutans, S. sanguis, S. mitis and C. albicans by the Punica granatum Linn in the presence of sucrose.
Kakiuchi et al. (20) and Pereira (9) demonstrated the specific antimicrobial action of Punica granatum Linn on dental biofilm bacteria, i.e., disturbance of polyglycan synthesis, thus acting on the adherence mechanisms of these organisms to dental surface. The present study evaluated the inhibitory capacity of adherence of a gel derived from Punica granatum Linn, which is a fruit rich in tannin and polyphenolics. The possibility that this component interfered with different mechanisms of toxicity or astringency was investigated on the adherence of one yeast and three bacterial stains to oral surfaces. The results indicated that the glucan synthesis and its antimicrobial action gave this gel an effective control of the already formed biofilm, which is considered the primary etiologic agent in caries disease and stomatitis.
Gebara et al. (19) reported that dental products containing natural substances have good market perspectives due to popular acceptance of phytotherapy, which represents an alternative to conventional treatments and could be introduced in the dental market as long as they are supported by scientific-based evidence.
The Punica granatum Linn (pomegranate) gel presented an inhibitory activity on the adherence of different bacterial strains and one yeast commonly found in the oral cavity. The findings of this study support the possibility that the Punica granatum Linn (pomegranate) gel might be used in the control of bacteria and yeasts responsible for oral infections such as caries, periodontal disease and stomatitis.
Accepted April 3, 2006
- 1. Marsh PD, BradshaW D.J. Physiological approaches to the control of oral biofilms. Adv Dent Res 1997;11:176-185.
- 2. Gibbons RJ. Adherent interactions which may affect microbial ecology in the mouth. J Dent Res 1984;63:378-385.
- 3. Jenkinson HF, Lala HC, Shepherd MG. Coaggregation of Streptococcus sanguis and other streptococci with Candida albicans Infect Immun 1990;58:1429-1436.
- 4. Nikawa H, Egusa H, Makihira S, Nishimura M, Ishida K, Furukawa M, Hamada T. A novel technique to evaluate the adhesion of Candida species to gingival epithelial cells. Mycoses 2002;46:384-389.
- 5. Makihira S, Nikawa H, Tamagami M, Hamada T, Nishimura H, Ishida K, Yamashiro H. Bacterial and Candida adhesion to intact and denatured collagen in vitro Mycoses 2002;45:389-392.
- 6. Nikawa H, Yamashiro H, Makihira S, Nishimura M, Egusa H, Furukawa M, Setijanto D, Hamada T. In vitro cariogenic potential of Candida albicans Mycoses 2003;46:471-478.
- 7. Klinke TH, Klimm W. Induction of caries-like lesions by Candida albicans in an artificial mouth. Caries Res 2002;36:3 (Abstrs.195-196).
- 8. Nostro A, Canntelli MA, Crisafi G, Musolino AD, Procopio F, Alonzo V. modifications of hydrophobicity, in vitro adherence and cellular aggregation of Streptococcus mutans by Helichrysum italicum extract. Lett Appl Microbial 2004;38:423-427.
- 9 Pereira JV, Pereira MSV, Sampaio FC, Sampaio MCC, Alves PM, Araújo CRF, Higino JS. In vitro antibacterial and antiadherence effect of Punica granatum Linn extract upon dental biofilm microorganisms. Braz J Pharmacogn 2006;16:88-93.
- 10. Vasconcelos LCS, Sampaio MCC, Sampaio FC, Higino JS. Use of Punica granatum Linn as an antifungal agent against candidosis associated with denture stomatitis. Mycoses 2003;46:192-196.
- 11. Haslam E. Natural polyphenols (vegetables tannins) as drugs: possible modes of action. J Nat Prod. 1996;59:205-215.
- 12. Scalbert A. Antimicrobial properties of tannins. Chemistry 1991;30:3875-3883.
- 13. Kulak Y, Arikan A, Kazazoglu E. Existence of Candida albicans and microorganisms in denture stomatitis patients. J. Oral Rehabil 1997;24:778-790.
- 14. Luo G, Samaranayake LP. Candida glabrata and emerging fungal pathogen, exhibits superior relative cell surface hydrophobicity and adhesion to denture acrylic surfaces compared with Candida albicans APMIS 2002;110:601-610.
- 15. Maza JL, Elguezabal N, Prado C, Ellacuría J, Soler I, Potón J. Candida albicans adherence to resin composite restorative dental material: influence of whole human saliva. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2002;94:589-592.
- 16. Shinada K, Ozaki F, Cordiero JG, Okada S, Shimoyama K, Nagao M, Ichinose S, Yamashita Y. A morphological study of interactions of Candida albicans and Streptococcus mutans Kokubyo Gakkai Zasshi 1995;62:281-286.
- 17. Guggenheim B, Guggenheim M, Gmur R, Giertsen E, Thurnheer T. Application of the Zurich biofilm model to problems of Cariology. Caries Res 2004;38:212-222.
- 18. Cotter G, Kavanagh K. Adherence Mechanisms of Candida albicans Br J Biomed Sci 2000;57:241-249.
- 19. Gebara ECE, Zardetto CGDC, Mayer MPA. In vitro study of the antimicrobial activity of natural substances against S. mutans and S. sobrinus Braz Oral Res (formerly Rev Odontol Univ São Paulo) 1996;10:251-256.
- 20. Kakiuchi N, Hattori M, Nishizawa M. Studies on dental caries prevention by traditional medicines. Inhibitory effect of various tannins on glucan synthesis by glycosyltransferase from Streptococcus mutans Chem Pharm Bull 1986;34:720-725.
Publication Dates
-
Publication in this collection
21 Dec 2006 -
Date of issue
2006
History
-
Accepted
03 Apr 2006 -
Received
03 Apr 2006