Abstract
The present study determined the hepatoprotective and antioxidant potential of the extract obtained from Amazon nut residues. The brown walnut shell of Bertholletia excelsa was collected and extracted sequentially for 48 h with different ethanol:water ratios and the dry extract was obtained by the spray dryer method. Antioxidant activities were evaluated by testing DPPH radicals, ABTS, total phenolics, flavonoids and cellular antioxidant. Subsequently, in vitro and in vivo tests were carried out to evaluate the protective effect of the extract after induction of liver damage with CCL4. Biochemical parameters of liver injury and biochemical markers of oxidative stress and antioxidant capacity were evaluated. In the mass spectrometry study, phenol and organic acids such as protocatechuic acid, gallic acid and citric acid were identified, which contributed to the elimination of free radicals, reducing DPPH and ABTS levels. The cell viability test after treatment with the extract on human fibroblast and human hepatocellular carcinoma cells showed no cytotoxicity. It was observed that the extract inhibited the production of free radicals in human fibroblasts. The dosage of 400 mg/kg was the most effective in reducing serum MDA levels. There was a significant reduction in hepatic biochemical markers in Hepg-2 with the extract tested at concentrations of 100 and 50 µg/mL and in rats there was a reduction after supplementation with the extract at a dose of 400 mg/kg, when subjected to oxidative stress with CCl4. From the results presented, it can be concluded that Bertholletia excelsa residues can be applied preventively against hepatotoxicity through the prevention of oxidative stress.
Keywords:
antioxidant; Bertholletia excelsa; hepatoprotection; liver injury; oxidative stress
Resumo
O presente estudo determinou o potencial hepatoprotetor e antioxidante do extrato obtido de resíduos de castanha da Amazônia. A casca da noz marrom de Bertholletia excelsa foi coletada e extraída sequencialmente por 48 h com diversas proporções etanol:água e o extrato seco foi obtido pelo método de spray dryer. As atividades antioxidantes foram avaliadas testando radicais DPPH, ABTS, fenólicos totais, flavonóides e antioxidante celular. Posteriormente, foram realizados testes in vitro e in vivo para avaliar o efeito protetor do extrato após indução de danos hepáticos com CCL4. Foram avaliados parâmetros bioquímicos de lesão hepática e marcadores bioquímicos de estresse oxidativo e capacidade antioxidante. No estudo de espectrometria de massas foram identificados fenóis como ácidos protocatecuico, ácido gálico e ácido quínico, que contribuíram para a eliminação de radicais livres, reduzindo os níveis de DPPH e ABTS. O teste de viabilidade celular após tratamento com o extrato em células de fibroblastos humanos e hepatocarcinoma humano não mostrou citotoxicidade. Observou-se que o extrato inibiu a produção de radicais livres em fibroblastos humanos. A dosagem de 400 mg/kg foi a mais eficaz na redução dos níveis séricos de MDA. Houve redução significativa dos marcadores bioquímicos hepáticos em Hepg-2 com o extrato testado na concentração de 100 e 50 µg/mL e em ratos houve uma redução após a suplementação com o extrato na dose de 400 mg/kg, quando submetidos ao estresse oxidativo com CCl4. A partir dos resultados apresentados pode-se concluir que os resíduos de Bertholletia excelsa podem ser aplicados preventivamente contra a hepatotoxicidade através da prevenção do estresse oxidativo.
Palavras-chave:
antioxidante; Bertholletia excelsa; hepatoproteção; lesão hepática; estresse oxidativo
1. Introduction
The use of plants, fruits and vegetables with medicinal properties, with the aim of treating, curing and preventing diseases, is one of the oldest medical practices. This context has attracted the interest of researchers and some pharmaceutical companies, which are dedicated to the study of medicinal plants and their active components (Harvey et al., 2015). Phytochemicals generate a wide variety of structurally diverse substances with a range of biological activities. They encompass a vast and diverse group of isolated, probiotic and dietary compounds, obtained from different sources with useful medicinal properties for the development of alternative or complementary therapies (Paine, 2020).
Medicinal plants, fruits and vegetables are rich in secondary metabolites that provide antioxidant properties. Phenolic compounds, terpenoids and alkaloids present in their composition are example of compunds responsibles for these activities. These components have distinct beneficial properties and actions, being widely used as antioxidants to protect and restore liver function, reduce inflammation and prevent oxidative stress (Barreto and Sahebkar, 2019). In some cases, these compounds may be present in fruit residues such as peels or seeds discarded by industries.
Bertholletia excelsa H.B.K. whose popular name is Castanha-do-Pará and Castanha-do-Brazil, is a plant species characteristic of Brazil, belonging to the Lecythidaceae family (Pena et al., 2015). It is known for its high concentration of selenium, proteins, fibers and a diversity of bioactive compounds, especially phenolic compounds, sterols and tocopherols (Huguenin et al., 2015). With regard to pharmacological aspects, the composition of the chestnut, rich in lipids, minerals and phytochemicals, has been related to the improvement of health indicators, contributing to the reduction of inflammatory markers, such as interleukins, NF-kβ transcription factor, prostaglandins , cyclooxygenases and reactive oxygen species. This highlights its anti-inflammatory, antioxidant, antiproliferative and hepatoprotective properties (González et al., 2021; Macan et al., 2022).
Hepatoprotection is related to the preservation of the functions and structure of hepatocytes, contributing to maintaining healthy liver function. Hepatoprotectors, which can be medicines or herbal medicines that play an important role in protecting liver cells against toxic agents, regardless of their origin, whether from external sources or internally generated metabolites, preventing the absorption of harmful substances, neutralizing the generation of free radicals, reducing hepatic inflammation and preserving the integrity of the hepatocyte membrane (Guan and He, 2015).
In this context, the objective of this work was to determine the hepatoprotective and antioxidant activity of an extract rich in phenolics obtained from Bertholletia excelsa nut residues. For this, antioxidant analyses, quantification of malonaldehyde (MDA) and measurement of liver enzymes (AST, ALT, FA, LDH) were carried out in vitro and in vivo models.
2. Materials and Methods
2.1. Sampling
The Brazil nuts (Bertholletia excelsa) were kindly provided by the “Cooperativa Agrícola Coari-Itapeua” located in the city of Coari, state of Amazonas, Brazil. All ripe fruits were cut in half and manually removed from the skins and nuts. The peels were washed three times with distilled water to remove contaminants, then dried in an oven with air circulation at 40 °C for 48 h and subsequently crushed in a knife mill.
2.2. Extract preparation
Brazil nut shells were extracted with a hydroethanolic solution (1:1 ethanol:water) in a ratio of 1:10 weight/solvent for biological testing. The method used was cold maceration, where 50 g of plant material were added to 500 mL of solvent extractor separately at room temperature for 48 h. The extract was dried in a spray dryer (MD 01, Labmaq do Brasil, Ribeirão Preto, Brazil) with an inlet and outlet temperature of 120 ºC and 80 ºC, respectively. The flow rate used was 0.6 L per hour with a 1mm diameter atomizer. The dry extract was kept under refrigeration until analysis.
2.3. Mass spectrometry analysis
The extract was characterized by mass spectrometry. The analyzes were carried out in the Analytical Center Laboratory of the Multidisciplinary Support Center (CAM). The high-resolution mass spectrum was obtained on a quadrupole time-of-flight (QTOF), MicroTOF-QII (Bruker Daltonics, Bremen, Germany), equipped with an electrospray source (ESI), operating in negative ionization mode. For direct injection MS measurement, the sample solution was delivered using a syringe pump at a rate of 180 μL/min. The QTOF system was calibrated using sound format calibrant with acceptance being <1.5 ppm.
2.4. Total phenolics dosage
The concentration of total phenols was quantified using the method described by Singleton and Rossi Junior (1965) with some modifications. Initially, 10 µL of B. excelsa residue extract (1 mg/mL) plus 50 µL of Folin-Ciocalteu solution (1:10) were added to the microplates and incubated for 8 min, then sodium carbonate was added to 0.4% and again incubated for 3 min. Then, absorbance was measured at 620 nm in a microplate reader (DTX 800, Beckman Coulter, CA, USA). The standard used was gallic acid.
2.5. Total flavonoids dosage
Total flavonoids were quantified using the method described by Zhishen et al. (1999) with modifications. Initially, 30 µL of B. excelsa residue extract (1 mg/mL), 90 µL of ethanol, 6 µL of 10% aluminum chloride and 6 µL of potassium acetate were added to the microplate wells, which were then incubated for 30 min. After the incubation time, absorbance was measured at 765 nm in a microplate reader (DTX 800, Beckman Coulter, CA, USA), the flavonoid content was calculated using a standard curve and quercetin was used as standard.
2.6. DPPH Assay
The test was conducted according to the methodology described by Molyneux (2004), using the 2,2-diphenyl1-picrylhydrazyl (DPPH) free radical scavenging method (Sigma-Aldrich, USA), with some modifications. An aliquot of 270 µL of DPPH solution (2 mg of DPPH dissolved in 12 mL of absolute ethanol) was added to a microplate along with 30 µL of B. excelsa residue extract (1 mg/mL) and the mixture was incubated for 30 min at room temperature protected from light. After this period, reading was performed at 517 nm on a microplate reader (DTX 800, Beckman Coulter, CA, USA). Gallic acid and DMSO were used as standard and negative controls, respectively.
2.7. ABTS assay
The test was carried out according to the methodology described by Re et al. (1999), with modifications. The ABTS solution (Sigma-Aldrich, USA) was prepared using the reaction of 0.7 mM of the radical dissolved in deionized water and 2.4 mM of potassium persulfate and the mixture was incubated at room temperature in the absence of light for 16 h. For the test, 30 µL of the B. excelsa residue extract and 270 µL of the ABTS solution were added and the plate was incubated under protection from light for 15 min. After this period, reading was performed at 630 nm on a microplate reader (DTX 800, Beckman Coulter, CA, USA). Gallic acid (Sigma-Aldrich, USA) was used as standard and DMSO as a negative control.
2.8. Cell culture
Human fibroblast (MRC-5) and human hepatocellular carcinoma (HEPG-2) cell lines were acquired from the cell bank of the Faculty of Pharmaceutical Sciences (FCF) of the Federal University of Amazonas (UFAM). At the FCF Cell Culture Laboratory (FCF/UFAM), cells were cultured with Dulbecco's Modified Eagle Medium (DMEM) with high glucose content (Gibco, San Jose, CA, USA), supplemented with 10% serum fetal bovine (FBS) (Gibco, USA) and 1% penicillin-streptomycin (Gibco, USA), in an oven at 37 ºC, with a humidified atmosphere and injection of 5% CO2.
2.9. Cytotoxicity
The cytotoxicity assay in fibroblast cell lines (MRC-5) and human hepatocellular carcinoma (HEPG-2) was performed by the Alamar blue method, using resazurin sodium salt (Sigma-Aldrich, USA) according to the method described by Ahmed et al. (1994). Cells were plated at a concentration of 0.5x104 cells per well in 96-well microplates. After 24 h of incubation and cell adhesion, the cells were treated with the B. excelsa destruction extract for a period of 72 h, at concentrations of 100, 50, 25, 12.5, 6.25, 3.125, 1.5625 µG/mL. As a negative control, 0.01% DMSO culture medium was used. After the treatment period, 10 μL of 0.4% resazurin (1:20 dilution) was added. The standardized incubation period for the MRC-5 cell line is 3 h and for HEPG-2 it is 2 h. After incubation, the microplates were checked using the fluorescence mode (540 nm exchange filter and 585 nm emission filter) in a microplate reader (DTX 800, Beckman Coulter, CA, USA).
2.10. Cellular antioxidant activity
The evaluation of cellular antioxidant activity was carried out using the methodology of Wolfe and Liu (2007), based on the detection of intracellular ROS production through the use of the fluorescent compound 2'7'-dichlorofluorescein-diacetate (DCFHDA) (Sigma-Aldrich, USA ). In this technique, cells from the fibroblast lineage (MRC-5) were used, which were seeded at a concentration of 6x104 cells per well and incubated for 24 h. After this period, the culture medium was removed and the wells were washed with phosphate-buffered saline (PBS). 100 µL of a solution of 2,7-dichlorofluorescein-diacetate (DCFH-DA) at 25 µM, dissolved in Hank's buffer, containing the extract of B. excelsa residue (12.5, 25, 50, 100) was added to the microplate wells. µg/mL), and incubated for 60 min at 37 °C and 5% CO2. The wells were washed again with PBS and, shortly after, a solution of 2,2'-azobis (2-methylpropionamidine) dihydrochloride (AAPH) (Sigma-Aldrich, USA) at 600 µM, dissolved in Hanks' buffer, was added. to the wells. Then, the microplate was read and fluorescence was measured at 485 excitation and 520 nm emission wavelengths for 60 min at 5 min intervals. As a positive control, rutin was used.
2.11. Hepatoprotective activity
2.11.1. Protective effect on HEPG-2 after toxicity induction with CCL4
Human hepatocellular carcinoma cells (HEPG-2) were seeded in a 6-well plate (5 x 105 cells/well) and left in the oven at 37 °C with 5% CO2 for 24 h. After this period, the cells were treated for 24 h with 25, 50 and 100 µg/mL of B. excelsa residue extract, after 24 h they were treated with CCL4 (10mM) to induce hepatotoxicity and incubated for 24 h. At the end of the treatment time, the supernatant was collected for subsequent biochemical analyzes (Ginting et al., 2021).
2.11.2. Measurement of AST, FA and LDH activities
After collecting the supernatant from HEPG-2 cells, the activities of aspartate aminotransferase (AST), alkaline phosphatase (FA) and lactate dehydrogenase (LDH) were measured immediately using the Kinetic-UV Test method using test kits. assay, following the manufacturer's guidelines (Vida Biotecnologia) which quantitatively determined the enzymatic activities of each marker to evaluate liver functions. The results are expressed in U /L.
2.11.3. Protective effect in vivo after toxicity induction with CCL4
25 male Balb mice aged 6-8 weeks, weighing 30-40 g, were used. These animals were provided by the UFAM bioterium and were housed in standard cages, in temperature-controlled rooms 22 °C, with a 12 h light/dark cycle and received water and food ad libitum. In this experimental model, the animals were divided into 5 groups, each group containing 5 animals. Group one (control) and group two (animals treated with 20% CCL4) were treated with only 0.2 mL of saline solution. Groups three, four and five were pretreated with B. excelsa residue extract (100, 200 and 400 mg/kg body weight). On the 15th day, groups 2, 3, 4 and 5 received the administration of CCL4 to induce hepatotoxicity. CCL4 was used at a final concentration of 20% v/v. This solution was administered to the mice by gavage with 150 µL of 20% CCl4 solution, three times a week for 2 weeks. On the 29th day, after completing 4 weeks, the mice were euthanized by cervical dislocation after anesthesia and blood samples were collected to measure biochemical parameters involving enzymatic markers of liver damage such as AST and ALT transaminases. The dosage was carried out through the blood plasma of mice and using the Kinetic-UV Test method through assay kits.
2.12. Quantification of TBARs
After euthanasia of the mice, liver tissues were found for quantification of lipid peroxidation using the TBARs method in which malondialdehyde (MDA), a final product of lipid peroxidation, reacts with thiobarbituric acid (TBA) forming a pinkish chromophore quantified spectrophotometrically, as shown. methodology described by Draper and Hadley (1990), with modifications. First, the liver tissue was homogenated by leaching and then resuspended in 150 µM NaCl. 100 µL of the homogenate was added to 1 mL of a mixture solution containing 400 µL of 1.3 M acetate in 0.27 M HCl, pH 3.4; 400 µL of 0.8% TBA and 200 µL of 8.1% SDS. Then, the mixture was incubated in a digester at 96 ºC for 60 min. Next, the samples were centrifuged at 3500 rpm for 10 min. Absorbance was measured at 535 nm and total malondialdehyde (MDA) content was quantified using MDA standard curve engineering. The results were expressed in µmoL/L.
2.13. Statistical analysis
The results were expressed as mean ± SD (standard deviation from the mean). The means were analyzed using the GraphPad Prisma® software 6.0 via two-way ANOVA followed by the Dunnett test of multiple comparisons with a significance level of p < 0.05.
3. Results and discussion
3.1. Mass spectrometry analysis
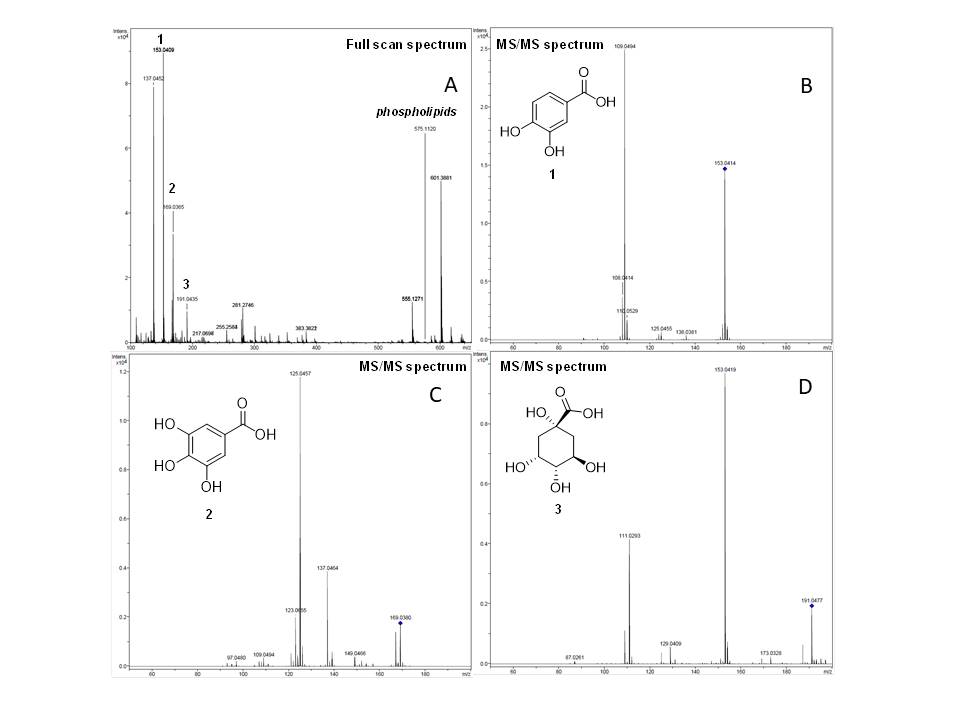
In the mass spectrometry analysis of the B. excelsa residue extract, phenolic compounds and organic acids were identified, such as protocatechuic acid (m/z 153), gallic acid (m/z 169) and citric acid (m/z 191), according to the MS/MS data (Figure 1 and Table 1). According to a study carried out by Asejeje et al. (2023) protocatechuic acid has a hepatoprotective effect against liver damage induced by dimethyl nitrosamine, a potent hepatotoxin, through its ability to suppress oxidative stress, inflammation and modulate the activities of cytochrome P-450.
Full scan spectrum and MS/MS spectra of Extract (A) protocatechuic acid (B), gallic acid (C) and citric acid (D) found in mass spectrometry analysis of B. excelsa residue extract.
Bashar et al. (2021) demonstrate that gallic acid provides significant protection against type 2 diabetes mellitus-mediated liver injury through increased hepatic mRNA expression of GLUT-4, Wnt1, and β-catenin with inhibitory effects on elevated expression of ERK1/2/ NF-κB. Quinic acid, according to Xue et al. (2023), belonging to the family of chlorogenic acids (CGAs), have hepatoprotective effects against various liver diseases through their antioxidant and anti-inflammatory activities.
3.2. Antioxidant activity
During the activity of the biological system, cellular metabolism generates countless free radicals, many of these radicals are produced in the form of reactive oxygen species. Excessive production of ROS in the liver will lead to progressive functional impairment of the liver parenchyma, stimulating inflammatory processes responsible for the development of liver diseases (Mohammed et al., 2021).
In view of this, antioxidant tests were carried out to evaluate the antioxidant potential of the B. excelsa residue extract, where the extract (concentration of 100 µg/mL) presented flavonoid contents of 0.57 ± 0.09% and 4.44 ± 0.22% in phenols (Table 2). And in the free radical capture assays, in which the percentage of antioxidant activity corresponds to the amount of DPPH and ABTS consumed by the antioxidant substance, the extract (concentration of 100 µg/mL) showed 81.5±1.47% inhibition in the DPPH and 76.7±1.29% in the ABTS assay (Table 2).
Phenolics and flavonoids content and antioxidant activity in vitro of B. excelsa residues extracts.
The cellular antioxidant activity evaluation assay was also carried out, which is based on the detection of inhibition of intracellular ROS production using the fluorescent compound: 2'7'-dichloro-fluorescein-diacetate (DCFH-DA) in fibroblast cell lines (Figure 2). As a result of this test, the B. excelsa residue extract showed 82.1 ± 3.72% ROS inhibition when tested at a concentration of 100 µg/mL, 77.1 ± 2.70% at 50 µg/mL, 63.1 ± 4.03% at 25 µg/mL and 55.6 ± 2.76% at 12.5 µg/mL.
Result of the cellular antioxidant activity assay in a fibroblast line treated with the B. excelsa residue extract and Quercetin, at concentrations of 100, 50, 25, 12.5 µg/mL. Data are expressed as percentage of ROS inhibition with mean ± standard deviation and analyzed by two-way ANOVA followed by Dunnett's test. *p < 0.05.
In the cellular antioxidant assay, the extract demonstrated a concentration-dependent antioxidant activity where the percentage of inhibition decreases as the concentration of the extract decreases. Quercetin has potent antioxidant activity and was used in this trial as a comparison standard. The association of polyphenols present in the extract contributes to the antioxidant potential and constancy of this activity.
Silva et al. (2023) evaluated the anti-inflammatory and antioxidant properties of Bertholletia excelsa bark extract in which the extract efficiently reduced oxidative stress through the inhibition of ROS due to its antioxidant properties that protect cells from cell death induced by H2O2, inducing enzymes antioxidants, including GPX1.
3.3. Cell viability
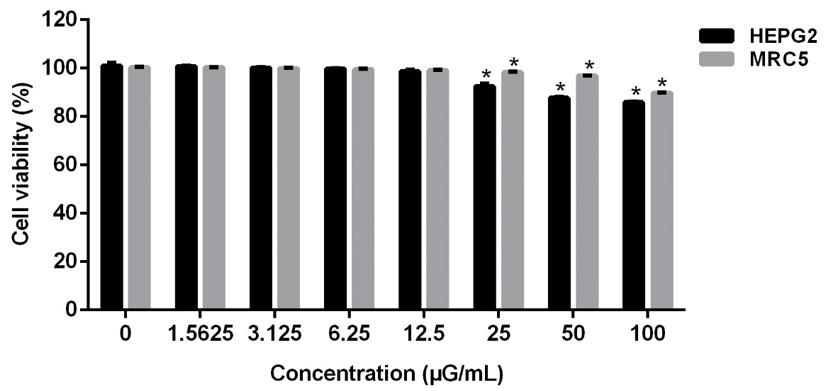
To verify possible cytotoxicity of the extracts, a cell viability assay was carried out with human hepatocarcinoma cell lines (HEPG-2) and human fibroblasts (MRC-5) using the alamar blue dye to determine viability after incubation of the extract. residue from B. excelsa with the cells in 7 different concentrations ranging from 1.5625 to 100 µg/mL for a period of 72 h. This assay is based on resazurin staining, which displays colorimetric and fluorimetric changes related to cellular metabolic activity.
According to the graphic representation shown in Figure 3, the cell lines showed an average cell viability above 80% when exposed to the B. excelsa residue extract for a period of 72 h. At a concentration of 100 µg/mL, HEPG-2 presented a percentage of 85.9 ± 0.25% of viable cells and MRC-5 89.8 ± 0.14%. At other concentrations, cells showed an average viability percentage above 90% in both lines.
Cell viability assay of B. excelsa residue extract. HEPG-2 and MRC-5 cells were exposed with the B. excelsa residue extract in a concentration curve of 100, 50, 25, 12.5, 6.25, 3.125, 1.5625 µG/mL over a treatment time of 72 h. Data are expressed as percentage of cell viability with mean ± standard deviation and analyzed by two-way ANOVA followed by Dunnett's test. *p < 0.05.
Based on the analysis of the results obtained, it is possible to conclude that the B. excelsa residue extract does not present cytotoxicity at the concentrations to which the MRC-5 and HEPG-2 cell lines were exposed.
3.4. Quantification of TBARs
TBARs analysis is used to evaluate lipid oxidation through the quantification of malonaldehyde (MDA), which is an important biomarker used in the evaluation of oxidative stress. Evaluating oxidative stress in the experimental model in mice, the concentration of MDA (µMol/L) was measured through liver tissue samples. In Figure 4, we can see that the groups treated with the B. excelsa residue extract at concentrations of 100, 200 and 400 mg/Kg had lower MDA levels than the group treated with CCL4 (inducer of hepatotoxicity). The MDA concentration values obtained from animals treated with the extract at the respective doses were 0.92 ± 0.07; 0.60 ± 0.05; 0.44 ± 0.06 µMol/L.
Quantification of TBARs in the plasma of mice intoxicated by CCL4 and supplemented with the residual extract of B. excelsa at doses of 100, 200 and 400 mg/Kg. Data are expressed as mean ± standard deviation and analyzed by two-way ANOVA followed by Dunnett's test. *p < 0.05.
MDA is the main marker of lipid oxidation as it is the most abundant final product in plasma after lipid peroxidation. This aldehyde has a deleterious effect on proteins and can cause DNA fragmentation (Nucci et al., 2013). The B. excelsa residue extract reduced the concentration of this aldehyde in liver tissues, promoting an antioxidant and hepatoprotective action, effectively reducing the concentration of this compound in animals treated with the extract, due to the difference between the group treated with the extract and the group treated with the extract. only with CCL4.
3.5. Hepatoprotective activity
3.5.1. Protective effect on HEPG-2 after toxicity induction with CCL4
The administration of CCl4 generates excessive production of ROS in the liver, leading to progressive functional impairment of the liver parenchyma. Damaged liver cells lose their integrity, releasing specific enzymes into the bloodstream, such as alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (FA) and lactate dehydrogenase (LDH), often used as biochemical indicators of liver damage (Colak et al., 2016; Otrubova et al., 2018).
Therefore, hepatotoxicity was induced with CCl4 in HEPG-2 cells after previous treatment with the B. excelsa residue extract, in order to evaluate its hepatoprotective/cytoprotective effect. In this way, the cells were exposed to the extract at concentrations of 100, 50 and 25 µg/mL, treated 24 h before treatment with 10 mM CCl4.
The results of this analysis are represented through the graphs in Figure 5, showing better results in reducing the levels of AST, FA and LDH enzyme activities in treatment with the extract at concentrations of 100 and 50 µg/mL, presenting 210 and 549 U /L of AST, 98 and 211 U/L of FA, 271 and 456 U/L of LDH, respectively, when compared with the positive hepatotoxicity control (cells treated only with 10 mM CCl4).
Result of the in vitro hepatoprotective effect of the B. excelsa residual extract, through the measurement of hepatic biochemical markers (AST, FA, LDH) as a result of hepatotoxicity induced by CLL4 in a HEPG-2 cell line. The negative control is cells treated with culture medium, the positive control is cells treated with CCL4. Graph A represents the AST dosage, graph B the alkaline phosphatase dosage and graph C the LDH dosage. Data are expressed as mean ± standard deviation and analyzed by two-way ANOVA followed by Dunnett's test. *p < 0.05.
3.6. Protective effect in vivo after toxicity induction with CCL4
The action of free radicals can cause damage to certain tissues, such as the liver, causing changes in metabolic and enzymatic processes (Kisseleva, 2017). CCL4 is considered an extremely hepatotoxic substance, as its metabolites cause cirrhosis and liver fibrosis (Andrade et al., 2019).
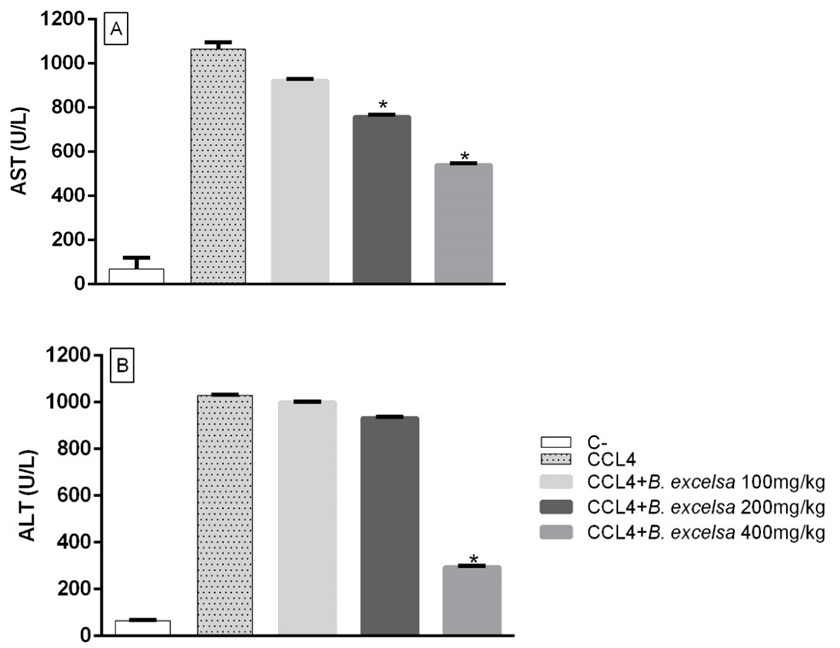
Evaluating hepatoprotection in the experimental model in mice, mouse blood plasma was used to measure alanine aminotransferase (ALT) and aspartate aminotransferase (AST), which are biochemical indicators of liver damage.
According to Figure 6, it was observed that the animals treated with the B. excelsa residue extract had their transaminase activity profile improved when compared to the group treated with CLL4 (inducer of hepatotoxicity), this effect was more significant in the animals treated with a dose of 400mg/kg of the extract. (p<0.05). The results obtained in the quantification of AST and ALT at a dose of 400 mg/Kg were 540 and 294 U/L, respectively.
Result of the measurement of hepatic biochemical markers (AST and ALT) of mice supplemented with residual extract of B. excelsa at doses of 100, 200 and 400 mg/Kg, and subjected to oxidative stress with CCl4. Graph A represents the AST dosage and B the ALT dosage. Data are expressed as mean ± standard deviation and analyzed by two-way ANOVA followed by Dunnett's test. *p < 0.05.
This capacity can be attributed to phenolic compounds that can reduce the concentration of these biomarkers, acting to inhibit the oxidation of tissues, especially the liver, promoting an antioxidant and hepatoprotective effect (Hui et al., 2020).
4. Conclusion
In conclusion, the results obtained from the present study demonstrated that the B. excelsa residue extract presented antioxidant and hepatoprotective activity in in vitro and in vivo models. The extract was rich in phenolic compounds such as protocatechuic acid, gallic acid and citric acid as the main compounds. These results indicate that Brazil nut seed shell extract can be applied against hepatotoxicity through the prevention and inhibition of oxidative stress in products intended for human or animal health.
Acknowledgements
Authors would like to thank Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – CAPES, Fundação de Amparo à Pesquisa do Estado do Amazonas – FAPEAM and Conselho Nacional de Desenvolvimento Científico e Tecnológico - CNPq for financial support and fellowships. We are also grateful to the staff at the Analytical Center at UFAM for making their infrastructure available.
References
-
AHMED, S.A., GOGAL JUNIOR, R.M. and WALSH, J.E., 1994. A new rapid and simple non-radioactive assay to monitor and determine the proliferation of lymphocytes: an alternative to [3H]thymidine incorporation assay. Journal of Immunological Methods, vol. 170, no. 2, pp. 211-224. http://doi.org/10.1016/0022-1759(94)90396-4 PMid:8157999.
» http://doi.org/10.1016/0022-1759(94)90396-4 -
ANDRADE, R.J., CHALASANI, N., BJÖRNSSON, E.S., SUZUKI, A., KULLAK-UBLICK, G.A., WATKINS, P.B., DEVARBHAVI, H., MERZ, M., LUCENA, M.I., KAPLOWITZ, N. and AITHAL, G.P., 2019. Drug-induced liver injury. Nature Reviews Disease Primers, vol. 22, no. 5, pp. 58. https://dx.doi.org/10.1038/s41572-019-0105-0 PMid: 31439850.
» https://dx.doi.org/10.1038/s41572-019-0105-0 -
ASEJEJE, F., ETIM, S., ASEJEJE, G., IWUOH, B.C., AKINTADE, S.I., ADEDARA, I. and FAROMBI, E.O., 2023. Protocatechuic acid modulates hepatic oxidative stress and inflammation linked to DMN exposure in rat. Nigerian Journal of Physiological Sciences; Official Publication of the Physiological Society of Nigeria, vol. 38, no. 2, pp. 145-155. http://doi.org/10.54548/njps.v38i2.4 PMid:38696681.
» http://doi.org/10.54548/njps.v38i2.4 -
BARRETO, G. and SAHEBKAR, A., 2019. Pharmacological properties of plant-derived natural products and implications for human health. In: H. DONG, N. REZAEI, O. STEINLEIN, J. XIAO, A. ROSENHOUSE-DANTSKER and R. GERLAI, eds. Advances in experimental medicine and biology Switzerland: Springer Nature, vol. 1308., pp. 249-255. https://dx.doi.org/10.1007/978-3-030-64872-5
» https://dx.doi.org/10.1007/978-3-030-64872-5 -
BASHAR, S.M., ELHADIDY, M.G., MOSTAFA, A.F., HAMED, B., HELMY, S. and ABD-ELMONIEM, H.A., 2021. Hepatoprotective effect of gallic acid against type 2 induced diabetic liver injury in male rats through modulation of fetuin-A and GLP-1 with involvement of ERK1/2/NF-κB and Wnt1/β-catenin signaling pathways. General Physiology and Biophysics, vol. 40, no. 3, pp. 221-234. http://doi.org/10.4149/gpb_2021005 PMid:34100378.
» http://doi.org/10.4149/gpb_2021005 -
COLAK, E., USTUNER, M.C., TEKIN, N., COLAK, E., BURUKOGLU, D., DEGIRMENCI, I. and GUNES, H.V., 2016. The hepatocurative effects of Cynara scolymus L. leaf extract on carbon tetrachloride-induced oxidative stress and hepatic injury in rats. Springer Plus, vol. 5, pp. 216. https://dx.doi.org/10.1186/s40064-016-1894-1 PMid: 27026910.
» https://dx.doi.org/10.1186/s40064-016-1894-1 -
DRAPER, H.H. and HADLEY, M., 1990. Malondialdehyde determination as index of lipid peroxidation. Methods in Enzymology, vol. 186, pp. 421-431. http://doi.org/10.1016/0076-6879(90)86135-I PMid:2233309.
» http://doi.org/10.1016/0076-6879(90)86135-I -
GINTING, C.N., LISTER, I.N.E., GIRSANG, E., WIDOWATI, W., YUSEPANY, D.T., AZIZAH, A.M. and KUSUMA, H.S.W., 2021. Hepatotoxicity prevention in Acetaminophen-induced HepG2 cells by red betel (Piper crocatumRuiz and Pav) extract from Indonesia via antioxidant, anti-inflammatory, and anti-necrotic. Heliyon, vol. 4, no. 1, pp. e05620. http://doi.org/10.1016/j.heliyon.2020.e05620 PMid:33474504.
» http://doi.org/10.1016/j.heliyon.2020.e05620 -
GONZÁLEZ, O., BAUTISTA, C.J., GONZÁLEZ, F., HERNANDEZ, A., CAMARENA, E., CRUZ, F. and TRUJANO, M.E., 2021. Bertholletia excelsaseeds reduce anxiety-like behavior, lipids, and overweight in mice. Molecules (Basel, Switzerland), vol. 26, no. 11, pp. 3212. http://doi.org/10.3390/molecules26113212 PMid:34072024.
» http://doi.org/10.3390/molecules26113212 -
GUAN, Y.S. and HE, Q., 2015. Plants consumption and liver health. Evidence-Based Complementary and Alternative Medicine, vol. 2015, pp. 824185. http://doi.org/10.1155/2015/824185 PMid:26221179.
» http://doi.org/10.1155/2015/824185 -
HARVEY, A.L., EDRADA-EBEL, R. and QUINN, R.J., 2015. The re-emergence of natural products for drug discovery in the genomics era. Nature Reviews. Drug Discovery, vol. 14, no. 2, pp. 111-129. http://doi.org/10.1038/nrd4510 PMid:25614221.
» http://doi.org/10.1038/nrd4510 -
HUGUENIN, G.V., OLIVEIRA, G.M., MOREIRA, A.S., SAINT'PIERRE, T.D., GONÇALVES, R.A., PINHEIRO-MULDER, A.R., TEODORO, A.J., LUIZ, R.R. and ROSA, G., 2015. Improvement of antioxidant status after Brazil nut intake in hypertensive and dyslipidemic subjects. Nutrition Journal, vol. 14, pp. 54. http://doi.org/10.1186/s12937-015-0043-y PMid:26022214.
» http://doi.org/10.1186/s12937-015-0043-y -
HUI, Y., WANG, X., YU, Z., FAN, X., CUI, B., ZHAO, T., MAO, L., FENG, H., LIN, L., YU, Q., ZHANG, J., WANG, B., CHEN, X., ZHAO, X. and SUN, C., 2020. Scoparone as a therapeutic drug in liver diseases: pharmacology, pharmacokinetics and molecular mechanisms of action. Pharmacological Research, vol. 160, pp. 105170. http://doi.org/10.1016/j.phrs.2020.105170 PMid:32877694.
» http://doi.org/10.1016/j.phrs.2020.105170 -
KISSELEVA, T., 2017. The origin of fibrogenic myofibroblasts in fibrotic liver. Hepatology, vol. 65, no. 3, pp. 1039-1043. https://dx.doi.org.10.1002/hep.28948 PMid: 27859502.
» https://dx.doi.org.10.1002/hep.28948 -
MACAN, T.P., AMORIM, T.A., DAMIANI, A.P., BERETTA, Â.C.D.L., MAGENIS, M.L., VILELA, T.C., TEIXEIRA, J.P. and ANDRADE, V.M., 2022. Brazil nut prevents oxidative DNA damage in type 2 diabetes patients. Drug and Chemical Toxicology, vol. 45, no. 3, pp. 1066-1072. http://doi.org/10.1080/01480545.2020.1808667 PMid:32811197.
» http://doi.org/10.1080/01480545.2020.1808667 -
MOHAMMED, S., NICKLAS, E.H., THADATHIL, N., SELVARANI, R., ROYCE, G.H., KINTER, M., RICHARDSON, A. and DEEPA, S.S., 2021. Role of necroptosis in chronic hepatic inflammation and fibrosis in a mouse model of increased oxidative stress. Free Radical Biology and Medicine, vol. 164, pp. 315-328. http://doi.org/10.1016/j.freeradbiomed.2020.12.449 PMid:33429022.
» http://doi.org/10.1016/j.freeradbiomed.2020.12.449 - MOLYNEUX, P., 2004. The use of the stable free radical diphenylpicrylhydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin Journal of Science and Technology, vol. 26, no. 2, pp. 211-219.
- NUCCI, C., DI-PIERRO, D., VARESI, C., CIUFFOLETTI, E., RUSSO, R., GENTILE, R. and MANCINO, R., 2013. Increased malondialdehyde concentration and reduced total antioxidant capacity in aqueous humor and blood samples from patients with glaucoma. Molecular Vision, vol. 19, pp. 1841-1846. PMid:23946639.
-
OTRUBOVA, O., JERIGOVA, M., HALASZOVA, S., TURECKY, L., MUCHOVA, J. and VELIC, D., 2018. Rat liver intoxication with CCl4: biochemistry, histology, and mass spectrometry. General Physiology and Biophysics, vol. 37, no. 5, pp. 527-535. https://dx.doi.org.10.4149/gpb_2018011 PMid: 30307403.
» https://dx.doi.org.10.4149/gpb_2018011 -
PAINE, M.F., 2020. Natural products: experimental approaches to elucidate disposition mechanisms and predict pharmacokinetic drug interactions. Drug Metabolism and Disposition: the Biological Fate of Chemicals, vol. 48, no. 10, pp. 956-962. http://doi.org/10.1124/dmd.120.000182 PMid:32816868.
» http://doi.org/10.1124/dmd.120.000182 -
PENA, M.M.A., FERREIRA, S.M.N., DA-COSTA, C.E., MORAIS, L., LAMARÃO, M.L., RIBEIRO-COSTA, R.M. and SILVA-JÚNIOR, J.O., 2015. Physicochemical characterization, fatty acid composition, and thermal analysis of Bertholletia excelsa HBK oil. Pharmacognosy Magazine, vol. 11, no. 41, pp. 147-151. http://doi.org/10.4103/0973-1296.149730 PMid:25709225.
» http://doi.org/10.4103/0973-1296.149730 -
RE, R., PELLEGRINI, N., PROTEGGENTE, A., PANNALA, A., YANG, M. and EVANS, C.R., 1999. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biology & Medicine, vol. 26, no. 9-10, pp. 1231-1237. http://doi.org/10.1016/S0891-5849(98)00315-3 PMid:10381194.
» http://doi.org/10.1016/S0891-5849(98)00315-3 -
SILVA, M.J., BOLETI, A.P., ACHO, L.D., CAMPOS, J.F., NETO, J.P., GUIMARAES, A., SILVA, F.M., KOOLEN, H.F., SANTOS, E.L. and LIMA, E.S., 2023. Anti-inflammatory and antioxidant properties of Bertholletia excelsa (H.B.K) bark extract. Boletín Latinoamericano y del Caribe de Plantas Medicinales y Aromáticas, vol. 22, no. 4, pp. 472-487. http://doi.org/10.37360/blacpma.23.22.4.35
» http://doi.org/10.37360/blacpma.23.22.4.35 -
SINGLETON, V.L. and ROSSI JUNIOR, J.A., 1965. Colorimetry of total phenolic with phosphomolybdic-phosphotungstic acid reagents. American Journal of Enology and Viticulture, vol. 16, no. 3, pp. 144-158. http://doi.org/10.5344/ajev.1965.16.3.144
» http://doi.org/10.5344/ajev.1965.16.3.144 -
SOONG, Y.Y. and BARLOW, P.J., 2005. Isolation and structure elucidation of phenolic compounds from longan (Dimocarpus longan Lour.) seed by high-performance liquid chromatography–electrospray ionization mass spectrometry. Journal of Chromatography. A, vol. 1085, no. 2, pp. 270-277. http://doi.org/10.1016/j.chroma.2005.06.042 PMid:16106708.
» http://doi.org/10.1016/j.chroma.2005.06.042 - TAHIR, N.I., SHAARI, K., ABAS, F., PARVEEZ, G.K.A., HASHIM, A.T. and RAMLI, U.S., 2013. Identification of oil palm (Elaeis guineensis) spear leaf metabolites using mass spectrometry and neutral loss analysis. Journal of Oil Palm Research, vol. 25, no. 1, pp. 72-83.
-
VITAGLIONE, P., DONNARUMMA, G., NAPOLITANO, A., GALVANO, F., GALLO, A., SCALFI, L. and FOGLIANO, V., 2007. Protocatechuic acid is the major human metabolite of cyanidin-glucosides3. The Journal of Nutrition, vol. 137, no. 9, pp. 2043-2048. http://doi.org/10.1093/jn/137.9.2043 PMid:17709440.
» http://doi.org/10.1093/jn/137.9.2043 -
WOLFE, K.L. and LIU, R.H., 2007. Cellular antioxidant activity (CAA) assay for assessing antioxidants, foods, and dietary supplements. Journal of Agricultural and Food Chemistry, vol. 55, no. 22, pp. 8896-8907. http://doi.org/10.1021/jf0715166 PMid:17902627.
» http://doi.org/10.1021/jf0715166 -
XUE, H., WEI, M. and JI, L., 2023. Chlorogenic acids: a pharmacological systematic review on their hepatoprotective effects. Phytomedicine, vol. 118, pp. 154961. http://doi.org/10.1016/j.phymed.2023.154961 PMid:37453191.
» http://doi.org/10.1016/j.phymed.2023.154961 -
ZHISHEN, J., MENGCHENG, T. and JIANMING, W., 1999. The determination of flavonoidcontents in mulberry and their scavenging effects on superoxide radicals. Food Chemistry, vol. 64, no. 4, pp. 555-559. http://doi.org/10.1016/S0308-8146(98)00102-2
» http://doi.org/10.1016/S0308-8146(98)00102-2
Publication Dates
-
Publication in this collection
22 Nov 2024 -
Date of issue
2024
History
-
Received
29 July 2024 -
Accepted
15 Sept 2024

 Hepatoprotective and antioxidant activities of phenolic-rich extract from shell of nut Brazil (Bertholletia excelsa H.B.K.)
Hepatoprotective and antioxidant activities of phenolic-rich extract from shell of nut Brazil (Bertholletia excelsa H.B.K.)