Abstract
Promising bioactivities of silver nanoparticles SNP urged researchers of different specialties to evaluate their field-respective activities. Bioactivity towards agricultural pests were the subject of limited publications. In the current study, SNP were synthesized and miticidal activity was evaluated towards old world date mite Oligonychus afrasiaticus (McGregor) (Acari: Tetranychidae) and an associated predatory mite Neoseiulus barkeri Hughes (Phytoseiidae). Under laboratory conditions, SNP displayed significantly higher activity towards O. afrasiaticus (LC50 was 39.7 µg/mL) than N. barkeri (LC50 was 1587.9 µg/mL) which accounts for about 40 folds of selectivity against the pest. SNP exhibited ovicidal activity against laid eggs of O. afrasiaticus (LC50 was 67.8 µg/mL). In field, SNP (at 216 µg/mL) achieved slightly higher efficiency than in laboratory study, 86.5% of population reduction of O. afrasiaticus was achieved and only 18.5% of N. barkeri population was affected. SNP suppressed hatching of 57.1% of laid eggs of O. afrasiaticus. Residues of silver were determined using ICP-OES spectrometry. Initial residues reached 1.83 µg/mL after application then declined with time passing. Estimated daily intake (EDI) reached 1.28 µg/kg/day, calculated for the highest residues obtained and the highest consumption rate of date in the world. Hazard index (Hi) was 0.17 in average. The obtained level of residues appeared to be safe in terms of acute and chronic toxicity references.
Keywords:
miticidal activity of SNP; Oligonychus afrasiaticus; Neoseiulus barkeri; selectivity; risk assessment
Resumo
Bioatividades promissoras de nanopartículas de prata (SNPs) incitaram pesquisadores de diferentes especialidades a avaliar suas atividades em campo. A bioatividade contra pragas agrícolas foi objeto de publicações limitadas. No presente estudo, SNPs foram sintetizadas, e a atividade miticida foi avaliada em relação ao ácaro Oligonychus afrasiaticus (McGregor) (Acari: Tetranychidae) e um ácaro predador associado, Neoseiulus barkeri Hughes (Phytoseiidae). Em condições de laboratório, SNP apresentou atividade significativamente maior para O. afrasiaticus (LC50 foi de 39,7 µg/mL) do que N. barkeri (LC50 foi de 1.587,9 µg/mL), o que representa cerca de 40 vezes de seletividade contra a praga. O SNP exibiu atividade ovicida contra ovos postos de O. afrasiaticus (LC50 foi de 67,8 µg/mL). Em campo, o SNP (a 216 µg/mL) alcançou eficiência ligeiramente maior do que em estudo de laboratório; 86,5% de redução populacional de O. afrasiaticus foram alcançados e apenas 18,5% da população de N. barkeri foram afetados. O SNP suprimiu a eclosão de 57,1% dos ovos postos de O. afrasiaticus. Os resíduos de prata foram determinados usando espectrometria ICP-OES. Os resíduos iniciais atingiram 1,83 µg/mL após a aplicação e depois diminuíram com o passar do tempo. A ingestão diária estimada (IDE) atingiu 1,28 µg/kg/dia, calculada para os maiores resíduos obtidos e a maior taxa de consumo de tâmaras do mundo. O índice de risco (Hi) foi de 0,17 em média. O nível de resíduos obtido mostrou-se seguro em termos de referências de toxicidade aguda e crônica.
Palavras-chave:
atividade miticida do SNP; Oligonychus afrasiaticus; Neoseiulus barkeri; seletividade; avaliação de risco
1. Introduction
Date palm (Phoenix dactylifera L.) is cultivated in forty countries that distributed in all continents. World production of date exceeded nine million tonnes in the year 2019, of which 16% was produced in Saudi Arabia with a value of about 3.5 billion US Dollars (FAO, 2019FOOD AND AGRICULTURE ORGANIZATION – FAO, 2019 [viewed 15 October 2021]. FAOSTAT [online]. Available from: http://www.fao.org/faostat/
http://www.fao.org/faostat/...
). World production is increasing every year by cultivating more palm trees. Pests play an infamous role in agricultural production as a limiting factor. Old world date mite O. afrasiaticus or (dust mite of palm) is a key pest of date palm in The Middle East and North Africa (Talhouk, 1991TALHOUK, A.S., 1991. On the management of the date palm and its arthropod enemies in the Arabian Peninsula. Journal of Applied Entomology, vol. 111, no. 1-5, pp. 514-520. http://dx.doi.org/10.1111/j.1439-0418.1991.tb00354.x.
http://dx.doi.org/10.1111/j.1439-0418.19...
; Blumberg, 2008BLUMBERG, D., 2008. Review: date palm arthropod pests and their management in Israel. Phytoparasitica, vol. 36, no. 5, pp. 411-448. http://dx.doi.org/10.1007/BF03020290.
http://dx.doi.org/10.1007/BF03020290...
; El-Shafie, 2012EL-SHAFIE, H.A.F., 2012. Review: list of arthropod pests and their natural enemies identified worldwide on date palm, Phoenix dactylifera L. Agriculture and Biology Journal of North America, vol. 3, no. 13, pp. 516-524. http://dx.doi.org/10.5251/abjna.2012.3.12.516.524.
http://dx.doi.org/10.5251/abjna.2012.3.1...
). It is responsible for great losses of date produce in terms of quantity and quality (Jeppson et al., 1975JEPPSON, L.R., KEIFER, H.H. and BAKER, E.W., 1975. Mites injurious to economic plants. Berkeley: University of California Press, 614 p. http://dx.doi.org/10.1525/9780520335431.
http://dx.doi.org/10.1525/9780520335431...
; Alatawi, 2020ALATAWI, F.J., 2020. Field studies on occurrence, alternate hosts and mortality factors of date palm mite, Oligonychus afrasiaticus (McGregor) (Acari: tetranychidae). Journal of the Saudi Society of Agricultural Sciences, vol. 19, no. 2, pp. 146-150. http://dx.doi.org/10.1016/j.jssas.2018.08.003.
http://dx.doi.org/10.1016/j.jssas.2018.0...
). It develops netting of thin filament produced by nymphs and adults where dust and remains of mites accumulate (Yahia and Kader, 2011YAHIA, E.M. and KADER, A.A. 2011. Date (Phoenix dactylifera L.). In: E.M. YAHIA, ed. Postharvest biology and technology of tropical and sub-tropical fruits. Cambridge: Woodhead Publishing, pp. 41-79. http://dx.doi.org/10.1533/9780857092885.41.
http://dx.doi.org/10.1533/9780857092885....
). It causes fruit drop while still immature (Yahia and Kader, 2011YAHIA, E.M. and KADER, A.A. 2011. Date (Phoenix dactylifera L.). In: E.M. YAHIA, ed. Postharvest biology and technology of tropical and sub-tropical fruits. Cambridge: Woodhead Publishing, pp. 41-79. http://dx.doi.org/10.1533/9780857092885.41.
http://dx.doi.org/10.1533/9780857092885....
). Palm dust mite affects date fruits mainly at the early stages of development and continues at later stages (Alatawi, 2020ALATAWI, F.J., 2020. Field studies on occurrence, alternate hosts and mortality factors of date palm mite, Oligonychus afrasiaticus (McGregor) (Acari: tetranychidae). Journal of the Saudi Society of Agricultural Sciences, vol. 19, no. 2, pp. 146-150. http://dx.doi.org/10.1016/j.jssas.2018.08.003.
http://dx.doi.org/10.1016/j.jssas.2018.0...
). According to Codex Alimentarius, date fruits should be practically free of any visible extraneous matter such as, dust, living or dead pests of different life-stages and remnants of pests per se or their life’s products (FAO, 2017FOOD AND AGRICULTURE ORGANIZATION – FAO. Codex Alimentarius Commission, 2017 [viewed 26 December 2021]. Joint FAO/WHO food standards program Codex Committee on fresh fruits and vegetables. 20th Session Kampala, Uganda, 2-6 October, 2017. Proposed draft standard for fresh dates [online]. Available from: http://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FMeetings%252FCX-731-21%252FWorking%2Bdocuments%252Fffv21_07e.pdf
http://www.fao.org/fao-who-codexalimenta...
). Pesticides are applied to control date pests and to enhance the quality of the produce. Several pesticides are recommended, by authorities, to control date palm mite (i.e. ethion, bifenthrin, abamectin and sulfur). Residues of pesticides in crops are proportional to rates and times of application that increase with incidence of pesticide resistance. The exceeding acceptable limits residues of pesticides in palm dates present a risk to consumers (Abdel Ghani et al., 2018ABDEL GHANI, S.B., ALHEWAIRINI, S.S. and HROUZKOVA, S., 2018. A fast and easy QuEChERS-DLLME method combined with GC-MS for ethion and bifenthrin residues determination and study of their dissipation dynamics in palm dates. Food Analytical Methods, vol. 11, no. 12, pp. 3542-3550. http://dx.doi.org/10.1007/s12161-018-1333-8.
http://dx.doi.org/10.1007/s12161-018-133...
). New selective and effective pesticides are always looked-for to accomplish control of normal and resistant pests. Silver nanoparticles SNP showed activity towards various arthropods (Govindarajan et al., 2016GOVINDARAJAN, M., RAJESWARY, M., VEERAKUMAR, K., MUTHUKUMARAN, U., HOTI, S.L., MEHLHORN, H., BARNARD, D.R. and BENELLI, G., 2016. Novel synthesis of silver nanoparticles using Bauhinia variegata: a recent eco-friendly approach for mosquito control. Parasitology Research, vol. 115, no. 2, pp. 723-733. http://dx.doi.org/10.1007/s00436-015-4794-3. PMid:26490683.
http://dx.doi.org/10.1007/s00436-015-479...
; Pavela et al., 2017PAVELA, R., MURUGAN, K., CANALE, A. and BENELLI, G., 2017. Saponaria officinalis-synthesized silver nanocrystals as effective biopesticides and oviposition inhibitors against Tetranychus urticae Koch. Industrial Crops and Products, vol. 97, pp. 338-344. http://dx.doi.org/10.1016/j.indcrop.2016.12.046.
http://dx.doi.org/10.1016/j.indcrop.2016...
; Avinash et al., 2017AVINASH, B., VENU, R., ALPHA, R.M., SRINIVASA, R.K., SRILATHA, C.H. and PRASAD, T.N.V.K.V., 2017. In vitro evaluation of acaricidal activity of novel green silver. nanoparticles against deltamethrin resistance Rhipicephalus (Boophilus) microplus. Veterinary Parasitology, vol. 237, pp. 130-136. http://dx.doi.org/10.1016/j.vetpar.2017.02.017. PMid:28246003.
http://dx.doi.org/10.1016/j.vetpar.2017....
). As a metal, SNP may bind to several enzymes and impair vital biological systems (Benelli, 2018BENELLI, G., 2018. Mode of action of nanoparticles against insects. Environmental Science and Pollution Research International, vol. 25, no. 13, pp. 12329-12341. http://dx.doi.org/10.1007/s11356-018-1850-4. PMid:29611126.
http://dx.doi.org/10.1007/s11356-018-185...
). It was reported that SNP can affect superoxide dismutase (Nair et al., 2013NAIR, P.M.G., PARK, S.Y. and CHOI, J., 2013. Evaluation of the effect of silver nanoparticles and silver ions using stress responsive gene expression in Chironomus riparius. Chemosphere, vol. 92, no. 5, pp. 592-599. http://dx.doi.org/10.1016/j.chemosphere.2013.03.060. PMid:23664472.
http://dx.doi.org/10.1016/j.chemosphere....
), glutathione s transferase (Nair and Choi, 2011NAIR, P.M.G. and CHOI, J., 2011. Identification, characterization and expression profiles of Chironomus riparius glutathione S-transferase (GST) genes in response to cadmium and silver nanoparticles exposure. Aquatic Toxicology, vol. 101, no. 3-4, pp. 550-560. http://dx.doi.org/10.1016/j.aquatox.2010.12.006. PMid:21276481.
http://dx.doi.org/10.1016/j.aquatox.2010...
), acetylcholine esterase, phosphatase, carboxylesterase (Fouad et al., 2018FOUAD, H., HONGJIE, L., HOSNI, D., WEI, J., ABBAS, G., GA’AL, H. and JIANCHU, M., 2018. Controlling Aedes albopictus and Culex pipiens pallens using silver nanoparticles synthesized from aqueous extract of Cassia fistula fruit pulp and its mode of action. Artificial Cells, Nanomedicine, and Biotechnology, vol. 46, no. 3, pp. 558-567. http://dx.doi.org/10.1080/21691401.2017.1329739. PMid:28541740.
http://dx.doi.org/10.1080/21691401.2017....
) as well as genes’ expression (Nair et al., 2011NAIR, P.M.G., PARK, S.Y., LEE, S.W. and CHOI, J., 2011. Differential expression of ribosomal protein gene, gonadotrophin releasing hormone gene and Balbiani ring protein gene in silver nanoparticles exposed Chironomus riparius. Aquatic Toxicology, vol. 101, no. 1, pp. 31-37. http://dx.doi.org/10.1016/j.aquatox.2010.08.013. PMid:20870301.
http://dx.doi.org/10.1016/j.aquatox.2010...
; Nair and Choi, 2012NAIR, P.M.G. and CHOI, J., 2012. Modulation in the mRNA expression of ecdysone receptor gene in aquatic midge, Chironomus riparius upon exposure to nonylphenol and silver nanoparticles. Environmental Toxicology and Pharmacology, vol. 33, no. 1, pp. 98-106. http://dx.doi.org/10.1016/j.etap.2011.09.006. PMid:22196049.
http://dx.doi.org/10.1016/j.etap.2011.09...
). This multi-target biosite mechanism of action may qualify SNP to be implemented in agricultural pest control programs. SNP is already registered in USA as an algicide and bactericide in water (EPA, 1992ENVIRONMENTAL PROTECTION AGENCY – EPA, 1992 [viewed 20 November 2021]. Registration eligibility decision for silver [online]. Available from: https://www3.epa.gov/pesticides/chem_search/reg_actions/reregistration/red_G-75_5-Sep-07.pdf
https://www3.epa.gov/pesticides/chem_sea...
). SNP are produced on an industrial level to supply the increasing world demand that has exceeded 70 tonnes in the year 2017 and it is expected to be 160 tonnes in the year 2020 for the industries of electronics, electrical, healthcare products, food processing and storing gadgets, textile and others (Syafiuddin et al., 2017SYAFIUDDIN, A., SALMIATI., SALIM, M.R., BENG HONG KUEH, A., HADIBARATA, T. and NUR, H., 2017. A review of silver nanoparticles: research trends, global consumption, synthesis, properties, and future challenges. Journal of the Chinese Chemical Society (Taipei), vol. 64, no. 7, pp. 732-756. http://dx.doi.org/10.1002/jccs.201700067.
http://dx.doi.org/10.1002/jccs.201700067...
). All these amounts are released at some point into the environment as it is in zero-valent form or as silver ions, oxidized to Ag2O or sulfidized Ag2S (Kaegi et al., 2013KAEGI, R., VOEGELIN, A., ORT, C., SINNET, B., THALMANN, B., KRISMER, J., HAGENDORFER, H., ELUMELU, M. and MUELLER, E., 2013. Fate and transformation of silver nanoparticles in urban wastewater systems. Water Research, vol. 47, no. 12, pp. 3866-3877. http://dx.doi.org/10.1016/j.watres.2012.11.060. PMid:23571111.
http://dx.doi.org/10.1016/j.watres.2012....
). Information about factual released amounts, existing concentrations, rate of transformations and environmental impacts are lacking (Syafiuddin et al., 2017SYAFIUDDIN, A., SALMIATI., SALIM, M.R., BENG HONG KUEH, A., HADIBARATA, T. and NUR, H., 2017. A review of silver nanoparticles: research trends, global consumption, synthesis, properties, and future challenges. Journal of the Chinese Chemical Society (Taipei), vol. 64, no. 7, pp. 732-756. http://dx.doi.org/10.1002/jccs.201700067.
http://dx.doi.org/10.1002/jccs.201700067...
). SNP may affect marine organisms and humans (Skebo et al., 2007SKEBO, J.E., GRABINSKI, C.M., SCHRAND, A.M., SCHLAGER, J.J. and HUSSAIN, S.M., 2007. Assessment of metal nanoparticle agglomeration, uptake, and interaction using high-illuminating system. International Journal of Toxicology, vol. 26, no. 2, pp. 135-141. http://dx.doi.org/10.1080/10915810701226248. PMid:17454253.
http://dx.doi.org/10.1080/10915810701226...
). Provision of information concerning bioactivity, efficiency, selectivity, residues and environmental behavior and toxicological data, is required for development of SNP to be used for agricultural purposes.
Quite few investigations were conducted to test the activity of SNP towards phytophagous mites (Jalalizand et al., 2013JALALIZAND, A., GAVANJI, S., ESFAHANI, J.K., BESHARATNEJAD, M.H., EMAMI, M.S. and LARKI, B., 2013 [viewed 15 October 2021]. The effect of Silver nanoparticles on Tetranychus urticae. International Journal of Agriculture and Crop Sciences [online], vol. 5, pp. 820-827. Available from: http://www.ijagcs.com
http://www.ijagcs.com...
; Pavela et al., 2017PAVELA, R., MURUGAN, K., CANALE, A. and BENELLI, G., 2017. Saponaria officinalis-synthesized silver nanocrystals as effective biopesticides and oviposition inhibitors against Tetranychus urticae Koch. Industrial Crops and Products, vol. 97, pp. 338-344. http://dx.doi.org/10.1016/j.indcrop.2016.12.046.
http://dx.doi.org/10.1016/j.indcrop.2016...
; Al-Azzazy et al., 2019AL-AZZAZY, M.M., ABDEL GHANI, S.B. and ALHEWAIRINI, S.S., 2019 [viewed 20 November 2021]. Field evaluation of the efficacy of Silver nanoparticles (AgNP) against mites associated with tomato plants in greenhouses. Pakistan Journal of Agricultural Sciences [online], vol. 56, no. 1, pp. 283-288. Available from: https://pakjas.com.pk/papers/2953.pdf
https://pakjas.com.pk/papers/2953.pdf...
). The current study was dedicated to assessing the activity of SNP against O. afrasiaticus mite, one of the key pests of date palm, and give special focus to one of its natural enemies, the predatory mite N. barkeri in laboratory and in field. In addition, shed light on the concerns of residues of the SNP in the produce and presumed risks to the consumer.
2. Materials and Methods
2.1. Synthesis and characterization of silver nanoparticles
Silver nano particles (SNP) were synthesized using the method described by Lee and Meisel (1982)LEE, P.C. and MEISEL, D., 1982. Adsorption and surface-enhanced Raman of dyes on silver and gold sols. Journal of Physical Chemistry, vol. 86, no. 17, pp. 3391-3395. http://dx.doi.org/10.1021/j100214a025.
http://dx.doi.org/10.1021/j100214a025...
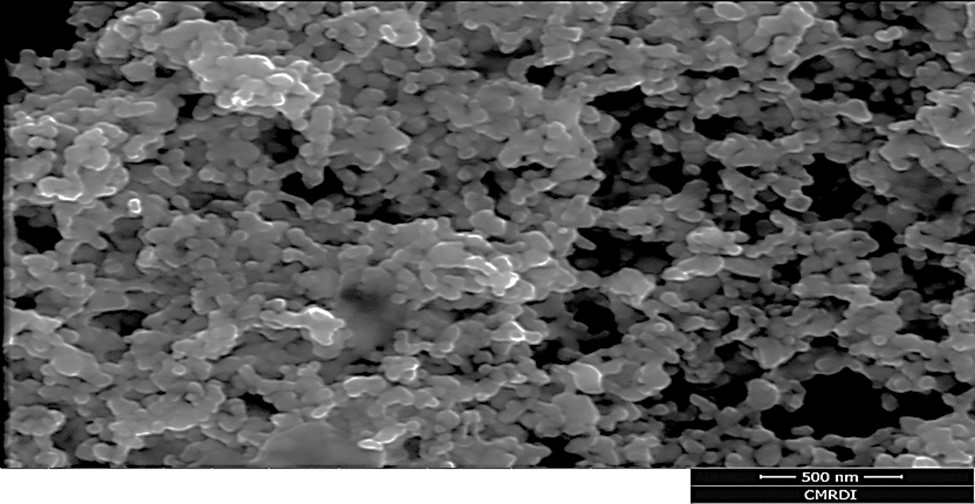
. Spectroscopic characterization of the synthesized SNP was performed to confirm the achievement of the required particles in terms of size and shape. A particle size of 40 nm (average) was obtained, as shown from the laser particle size analyzer (S3500 Microtrac Bluewave, USA). EDX analysis confirmed the existence of the silver metal. Scanning electron microscopy (JEOL SEM, USA) was also run to show, basically, the particle shape and its dimension as well. Particles were mostly spherical (Figure 1).
2.2. Evaluation of SNP activity on infested dates in laboratory
Bunches of infested date fruits were brought from the Experimental Farm of Qassim University, Qassim, Saudi Arabia. Dates were naturally infested with the phytophagous mite O. afrasiaticus. In addition, the predacious mite N. barkeri was also found. Both mites were identified according to Meyer (1987)MEYER, M.K.P. S., 1987. African Tetranychidae (Acari: Prostigmata) with reference to the world genera. Pretoria: Department of Agriculture and Water Supply, 175 p. Entomology Memoir, no. 69.; Chant and McMurtry (2003CHANT, D.A. and MCMURTRY, J.A., 2003. A review of the subfamily Amblyseiinae Muma (Acari: Phytoseiidae). Part I. Neoseiulini new tribe. International Journal of Acarology, vol. 29, no. 1, pp. 3-46. http://dx.doi.org/10.1080/01647950308684319.
http://dx.doi.org/10.1080/01647950308684...
, 2005CHANT, D.A. and MCMURTRY, J.A., 2005. A review of the subfamily Amblyseiinae Muma (Acari: Phytoseiidae): Part V. Tribe Amblyseiini, Subtribe Proprioseiopsina. International Journal of Acarology, vol. 31, no. 1, pp. 3-22. http://dx.doi.org/10.1080/01647950508684412.
http://dx.doi.org/10.1080/01647950508684...
, 2007CHANT, D.A. and MCMURTRY, J.A., 2007. Illustrated keys and diagnoses for the genera and subgenera of the Phytoseiidae of the World (Acari: Mesostigmata). Michigan: Indira Publishing House.). Dates were separated from the strands and placed in plastic plates. Pre-treatment counts of moving individuals of both mites and laid eggs (for only O. afrasiaticus) were recorded before treatment. Serial concentrations of SNP i.e. 13.5, 27, 54, 108 and 216 µg/mL were freshly prepared from the stock solution and sprayed using a one-liter volume hand atomizer. Untreated control was sprayed with tap water. Ten replicates were employed for each concentration of SNP and untreated control. Numbers of moving stages were recorded after three days of treatment. Hatched larvae were monitored for 7 days or until untreated control was fully hatched and newly hatched larvae were removed daily. Corrected mortality percent in each treatment was computed using Equation 1 (Henderson and Tilton, 1955HENDERSON, C.F. and TILTON, E.W., 1955. Test with acaricides against the brown wheat mite. Journal of Economic Entomology, vol. 48, no. 2, pp. 157-161. http://dx.doi.org/10.1093/jee/48.2.157.
http://dx.doi.org/10.1093/jee/48.2.157...
).
where: Cb is the number of mites or laid eggs in untreated control before spray, Ca is the number of mites or hatched larvae in untreated control after spray, Tb is the number of mites or laid eggs in treatment before spray and Ta is the number of mites or hatched larvae in treatment after spray.
2.3. Evaluation of contact activity of SNP in petri dishes
In order to assess the contact activity of SNP on both studied mites. Moving individuals of the phytophagous mite O. afrasiaticus and the predacious mite N. barkeri (100 individuals) were placed in petri dishes separately in triplicates. Mites were sprayed with SNP solutions viz 13.5, 27, 54, 108 and 216 µg/mL using a one-liter hand atomizer. Mortality was checked after 24 h. Corrected mortality was calculated using Abbot Formula Equation 2 (Abbott, 1925ABBOTT, W.S., 1925. A method of computing the effectiveness of an insecticide. Journal of Economic Entomology, vol. 18, no. 2, pp. 265-267. http://dx.doi.org/10.1093/jee/18.2.265a.
http://dx.doi.org/10.1093/jee/18.2.265a...
).
nT is the number of mites in treatment and nC is the number of mites in untreated control.
2.4. Evaluation of the miticidal activity of SNP in the field
The experiment was conducted using short heighted medium-laden date palm trees of Barhi variety in the Experimental Farm of Qassim University, from August to September 2020. The selected trees were not sprayed with any pesticide during the season and were already infested with the phytophagous mite O. afrasiaticus. The predacious mite N. barkeri was also identified on the selected palm trees. Palm trees were sprayed once with SNP solution (216 µg/mL). Spraying solution were applied using a ten-liter fixed pressure hand sprayer (obtained from the local market). Spray solution was directed mainly to date bunches until run-off. Untreated control trees were sprayed with the available irrigation well water. Each SNP or control treatments were conducted in triplicate using randomly selected palm trees. Date fruits, representing all bunches, were picked randomly from each tree before treatment to determine the initial population of both mites. Post treatment population was counted after three days of application. Date samples were transferred to the laboratory for counting of moving individuals of both mites. Hatching of laid eggs of O. afrasiaticus was also recorded. Corrected mortality percent in each treatment was calculated using Equation 1.
2.5. Determination of silver residues in dates and risk assessment
In order to determine residues of silver in date fruits, bulk representative samples of about 1 Kg of date fruits from the treated date palm trees were collected at zero time (after 3 h), 7, 15 days of application. Untreated date samples were also collected once. Samples were transferred to an (ISO 17025) accredited laboratory for analysis. Samples were digested and then subjected to determination using Varian 720ES ICP-OES Spectrometer.
To assess the risk caused by silver residues in date fruits, Hazard index (Hi) was calculated using Equations 3 and 4 (FAO, 2016FOOD AND AGRICULTURE ORGANIZATION – FAO, 2016 [viewed 21 November 2021]. Submission and evaluation of pesticide residues data for the estimation of maximum residue levels in food and feed [online]. FAO Plant Production and Protection Paper, no. 225. Available from: https://www.fao.org/3/i5452e/I5452E.pdf
https://www.fao.org/3/i5452e/I5452E.pdf...
).
where EDI is Estimated Daily Intake (µg/Kg body weight/day) and RfD is the Reference Dose (µg/Kg body weight/day) of silver.
where AgRC is Silver Residues Concentration (µg/Kg) in dates generated from field trial and FC is Food Consumption (g/day).
2.6. Statistical analysis
Statistical tests were performed using IBM SPSS Statistics version 23. All data were initially investigated through the Shapiro-Wilk’s test for normality (Shapiro and Wilk, 1965SHAPIRO, S.S. and WILK, M.B., 1965. An analysis of variance test for normality (complete samples). Biometrika, vol. 52, no. 3-4, pp. 591-611. http://dx.doi.org/10.1093/biomet/52.3-4.591.
http://dx.doi.org/10.1093/biomet/52.3-4....
). One way ANOVA was performed for statistical analysis within the same treatment using transformed corrected mortality percentage data. Differences in the means were considered statistically significant when P ≤ 0.05. Probit regression analysis was performed, according to Finney (1971)FINNEY, D.J., 1971. Probit analysis. 3rd ed. London: Cambridge University Press, 272 p., using average mortality percent versus the normal logarithm of applied concentration.
3. Results and Discussion
3.1. Evaluation of efficiency of SNP towards moving individuals on date fruits in laboratory
Probit analysis of the acquired mortality versus used concentrations was performed for SNP against both tested phytophagous and predatory mites. Toxicity parameters were calculated and reported in Table 1. All obtained values of Chi-square significance were higher than 0.47 and values of R2 were higher than 0.95 proving goodness of the obtained regression lines.
Acaricidal activity of SNP towards moving individuals of O. afrasiaticus and N. barkeri and ovicidal activity against O. afrasiaticus on date fruits in laboratory.
SNP showed very low LC50 (39.7 µg/mL) and LC90 (196.4 µg/mL) for O. afrasiaticus, while the LC50 and LC90 values for N. barkeri were 1587.9 and 36578.5 µg/mL, respectively. Confidence Limits at 95% (CL95) for both LC50 and LC90 of O. afrasiaticus were not overlapping with the corresponding ones for N. barkeri, implying a significant difference between the effect of SNP towards O. afrasiaticus and N. barkeri. The intercept of O. afrasiaticus and N. barkeri are rather similar. SNP showed a selectivity index of about 40. Toxicity lines of SNP for phytophagous and predacious mites did not overlap (significantly different) (Table 1). In addition, the ANOVA test showed a significant difference between O. afrasiaticus and N. barkeri (P < 0.001), indicating lower toxicity of SNP towards non-target predacious mites. SNP may work as digestive and /or contact poison. As well, body openings or interskeletal membranes might be an easy route to enter inside a mite’s body. This can explain the lower activity towards predatory mites that possess thicker body wall with shields on dorsal and ventral sides (Zannou et al., 2007ZANNOU, I.D., MORAES, G.J., UECKERMANN, E.A., OLIVEIRA, A.R., YANINEK, J.S. and HANNA, R., 2007. Phytoseiid mites of the subtribe Amblyseiina (Acari: Phytoseiidae: Amblyseiini) from sub-Saharan Africa. Zootaxa, vol. 1550, no. 1, pp. 1-47. http://dx.doi.org/10.11646/zootaxa.1550.1.1.
http://dx.doi.org/10.11646/zootaxa.1550....
) preventing the penetration of the nanoparticles. In addition, predatory mites feed only on alive phytophagous mites and it is unlikely to feed on intoxicated individuals. Thus decreasing the dose of SNP received by predatory mites. This is not the case for phytophagous mites that have thin body wall and feed on plant sap. Tripathi and coworkers reported that SNP translocated through vascular tissues in plant in Ag0/Ag+ form (Tripathi et al., 2017TRIPATHI, D.K., TRIPATHI, A., SHWETA., SINGH, S., SINGH, Y., VISHWAKARMA, K., YADAV, G., SHARMA, S., SINGH, V.K., MISHRA, R.K., UPADHYAY, R.G., DUBEY, N.K., LEE, Y. and CHAUHAN, D.K., 2017. Uptake, accumulation and toxicity of silver nanoparticle in autotrophic plants, and heterotrophic microbes: a concentric review. Frontiers in Microbiology, vol. 8, pp. 7. http://dx.doi.org/10.3389/fmicb.2017.00007. PMid:28184215.
http://dx.doi.org/10.3389/fmicb.2017.000...
), which might increase the exposure of plant sap-feeding mites to SNP by increasing the received dose.
Quite few studies have tested the miticidal activity of SNP towards phytophagous mites. Silver ion impregnated chitosan textile increased mortality of different dust mites by about 65% more than the only chitosan textile without Ag+ (Rahel et al., 2013RAHEL, J., JONASOVA, E., NESVORNA, M., KLUBAL, R., ERBAN, T. and HUBERT, J., 2013. The toxic effect of chitosan/metal-impregnated textile to synanthropic mites. Pest Management Science, vol. 69, no. 6, pp. 722-726. http://dx.doi.org/10.1002/ps.3428. PMid:23135827.
http://dx.doi.org/10.1002/ps.3428...
). Moving stages of Tetranychus urticae were moderately affected by commercial SNP of 18-34 nm diameter size (Jalalizand et al. 2013JALALIZAND, A., GAVANJI, S., ESFAHANI, J.K., BESHARATNEJAD, M.H., EMAMI, M.S. and LARKI, B., 2013 [viewed 15 October 2021]. The effect of Silver nanoparticles on Tetranychus urticae. International Journal of Agriculture and Crop Sciences [online], vol. 5, pp. 820-827. Available from: http://www.ijagcs.com
http://www.ijagcs.com...
). Plant extract assisted synthesis of SNP was achieved and the obtained silver particles showed miticidal activity towards eggs, nymphs and adults of T. urticae mite (Pavela et al., 2017PAVELA, R., MURUGAN, K., CANALE, A. and BENELLI, G., 2017. Saponaria officinalis-synthesized silver nanocrystals as effective biopesticides and oviposition inhibitors against Tetranychus urticae Koch. Industrial Crops and Products, vol. 97, pp. 338-344. http://dx.doi.org/10.1016/j.indcrop.2016.12.046.
http://dx.doi.org/10.1016/j.indcrop.2016...
). Synthesized SNP showed miticidal activity towards tomato russet mite Aculops lycopersici and two-spotted mite T. urticae (Al-Azzazy et al., 2019AL-AZZAZY, M.M., ABDEL GHANI, S.B. and ALHEWAIRINI, S.S., 2019 [viewed 20 November 2021]. Field evaluation of the efficacy of Silver nanoparticles (AgNP) against mites associated with tomato plants in greenhouses. Pakistan Journal of Agricultural Sciences [online], vol. 56, no. 1, pp. 283-288. Available from: https://pakjas.com.pk/papers/2953.pdf
https://pakjas.com.pk/papers/2953.pdf...
).
Acaricidal activity of SNP in ticks was previously tested against the cattle tick Rhipicephalus (Boophilus) microplus. Marimuthu and coworkers reported LC50 of 8.98 µg/mL (Marimuthu et al., 2011MARIMUTHU, S., RAHUMAN, A.A., RAJAKUMAR, G., SANTHOSHKUMAR, T., KIRTHI, A.V., JAYASEELAN, C., BAGAVAN, A., ZAHIR, A.A., ELANGO, G. and KAMARAJ, C., 2011. Evaluation of green synthesized silver nanoparticles against parasites. Parasitology Research, vol. 108, no. 6, pp. 1541-1549. http://dx.doi.org/10.1007/s00436-010-2212-4. PMid:21181192.
http://dx.doi.org/10.1007/s00436-010-221...
) and Rajakumar and Rahuman reported LC50 of 3.44 µg/mL of Manilkara zapota leaf extract-synthesized SNP (Rajakumar and Rahuman, 2012RAJAKUMAR, G. and RAHUMAN, A.A., 2012. Acaricidal activity of aqueous extract and synthesized silver nanoparticles from Manilkara zapota against Rhipicephalus (Boophilus) microplus. Research in Veterinary Science, vol. 93, no. 1, pp. 303-309. http://dx.doi.org/10.1016/j.rvsc.2011.08.001. PMid:21906765.
http://dx.doi.org/10.1016/j.rvsc.2011.08...
). Santhoshkumar and colleagues achieved LC50 and LC90 values of 7.61 and 22.68 µg/mL, respectively (Santhoshkumar et al., 2012SANTHOSHKUMAR, T., RAHUMAN, A.A., BAGAVAN, A., MARIMUTHU, S., JAYASEELAN, C., KIRTHI, A.V., KAMARAJ, C., RAJAKUMAR, G., ZAHIR, A.A., ELANGO, G., VELAYUTHAM, K., IYAPPAN, M., SIVA, C., KARTHIK, L. and RAO, K.V.B., 2012. Evaluation of stem aqueous extract and synthesized silver nanoparticles using Cissus quadrangularis against Hippobosca maculate and Rhipicephalus (Boophilus) microplus. Experimental Parasitology, vol. 132, no. 2, pp. 156-165. http://dx.doi.org/10.1016/j.exppara.2012.06.009. PMid:22750410.
http://dx.doi.org/10.1016/j.exppara.2012...
). Avinash and coworkers synthesized SNP using a chemical method and natural plant extract of neem. Values of LC50 ranged from 14.4 to 22.6 µg/mL in chemically synthesized SNP and 35. 4 µg/mL in neem extract synthesized-SNP (Avinash et al., 2017AVINASH, B., VENU, R., ALPHA, R.M., SRINIVASA, R.K., SRILATHA, C.H. and PRASAD, T.N.V.K.V., 2017. In vitro evaluation of acaricidal activity of novel green silver. nanoparticles against deltamethrin resistance Rhipicephalus (Boophilus) microplus. Veterinary Parasitology, vol. 237, pp. 130-136. http://dx.doi.org/10.1016/j.vetpar.2017.02.017. PMid:28246003.
http://dx.doi.org/10.1016/j.vetpar.2017....
).
Several studies investigated the effect of SNP on several insects (Benelli, 2018BENELLI, G., 2018. Mode of action of nanoparticles against insects. Environmental Science and Pollution Research International, vol. 25, no. 13, pp. 12329-12341. http://dx.doi.org/10.1007/s11356-018-1850-4. PMid:29611126.
http://dx.doi.org/10.1007/s11356-018-185...
). On a genetic level, SNP produced up and/or down-regulation of genes responsible for ribosomal protein gene (Nair et al., 2011NAIR, P.M.G., PARK, S.Y., LEE, S.W. and CHOI, J., 2011. Differential expression of ribosomal protein gene, gonadotrophin releasing hormone gene and Balbiani ring protein gene in silver nanoparticles exposed Chironomus riparius. Aquatic Toxicology, vol. 101, no. 1, pp. 31-37. http://dx.doi.org/10.1016/j.aquatox.2010.08.013. PMid:20870301.
http://dx.doi.org/10.1016/j.aquatox.2010...
), glutathione S-transferase (GST) (Nair and Choi, 2011NAIR, P.M.G. and CHOI, J., 2011. Identification, characterization and expression profiles of Chironomus riparius glutathione S-transferase (GST) genes in response to cadmium and silver nanoparticles exposure. Aquatic Toxicology, vol. 101, no. 3-4, pp. 550-560. http://dx.doi.org/10.1016/j.aquatox.2010.12.006. PMid:21276481.
http://dx.doi.org/10.1016/j.aquatox.2010...
), ecdysone receptor gene (Nair and Choi, 2012NAIR, P.M.G. and CHOI, J., 2012. Modulation in the mRNA expression of ecdysone receptor gene in aquatic midge, Chironomus riparius upon exposure to nonylphenol and silver nanoparticles. Environmental Toxicology and Pharmacology, vol. 33, no. 1, pp. 98-106. http://dx.doi.org/10.1016/j.etap.2011.09.006. PMid:22196049.
http://dx.doi.org/10.1016/j.etap.2011.09...
) and Mn superoxide dismutase (Nair et al., 2013NAIR, P.M.G., PARK, S.Y. and CHOI, J., 2013. Evaluation of the effect of silver nanoparticles and silver ions using stress responsive gene expression in Chironomus riparius. Chemosphere, vol. 92, no. 5, pp. 592-599. http://dx.doi.org/10.1016/j.chemosphere.2013.03.060. PMid:23664472.
http://dx.doi.org/10.1016/j.chemosphere....
) in Chironomus riparius midge. In relation to oxidative stress, SNP caused accumulation of reactive oxygen species (ROS) and related apoptosis in Drosophila melanogaster (Mao et al., 2018MAO, B.H., CHEN, Z.Y., WANG, Y.J. and YAN, S.J., 2018. Silver nanoparticles have lethal and sublethal adverse effects on development and longevity by inducing ROS-mediated stress responses. Scientific Reports, vol. 8, no. 1, pp. 2445. http://dx.doi.org/10.1038/s41598-018-20728-z. PMid:29402973.
http://dx.doi.org/10.1038/s41598-018-207...
). Regarding the activity of essential enzymes, SNP decreased the activity of esterase enzymes (carboxylesterase and acetylcholinesterase), phosphatase and a noticeable decrease in total protein levels, in general, in mosquitos (Fouad et al., 2018FOUAD, H., HONGJIE, L., HOSNI, D., WEI, J., ABBAS, G., GA’AL, H. and JIANCHU, M., 2018. Controlling Aedes albopictus and Culex pipiens pallens using silver nanoparticles synthesized from aqueous extract of Cassia fistula fruit pulp and its mode of action. Artificial Cells, Nanomedicine, and Biotechnology, vol. 46, no. 3, pp. 558-567. http://dx.doi.org/10.1080/21691401.2017.1329739. PMid:28541740.
http://dx.doi.org/10.1080/21691401.2017....
; Ga’al et al., 2018GA’AL, H., FOUAD, H., TIAN, J., HU, Y., ABBAS, G. and MO, J., 2018. Synthesis, characterization and efficacy of silver nanoparticles against Aedes albopictus larvae and pupae. Pesticide Biochemistry and Physiology, vol. 144, pp. 49-56. http://dx.doi.org/10.1016/j.pestbp.2017.11.004. PMid:29463408.
http://dx.doi.org/10.1016/j.pestbp.2017....
).
Mechanisms of toxicity of SNP were not explored in either phytophagous mites or animal parasitic ticks (Benelli et al., 2017BENELLI, G., MAGGI, F., ROMANO, D., STEFANINI, C., VASEEHARAN, B., KUMAR, S., HIGUCHI, A., ALARFAJ, A.A., MEHLHORN, H. and CANALE, A., 2017. Nanoparticles as effective acaricides against ticks: a review. Ticks and Tick-Borne Diseases, vol. 8, no. 6, pp. 821-826. http://dx.doi.org/10.1016/j.ttbdis.2017.08.004. PMid:28865955.
http://dx.doi.org/10.1016/j.ttbdis.2017....
). Such studies are instrumental in comprehending how SNP, as a control agent, work and unveiling real active site(s).
3.2. Evaluation of the ovicidal activity of SNP in laboratory
The ovicidal activity of SNP was investigated and dose-response regression lines were composed and data were summarized in Table 1. Chi-square significance and R-square values indicated well-fitness of the regression lines. SNP showed ovicidal activity towards O. afrasiaticus egg hatching with LC50 of 67.8 µg/mL. Ovicidal activities of SNP were reported by chemical as well as biological-based synthesized SNP. Egg hatching was fully suppressed in Aedes aegypti, Anopheles stephensi and Culex quinquefasciatus mosquitos using 30 µg/mL of marine plant synthesized SNP (Madhiyazhagan et al., 2015MADHIYAZHAGAN, P., MURUGAN, K., KUMAR, A.N., NATARAJ, T., DINESH, D., PANNEERSELVAM, C., SUBRAMANIAM, J., KUMAR, P.M., SURESH, U., RONI, M., NICOLETTI, M., ALARFAJ, A.A., HIGUCHI, A., MUNUSAMY, M.A. and BENELLI, G., 2015. Sargassum muticum-synthesized silver nanoparticles: an effective control tool against mosquito vectors and bacterial pathogens. Parasitology Research, vol. 114, no. 11, pp. 4305-4317. http://dx.doi.org/10.1007/s00436-015-4671-0. PMid:26281786.
http://dx.doi.org/10.1007/s00436-015-467...
). Ovicidal activity of plant extract synthesized SNP towards eggs of T. urticae was demonstrated (Pavela et al., 2017PAVELA, R., MURUGAN, K., CANALE, A. and BENELLI, G., 2017. Saponaria officinalis-synthesized silver nanocrystals as effective biopesticides and oviposition inhibitors against Tetranychus urticae Koch. Industrial Crops and Products, vol. 97, pp. 338-344. http://dx.doi.org/10.1016/j.indcrop.2016.12.046.
http://dx.doi.org/10.1016/j.indcrop.2016...
). SNP (216 µg/mL) caused 70% reduction of hatching of laid eggs in T. urtica (Al-Azzazy et al., 2019AL-AZZAZY, M.M., ABDEL GHANI, S.B. and ALHEWAIRINI, S.S., 2019 [viewed 20 November 2021]. Field evaluation of the efficacy of Silver nanoparticles (AgNP) against mites associated with tomato plants in greenhouses. Pakistan Journal of Agricultural Sciences [online], vol. 56, no. 1, pp. 283-288. Available from: https://pakjas.com.pk/papers/2953.pdf
https://pakjas.com.pk/papers/2953.pdf...
). SNP affected moving individuals more significantly than egg hatching as no overlap is noted between the obtained probit lines of O. afrasiaticus for moving individuals and egg hatching (Table 1). Non-feeding and stationary behaviors of eggs may decrease the received dose of SNP. Upon contact, SNP can penetrate inside the developing ova. As mentioned above, SNP can affect several active sites in living organisms or cells.
3.3. Evaluation of contact activity of SNP in petri dishes in laboratory
In order to assess only the contact activity of SNP, moving individuals of both O. afrasiaticus and N. barkeri mites were placed in petri dishes separately without diet and were exposed to a spray of SNP at the same concentrations used above. Toxicity regression lines were plotted. Chi-square and R2 values showed the quality of regression assumption fitness (Table 2). LC50 of SNP for O. afrasiaticus (155.9 µg/mL) was significantly different than in case of N. barkeri (848.1 µg/mL) as appeared from the non-overlapping confidence limits and ANOVA (P = 0.001). In case of LC90s, a slight overlap in CL95 was noted; however, LC90 values were estimates and not practically tested. A significant difference is noted between toxicity regression lines of SNP for O. afrasiaticus in this petri dishes experiment (Table 2) and the above-mentioned (Table 1) one as both LC50 and LC90 confidence limits values were not overlapped. This might suggest that ingestion of SNP through the digestive system has a significant effect on toxicity of SNP towards phytophagous mites. However, there was an overlap of CL95 values, of both LC50 and LC90 values, in the case of N. barkeri implying that contact with SNP droplets might be the main rout of SNP’s entry in predatory mites, and supporting the opinion that predatory mites feed only on non-intoxicated and alive phytophagous mite individuals.
Acaricidal activity of SNP towards moving individuals of O. afrasiaticus and N. barkeri in petri dish in laboratory.
3.4. Evaluation of efficacy of SNP in the field
The efficacy of the highest concentration solution (216 µg/mL) used in the laboratory experiment was evaluated in the field. The obtained results are illustrated in Figure 2. In the case of O. afrasiaticus, SNP achieved a mortality percent of 86.5. For N. barkeri, SNP caused about 18.5% of mortality. Selectivity was about 4.7 times for O. afrasiaticus, over N. barkeri. In addition, SNP showed ovicidal activity of 57.1% against the laid eggs of O. afrasiaticus. These results of the field showed that SNP was more effective towards the target phytophagous mite and safer towards the non-target predacious mite. Ovicidal activity of SNP is an added benefit to the activity of SNP in control of the phytophagous mite O. afrasiaticus, making it a multi-life stage poison for mite pests. SNP could be used in mite control programs to control mite populations in place of the traditional non-selective pesticides or interchangeably with them to suppress the development of mite resistance for the sake of sustainable mite control. Studies on the evaluation of the biological activity of SNP in the field are scarce and more investigation is required.
Percent of population reduction of moving individuals of O. afrasiaticus and N. barkeri and egg hatching of O. afrasiaticus as affected by a single dose of SNP (216 µg/mL).
3.5. Residues of silver generated under field conditions and risk assessment
Silver is allowed to be topically applied to burned or damaged skin or mucous tissues for therapeutic purposes. Silver is also used as a coloring agent for food, beverages and medical food products under the code E174 (European Commission, 2000EUROPEAN COMMISSION. Health and Consumer Protection Directorate-General, 2000 [viewed 5 November 2021]. Opinion on toxicological data on colouring agents for medicinal products: E 174 Silver [online]. p. 13. Available from: https://ec.europa.eu/health/archive/ph_risk/committees/scmp/documents/out30_en.pdf
https://ec.europa.eu/health/archive/ph_r...
). Excessive exposure to silver via ingestion, inhalation and dermal applications may cause a medical condition called argyrosis or argyria (greyish-blue coloration of the exposed organs) (Chan et al., 2014CHAN, K., ZHANG, H.W. and LIN, X., 2014. Treatments used in complementary and alternative medicine. In: D.R. SIDHARTHA, ed. Side effects of drugs annual. San Diego: Elsevier, pp. 717-724. Side Effects of Drugs Annual, no. 36.). American Environmental Protection Agency EPA established the Reference Dose RfD for silver to be 5 µg/Kg/day, which is the estimate of the amount of silver to be consumed in life time course without presenting a risk to human health (EPA, 1992ENVIRONMENTAL PROTECTION AGENCY – EPA, 1992 [viewed 20 November 2021]. Registration eligibility decision for silver [online]. Available from: https://www3.epa.gov/pesticides/chem_search/reg_actions/reregistration/red_G-75_5-Sep-07.pdf
https://www3.epa.gov/pesticides/chem_sea...
), this dose accounts for 300 µg/day for the average person of 60 Kg bodyweight (EPA, 1992ENVIRONMENTAL PROTECTION AGENCY – EPA, 1992 [viewed 20 November 2021]. Registration eligibility decision for silver [online]. Available from: https://www3.epa.gov/pesticides/chem_search/reg_actions/reregistration/red_G-75_5-Sep-07.pdf
https://www3.epa.gov/pesticides/chem_sea...
). In order to assess the anticipated risk that might be caused by remained residues of silver in date fruits, silver’s residues were determined. Silver residues were not detected in the untreated date sample. The initial deposit of silver (zero interval) reached 1830 µg/Kg and decreased with time to 670 µg/Kg at 14 days of application. This decline might be attributed to dilution of residues with growing of the produce and/or systemic behavior of SNP (Tripathi et al., 2017TRIPATHI, D.K., TRIPATHI, A., SHWETA., SINGH, S., SINGH, Y., VISHWAKARMA, K., YADAV, G., SHARMA, S., SINGH, V.K., MISHRA, R.K., UPADHYAY, R.G., DUBEY, N.K., LEE, Y. and CHAUHAN, D.K., 2017. Uptake, accumulation and toxicity of silver nanoparticle in autotrophic plants, and heterotrophic microbes: a concentric review. Frontiers in Microbiology, vol. 8, pp. 7. http://dx.doi.org/10.3389/fmicb.2017.00007. PMid:28184215.
http://dx.doi.org/10.3389/fmicb.2017.000...
) decreasing its concentration via translocation towards leaves and stem. Further studies on the translocation of SNP are required. The Estimated Daily Intake (EDI) of silver via consumption of date fruits was calculated according to FAO Manual on the Submission and Evaluation of Pesticide Residues Data (FAO, 2016FOOD AND AGRICULTURE ORGANIZATION – FAO, 2016 [viewed 21 November 2021]. Submission and evaluation of pesticide residues data for the estimation of maximum residue levels in food and feed [online]. FAO Plant Production and Protection Paper, no. 225. Available from: https://www.fao.org/3/i5452e/I5452E.pdf
https://www.fao.org/3/i5452e/I5452E.pdf...
). The consumption rate of palm date per person per day is 41.8 g in Middle Eastern countries (WHO, 2003WORLD HEALTH ORGANIZATION – WHO, 2003 [viewed 20 October 2021]. Global Environment Monitoring System/Food Contamination Monitoring and Assessment Programme Regional per Capita Consumption of Raw and Semi-processed Agricultural Commodities [online]. Geneva: WHO. Available from: https://apps.who.int/iris/handle/10665/42833
https://apps.who.int/iris/handle/10665/4...
), which happens to be the highest consumption rate worldwide. Average body weight is 60 Kg in the Middle East. The highest found residues of silver (initial deposit) produced an EDI value of 1.28 (µg/Kg body weight/day) which is only 26% of the set RfD of silver intake implying that neither chronic nor acute toxicity is predicted under such exposure. Risk assessment as Hazard index (Hi) was estimated using the set RfD of 5 µg/Kg/day and consumer body weight of 60 Kg. Hi was calculated to be 0.26 at the initial deposit level (1830 µg/Kg), which is the highest expected exposure and the Hi values declined using residues data of 7 and 14 days after application. In average Hi was 0.17, meaning consumers, by consuming such silver-containing dates, are exposed to only 17% of the RfD. However, residues were determined as total silver regardless of its chemical and physical real form in date fruits. Further studies on the risk assessment of silver in nanoparticle form are crucial. Table 3 summarizes residues concentrations of silver in palm dates in successive time intervals and respective EDI and Hi values.
Residues of silver (µg/kg) in palm date, Estimated Daily Intake (EDI), and Hazard Index (Hi).
4. Conclusion
Nanoparticles of silver metal were synthesized using a chemical method. Evaluation of the miticidal activity of the synthesized particles against date palm phytophagous mite O. afrasiaticus and an associated predacious mite N. barkeri was the chief focus of this study. SNP showed miticidal activity against moving stages and laid eggs of the phytophagous mite. SNP showed miticidal activity towards O. afrasiaticus in both laboratory and field trials. SNP showed remarkable selectivity of about five folds towards the pest over the non-target predatory mite at the field application rate. SNP showed ovicidal activity for O. afrasiaticus that may increase its overall control efficacy. Field results confirmed the practical usefulness of SNP as an acaricide. Phytotoxic effects were not observed in both laboratory and field experiments. Risk assessment using hazard index showed that residues of metal silver remained in date fruits pose no risk to the consumer.
Acknowledgements
The authors gratefully acknowledge Qassim University, represented by the Deanship of Scientific Research, for the financial support of this research under the number (7817-cam-2019-1-1-Q) during the academic year 1440 AH /2019 AD.
References
- ABBOTT, W.S., 1925. A method of computing the effectiveness of an insecticide. Journal of Economic Entomology, vol. 18, no. 2, pp. 265-267. http://dx.doi.org/10.1093/jee/18.2.265a
» http://dx.doi.org/10.1093/jee/18.2.265a - ABDEL GHANI, S.B., ALHEWAIRINI, S.S. and HROUZKOVA, S., 2018. A fast and easy QuEChERS-DLLME method combined with GC-MS for ethion and bifenthrin residues determination and study of their dissipation dynamics in palm dates. Food Analytical Methods, vol. 11, no. 12, pp. 3542-3550. http://dx.doi.org/10.1007/s12161-018-1333-8
» http://dx.doi.org/10.1007/s12161-018-1333-8 - ALATAWI, F.J., 2020. Field studies on occurrence, alternate hosts and mortality factors of date palm mite, Oligonychus afrasiaticus (McGregor) (Acari: tetranychidae). Journal of the Saudi Society of Agricultural Sciences, vol. 19, no. 2, pp. 146-150. http://dx.doi.org/10.1016/j.jssas.2018.08.003
» http://dx.doi.org/10.1016/j.jssas.2018.08.003 - AL-AZZAZY, M.M., ABDEL GHANI, S.B. and ALHEWAIRINI, S.S., 2019 [viewed 20 November 2021]. Field evaluation of the efficacy of Silver nanoparticles (AgNP) against mites associated with tomato plants in greenhouses. Pakistan Journal of Agricultural Sciences [online], vol. 56, no. 1, pp. 283-288. Available from: https://pakjas.com.pk/papers/2953.pdf
» https://pakjas.com.pk/papers/2953.pdf - AVINASH, B., VENU, R., ALPHA, R.M., SRINIVASA, R.K., SRILATHA, C.H. and PRASAD, T.N.V.K.V., 2017. In vitro evaluation of acaricidal activity of novel green silver. nanoparticles against deltamethrin resistance Rhipicephalus (Boophilus) microplus. Veterinary Parasitology, vol. 237, pp. 130-136. http://dx.doi.org/10.1016/j.vetpar.2017.02.017 PMid:28246003.
» http://dx.doi.org/10.1016/j.vetpar.2017.02.017 - BENELLI, G., 2018. Mode of action of nanoparticles against insects. Environmental Science and Pollution Research International, vol. 25, no. 13, pp. 12329-12341. http://dx.doi.org/10.1007/s11356-018-1850-4 PMid:29611126.
» http://dx.doi.org/10.1007/s11356-018-1850-4 - BENELLI, G., MAGGI, F., ROMANO, D., STEFANINI, C., VASEEHARAN, B., KUMAR, S., HIGUCHI, A., ALARFAJ, A.A., MEHLHORN, H. and CANALE, A., 2017. Nanoparticles as effective acaricides against ticks: a review. Ticks and Tick-Borne Diseases, vol. 8, no. 6, pp. 821-826. http://dx.doi.org/10.1016/j.ttbdis.2017.08.004 PMid:28865955.
» http://dx.doi.org/10.1016/j.ttbdis.2017.08.004 - BLUMBERG, D., 2008. Review: date palm arthropod pests and their management in Israel. Phytoparasitica, vol. 36, no. 5, pp. 411-448. http://dx.doi.org/10.1007/BF03020290
» http://dx.doi.org/10.1007/BF03020290 - CHAN, K., ZHANG, H.W. and LIN, X., 2014. Treatments used in complementary and alternative medicine. In: D.R. SIDHARTHA, ed. Side effects of drugs annual San Diego: Elsevier, pp. 717-724. Side Effects of Drugs Annual, no. 36.
- CHANT, D.A. and MCMURTRY, J.A., 2003. A review of the subfamily Amblyseiinae Muma (Acari: Phytoseiidae). Part I. Neoseiulini new tribe. International Journal of Acarology, vol. 29, no. 1, pp. 3-46. http://dx.doi.org/10.1080/01647950308684319
» http://dx.doi.org/10.1080/01647950308684319 - CHANT, D.A. and MCMURTRY, J.A., 2005. A review of the subfamily Amblyseiinae Muma (Acari: Phytoseiidae): Part V. Tribe Amblyseiini, Subtribe Proprioseiopsina. International Journal of Acarology, vol. 31, no. 1, pp. 3-22. http://dx.doi.org/10.1080/01647950508684412
» http://dx.doi.org/10.1080/01647950508684412 - CHANT, D.A. and MCMURTRY, J.A., 2007. Illustrated keys and diagnoses for the genera and subgenera of the Phytoseiidae of the World (Acari: Mesostigmata) Michigan: Indira Publishing House.
- EL-SHAFIE, H.A.F., 2012. Review: list of arthropod pests and their natural enemies identified worldwide on date palm, Phoenix dactylifera L. Agriculture and Biology Journal of North America, vol. 3, no. 13, pp. 516-524. http://dx.doi.org/10.5251/abjna.2012.3.12.516.524
» http://dx.doi.org/10.5251/abjna.2012.3.12.516.524 - ENVIRONMENTAL PROTECTION AGENCY – EPA, 1992 [viewed 20 November 2021]. Registration eligibility decision for silver [online]. Available from: https://www3.epa.gov/pesticides/chem_search/reg_actions/reregistration/red_G-75_5-Sep-07.pdf
» https://www3.epa.gov/pesticides/chem_search/reg_actions/reregistration/red_G-75_5-Sep-07.pdf - EUROPEAN COMMISSION. Health and Consumer Protection Directorate-General, 2000 [viewed 5 November 2021]. Opinion on toxicological data on colouring agents for medicinal products: E 174 Silver [online]. p. 13. Available from: https://ec.europa.eu/health/archive/ph_risk/committees/scmp/documents/out30_en.pdf
» https://ec.europa.eu/health/archive/ph_risk/committees/scmp/documents/out30_en.pdf - FINNEY, D.J., 1971. Probit analysis 3rd ed. London: Cambridge University Press, 272 p.
- FOOD AND AGRICULTURE ORGANIZATION – FAO, 2016 [viewed 21 November 2021]. Submission and evaluation of pesticide residues data for the estimation of maximum residue levels in food and feed [online]. FAO Plant Production and Protection Paper, no. 225. Available from: https://www.fao.org/3/i5452e/I5452E.pdf
» https://www.fao.org/3/i5452e/I5452E.pdf - FOOD AND AGRICULTURE ORGANIZATION – FAO. Codex Alimentarius Commission, 2017 [viewed 26 December 2021]. Joint FAO/WHO food standards program Codex Committee on fresh fruits and vegetables. 20th Session Kampala, Uganda, 2-6 October, 2017. Proposed draft standard for fresh dates [online]. Available from: http://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FMeetings%252FCX-731-21%252FWorking%2Bdocuments%252Fffv21_07e.pdf
» http://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FMeetings%252FCX-731-21%252FWorking%2Bdocuments%252Fffv21_07e.pdf - FOOD AND AGRICULTURE ORGANIZATION – FAO, 2019 [viewed 15 October 2021]. FAOSTAT [online]. Available from: http://www.fao.org/faostat/
» http://www.fao.org/faostat/ - FOUAD, H., HONGJIE, L., HOSNI, D., WEI, J., ABBAS, G., GA’AL, H. and JIANCHU, M., 2018. Controlling Aedes albopictus and Culex pipiens pallens using silver nanoparticles synthesized from aqueous extract of Cassia fistula fruit pulp and its mode of action. Artificial Cells, Nanomedicine, and Biotechnology, vol. 46, no. 3, pp. 558-567. http://dx.doi.org/10.1080/21691401.2017.1329739 PMid:28541740.
» http://dx.doi.org/10.1080/21691401.2017.1329739 - GA’AL, H., FOUAD, H., TIAN, J., HU, Y., ABBAS, G. and MO, J., 2018. Synthesis, characterization and efficacy of silver nanoparticles against Aedes albopictus larvae and pupae. Pesticide Biochemistry and Physiology, vol. 144, pp. 49-56. http://dx.doi.org/10.1016/j.pestbp.2017.11.004 PMid:29463408.
» http://dx.doi.org/10.1016/j.pestbp.2017.11.004 - GOVINDARAJAN, M., RAJESWARY, M., VEERAKUMAR, K., MUTHUKUMARAN, U., HOTI, S.L., MEHLHORN, H., BARNARD, D.R. and BENELLI, G., 2016. Novel synthesis of silver nanoparticles using Bauhinia variegata: a recent eco-friendly approach for mosquito control. Parasitology Research, vol. 115, no. 2, pp. 723-733. http://dx.doi.org/10.1007/s00436-015-4794-3 PMid:26490683.
» http://dx.doi.org/10.1007/s00436-015-4794-3 - HENDERSON, C.F. and TILTON, E.W., 1955. Test with acaricides against the brown wheat mite. Journal of Economic Entomology, vol. 48, no. 2, pp. 157-161. http://dx.doi.org/10.1093/jee/48.2.157
» http://dx.doi.org/10.1093/jee/48.2.157 - JALALIZAND, A., GAVANJI, S., ESFAHANI, J.K., BESHARATNEJAD, M.H., EMAMI, M.S. and LARKI, B., 2013 [viewed 15 October 2021]. The effect of Silver nanoparticles on Tetranychus urticae. International Journal of Agriculture and Crop Sciences [online], vol. 5, pp. 820-827. Available from: http://www.ijagcs.com
» http://www.ijagcs.com - JEPPSON, L.R., KEIFER, H.H. and BAKER, E.W., 1975. Mites injurious to economic plants Berkeley: University of California Press, 614 p. http://dx.doi.org/10.1525/9780520335431
» http://dx.doi.org/10.1525/9780520335431 - KAEGI, R., VOEGELIN, A., ORT, C., SINNET, B., THALMANN, B., KRISMER, J., HAGENDORFER, H., ELUMELU, M. and MUELLER, E., 2013. Fate and transformation of silver nanoparticles in urban wastewater systems. Water Research, vol. 47, no. 12, pp. 3866-3877. http://dx.doi.org/10.1016/j.watres.2012.11.060 PMid:23571111.
» http://dx.doi.org/10.1016/j.watres.2012.11.060 - LEE, P.C. and MEISEL, D., 1982. Adsorption and surface-enhanced Raman of dyes on silver and gold sols. Journal of Physical Chemistry, vol. 86, no. 17, pp. 3391-3395. http://dx.doi.org/10.1021/j100214a025
» http://dx.doi.org/10.1021/j100214a025 - MADHIYAZHAGAN, P., MURUGAN, K., KUMAR, A.N., NATARAJ, T., DINESH, D., PANNEERSELVAM, C., SUBRAMANIAM, J., KUMAR, P.M., SURESH, U., RONI, M., NICOLETTI, M., ALARFAJ, A.A., HIGUCHI, A., MUNUSAMY, M.A. and BENELLI, G., 2015. Sargassum muticum-synthesized silver nanoparticles: an effective control tool against mosquito vectors and bacterial pathogens. Parasitology Research, vol. 114, no. 11, pp. 4305-4317. http://dx.doi.org/10.1007/s00436-015-4671-0 PMid:26281786.
» http://dx.doi.org/10.1007/s00436-015-4671-0 - MAO, B.H., CHEN, Z.Y., WANG, Y.J. and YAN, S.J., 2018. Silver nanoparticles have lethal and sublethal adverse effects on development and longevity by inducing ROS-mediated stress responses. Scientific Reports, vol. 8, no. 1, pp. 2445. http://dx.doi.org/10.1038/s41598-018-20728-z PMid:29402973.
» http://dx.doi.org/10.1038/s41598-018-20728-z - MARIMUTHU, S., RAHUMAN, A.A., RAJAKUMAR, G., SANTHOSHKUMAR, T., KIRTHI, A.V., JAYASEELAN, C., BAGAVAN, A., ZAHIR, A.A., ELANGO, G. and KAMARAJ, C., 2011. Evaluation of green synthesized silver nanoparticles against parasites. Parasitology Research, vol. 108, no. 6, pp. 1541-1549. http://dx.doi.org/10.1007/s00436-010-2212-4 PMid:21181192.
» http://dx.doi.org/10.1007/s00436-010-2212-4 - MEYER, M.K.P. S., 1987. African Tetranychidae (Acari: Prostigmata) with reference to the world genera Pretoria: Department of Agriculture and Water Supply, 175 p. Entomology Memoir, no. 69.
- NAIR, P.M.G. and CHOI, J., 2011. Identification, characterization and expression profiles of Chironomus riparius glutathione S-transferase (GST) genes in response to cadmium and silver nanoparticles exposure. Aquatic Toxicology, vol. 101, no. 3-4, pp. 550-560. http://dx.doi.org/10.1016/j.aquatox.2010.12.006 PMid:21276481.
» http://dx.doi.org/10.1016/j.aquatox.2010.12.006 - NAIR, P.M.G. and CHOI, J., 2012. Modulation in the mRNA expression of ecdysone receptor gene in aquatic midge, Chironomus riparius upon exposure to nonylphenol and silver nanoparticles. Environmental Toxicology and Pharmacology, vol. 33, no. 1, pp. 98-106. http://dx.doi.org/10.1016/j.etap.2011.09.006 PMid:22196049.
» http://dx.doi.org/10.1016/j.etap.2011.09.006 - NAIR, P.M.G., PARK, S.Y. and CHOI, J., 2013. Evaluation of the effect of silver nanoparticles and silver ions using stress responsive gene expression in Chironomus riparius. Chemosphere, vol. 92, no. 5, pp. 592-599. http://dx.doi.org/10.1016/j.chemosphere.2013.03.060 PMid:23664472.
» http://dx.doi.org/10.1016/j.chemosphere.2013.03.060 - NAIR, P.M.G., PARK, S.Y., LEE, S.W. and CHOI, J., 2011. Differential expression of ribosomal protein gene, gonadotrophin releasing hormone gene and Balbiani ring protein gene in silver nanoparticles exposed Chironomus riparius. Aquatic Toxicology, vol. 101, no. 1, pp. 31-37. http://dx.doi.org/10.1016/j.aquatox.2010.08.013 PMid:20870301.
» http://dx.doi.org/10.1016/j.aquatox.2010.08.013 - PAVELA, R., MURUGAN, K., CANALE, A. and BENELLI, G., 2017. Saponaria officinalis-synthesized silver nanocrystals as effective biopesticides and oviposition inhibitors against Tetranychus urticae Koch. Industrial Crops and Products, vol. 97, pp. 338-344. http://dx.doi.org/10.1016/j.indcrop.2016.12.046
» http://dx.doi.org/10.1016/j.indcrop.2016.12.046 - RAHEL, J., JONASOVA, E., NESVORNA, M., KLUBAL, R., ERBAN, T. and HUBERT, J., 2013. The toxic effect of chitosan/metal-impregnated textile to synanthropic mites. Pest Management Science, vol. 69, no. 6, pp. 722-726. http://dx.doi.org/10.1002/ps.3428 PMid:23135827.
» http://dx.doi.org/10.1002/ps.3428 - RAJAKUMAR, G. and RAHUMAN, A.A., 2012. Acaricidal activity of aqueous extract and synthesized silver nanoparticles from Manilkara zapota against Rhipicephalus (Boophilus) microplus. Research in Veterinary Science, vol. 93, no. 1, pp. 303-309. http://dx.doi.org/10.1016/j.rvsc.2011.08.001 PMid:21906765.
» http://dx.doi.org/10.1016/j.rvsc.2011.08.001 - SANTHOSHKUMAR, T., RAHUMAN, A.A., BAGAVAN, A., MARIMUTHU, S., JAYASEELAN, C., KIRTHI, A.V., KAMARAJ, C., RAJAKUMAR, G., ZAHIR, A.A., ELANGO, G., VELAYUTHAM, K., IYAPPAN, M., SIVA, C., KARTHIK, L. and RAO, K.V.B., 2012. Evaluation of stem aqueous extract and synthesized silver nanoparticles using Cissus quadrangularis against Hippobosca maculate and Rhipicephalus (Boophilus) microplus. Experimental Parasitology, vol. 132, no. 2, pp. 156-165. http://dx.doi.org/10.1016/j.exppara.2012.06.009 PMid:22750410.
» http://dx.doi.org/10.1016/j.exppara.2012.06.009 - SHAPIRO, S.S. and WILK, M.B., 1965. An analysis of variance test for normality (complete samples). Biometrika, vol. 52, no. 3-4, pp. 591-611. http://dx.doi.org/10.1093/biomet/52.3-4.591
» http://dx.doi.org/10.1093/biomet/52.3-4.591 - SKEBO, J.E., GRABINSKI, C.M., SCHRAND, A.M., SCHLAGER, J.J. and HUSSAIN, S.M., 2007. Assessment of metal nanoparticle agglomeration, uptake, and interaction using high-illuminating system. International Journal of Toxicology, vol. 26, no. 2, pp. 135-141. http://dx.doi.org/10.1080/10915810701226248 PMid:17454253.
» http://dx.doi.org/10.1080/10915810701226248 - SYAFIUDDIN, A., SALMIATI., SALIM, M.R., BENG HONG KUEH, A., HADIBARATA, T. and NUR, H., 2017. A review of silver nanoparticles: research trends, global consumption, synthesis, properties, and future challenges. Journal of the Chinese Chemical Society (Taipei), vol. 64, no. 7, pp. 732-756. http://dx.doi.org/10.1002/jccs.201700067
» http://dx.doi.org/10.1002/jccs.201700067 - TALHOUK, A.S., 1991. On the management of the date palm and its arthropod enemies in the Arabian Peninsula. Journal of Applied Entomology, vol. 111, no. 1-5, pp. 514-520. http://dx.doi.org/10.1111/j.1439-0418.1991.tb00354.x
» http://dx.doi.org/10.1111/j.1439-0418.1991.tb00354.x - TRIPATHI, D.K., TRIPATHI, A., SHWETA., SINGH, S., SINGH, Y., VISHWAKARMA, K., YADAV, G., SHARMA, S., SINGH, V.K., MISHRA, R.K., UPADHYAY, R.G., DUBEY, N.K., LEE, Y. and CHAUHAN, D.K., 2017. Uptake, accumulation and toxicity of silver nanoparticle in autotrophic plants, and heterotrophic microbes: a concentric review. Frontiers in Microbiology, vol. 8, pp. 7. http://dx.doi.org/10.3389/fmicb.2017.00007 PMid:28184215.
» http://dx.doi.org/10.3389/fmicb.2017.00007 - WORLD HEALTH ORGANIZATION – WHO, 2003 [viewed 20 October 2021]. Global Environment Monitoring System/Food Contamination Monitoring and Assessment Programme Regional per Capita Consumption of Raw and Semi-processed Agricultural Commodities [online]. Geneva: WHO. Available from: https://apps.who.int/iris/handle/10665/42833
» https://apps.who.int/iris/handle/10665/42833 - YAHIA, E.M. and KADER, A.A. 2011. Date (Phoenix dactylifera L.). In: E.M. YAHIA, ed. Postharvest biology and technology of tropical and sub-tropical fruits Cambridge: Woodhead Publishing, pp. 41-79. http://dx.doi.org/10.1533/9780857092885.41
» http://dx.doi.org/10.1533/9780857092885.41 - ZANNOU, I.D., MORAES, G.J., UECKERMANN, E.A., OLIVEIRA, A.R., YANINEK, J.S. and HANNA, R., 2007. Phytoseiid mites of the subtribe Amblyseiina (Acari: Phytoseiidae: Amblyseiini) from sub-Saharan Africa. Zootaxa, vol. 1550, no. 1, pp. 1-47. http://dx.doi.org/10.11646/zootaxa.1550.1.1
» http://dx.doi.org/10.11646/zootaxa.1550.1.1
Publication Dates
-
Publication in this collection
10 June 2022 -
Date of issue
2024
History
-
Received
22 Feb 2022 -
Accepted
06 May 2022