Abstract
The increasing global consumption of plastic products has resulted in a growing accumulation of plastic waste, posing severe environmental challenges. The study aims to explore methods for recycling plastic macaque waste to produce carbon nanomaterials. Carbon nanomaterials were obtained via electric arc discharge from plastic waste processed at 1173 K in a nitrogen and water vapor environment. Key properties such as moisture, ash, and volatility were analyzed with a Thermoster Eltra analyzer. Pore volume, bulk density, pH, and adsorption activity were also assessed. This study addresses plastic waste pollution by converting it into porous carbon nanomaterials through pyrolysis at 900 °C. These materials, used as electrodes, produce graphene-forming nanomaterials via electric arc discharge. Analysis confirmed the composition using Raman spectroscopy, X-ray diffraction, and gas chromatography. The study reveals that the electrical conductivity of the synthesized carbon nanomaterials is close to that of graphite, with a reduction in electrical resistance of up to 3.6 times compared to the initial carbonized material. The process yields valuable products like nanomaterials, hydrogen, and flammable gases. This research presents an innovative and sustainable approach for the recycling of plastic waste into graphene-forming carbon nanomaterials using electric arc discharge.
Keywords:
plastic; carbonization; electric arc method; graphite; carbon
Resumo
O crescente consumo global de produtos plásticos resultou em um acúmulo crescente de resíduos plásticos, apresentando sérios desafios ambientais. O estudo visa explorar métodos para reciclar resíduos plásticos de macacos para produzir nanomateriais de carbono. Os nanomateriais de carbono foram obtidos por meio de descarga de arco elétrico de resíduos plásticos processados a 1.173 K em um ambiente de nitrogênio e vapor de água. Propriedades-chave, como umidade, cinzas e volatilidade, foram analisadas com um analisador Thermoster Eltra. Volume de poros, densidade aparente, pH e atividade de adsorção também foram avaliados. Este estudo aborda a poluição de resíduos plásticos convertendo-os em nanomateriais de carbono porosos por meio de pirólise a 900 °C. Esses materiais, usados como eletrodos, produzem nanomateriais formadores de grafeno por meio de descarga de arco elétrico. A análise confirmou a composição usando espectroscopia Raman, difração de raios X e cromatografia gasosa. O estudo revela que a condutividade elétrica dos nanomateriais de carbono sintetizados é próxima à do grafite, com uma redução na resistência elétrica de até 3,6 vezes em comparação ao material carbonizado inicial. O processo produz produtos valiosos, como nanomateriais, hidrogênio e gases inflamáveis. Esta pesquisa apresenta uma abordagem inovadora e sustentável para a reciclagem de resíduos plásticos em nanomateriais de carbono formadores de grafeno usando descarga de arco elétrico.
Palavras-chave:
plástico; carbonização; método de arco elétrico; grafite; carbono
1. Introduction
People periodically discover various materials that go beyond traditional ones (Kaewtrakulchai et al., 2019; Panomsuwan et al., 2022). Plastic is a revolutionary material often referred to as the “material of a thousand uses”. Plastic is ubiquitous in human life due to its versatile properties such as lightweight, high strength, flexibility, and low production cost. Despite all its advantages, plastic poses serious environmental and public health problems. Plastic can degrade into micro- and nanoplastics, and these tiny particles are more prevalent in the air, water, and soil (Kaewtrakulchai et al., 2024; Oyewale et al., 2023). Therefore, both terrestrial and aquatic animals are subjected to various negative impacts, such as ingestion, entanglement, ulcers, low reproduction rates, and oxidative stress. Microplastics also negatively impact human health, causing cardiovascular diseases, chronic kidney disease, birth defects, cancer, etc. Plastic pollution involves the accumulation of plastic products in the environment, which adversely affect wildlife, animal habitats, or humans. Plastic products are inexpensive and durable, making them almost ubiquitous. Plastic packaging is predominantly used in the food industry, with over 50% of all polymer packaging directed towards this sector, 25% of plastic products used in the chemical industry, easily explained by its strength and resistance to chemical reactions, and the remaining 25% distributed evenly among other industries, including paints, construction, and others (Kabeyi and Olanrewaju, 2023). The perspective of plastic waste without recycling involves disposal in landfills, the capacities of which are not limitless. Therefore, plastic recycling is one of the most pressing issues for modern society.
The specificity of plastic waste recycling is related to two main reasons: - diverse and complex chemical composition (polystyrene, polyvinyl chloride, polyphenylene, polyethylene terephthalate, polypropylene, etc.); - prolonged decomposition period (up to 100 years, and in some cases - up to 500 years).
The main objectives of plastic recycling are: reducing waste volumes; their neutralization; plastic recycling into fuel; creating raw materials for secondary use. The main and serious problem with this material is its very slow decomposition. A large part of plastic waste ends up in the World Ocean, and only a small fraction (just 5%) is recycled. Therefore, despite the numerous advantages of plastic products, there is a global environmental problem, namely the issue of proper disposal of polymer materials (Aipova and Dyuzhenkova, 2016).
As a result, a solid carbon material was obtained from the obtained solid carbon material using an electric arc method, the electrical conductivity of the initial material and the obtained nanomaterial were measured, and a conclusion was drawn.
Plastic possesses several valuable properties, such as lightweight, strength, high durability, good rigidity, versatile manufacturing capabilities, design capabilities, good thermal insulation, low electrical/thermal conductivity, and corrosion resistance (Oehlmann et al., 2009; Pan et al., 2020). Consumption and production of plastics have been increasing since its first production in the 1950s. About 4% of the world's oil and gas extraction is used as raw materials for plastics, and 3–4% is used to provide energy for their production. Plastics have a wide range of applications as they are versatile and relatively inexpensive. This study presents an in-depth analysis of solid plastic waste (SPW).
Plastic is widely known as a synthetic polymer developed by polymerizing monomers extracted from petrochemical products and blending them with other chemical substances (Torres-Agullo et al., 2021).
Monomers are repeating units of long polymers consisting of carbon, hydrogen, and oxygen held together by covalent bonds. Ethylene and propylene are common examples of light monomers widely used in plastic production (Hassan et al., 2022).
The specific chemical substances used in plastics vary depending on the type of plastic produced or the production process. Plastic production employs several additives such as fillers, plasticizers, pigments, foaming agents, processing aids, lubricants, thermal stabilizers, acid scavengers, antioxidants, UV stabilizers, flame retardants, and some antistatic agents in varying amounts to manage different functions such as enhancing properties, facilitating processing, improving strength, enhancing performance and appearance (Khoaele et al., 2023).
There are four main avenues available for plastic solid waste treatment, namely, reextrusion as a primary treatment, mechanical treatment as secondary measures, chemical treatment as a tertiary measure, and energy recovery as a quaternary measure. The pyrolysis oil has properties that are close to clean fuel and is, therefore, a substitute to fresh fossil fuel for power generation, transport, and other applications. The study showed that plastic wastes pyrolysis offers an alternative avenue for plastic waste disposal and an alternative source of fossil fuel to reduce the total demand of virgin oil. Through plastic pyrolysis, plastic wastes are thermally converted to fuel by degrading long-chain polymers into small complex molecules in the absence of oxygen, making it a technically and economically feasible process for waste plastic recycling. The process is advantageous because presorting is not required, and the plastic waste can be directly fed without pretreatment prior to the process. Products of plastic pyrolysis are pyrolysis oil, a hydrocarbon-rich gas, with a heating value of 25-45 MJ/kg, which makes it ideal for process energy recovery. Hence, the pyrolysis gas can be fed back to the process to extract the energy for the process-heating purpose, which substantially reduces the reliance on external heating sources (Kabeyi and Olanrewaju, 2023).
Currently, humanity is in danger of a sharp deterioration of its habitat, and the development of production, urbanization of regions, and population growth are leading to the depletion of all natural resources. Due to the threat of deterioration of the human environment, many phenomena cause catastrophe: loss of clean fresh air, death of many animals, plants, changes in air, soil and air, all as a result of human activities (Kassenova et al., 2024). The benefits of heavy industry, including plastic, include waste, more than 3 billion tons of plastic products (Adla et al., 2022; Jekal et al., 2024). Pyrolysis is one of the most effective methods for processing plastics. Using the pyrolysis method, waste is processed in an oxygen-free environment, in specially equipped rooms, at high temperatures. The chemical process produces gas, thermal energy and fuel oil (Czajczyńska et al., 2017; Kulikova and Tukacheva, 2017). When plastic waste is decomposed by pyrolysis, a proportion of gasoline is obtained, which can reach 80% of the mass of the raw material. The process involves thermal decomposition of plastic waste at various temperatures (300-900 °C) in the absence of oxygen, resulting in thermal decomposition and release of hydrogen particles in the plastic. A number of hydrocarbons are formed, which can be used as the basis for flammable substances (Al-Rumaihi et al., 2022; Arsentiev and Mikhailova, 2007; Escalante et al., 2022; Gudim and Golubev, 2009; Kabeyi and Olanrewaju, 2023; Petrov et al., 2015).
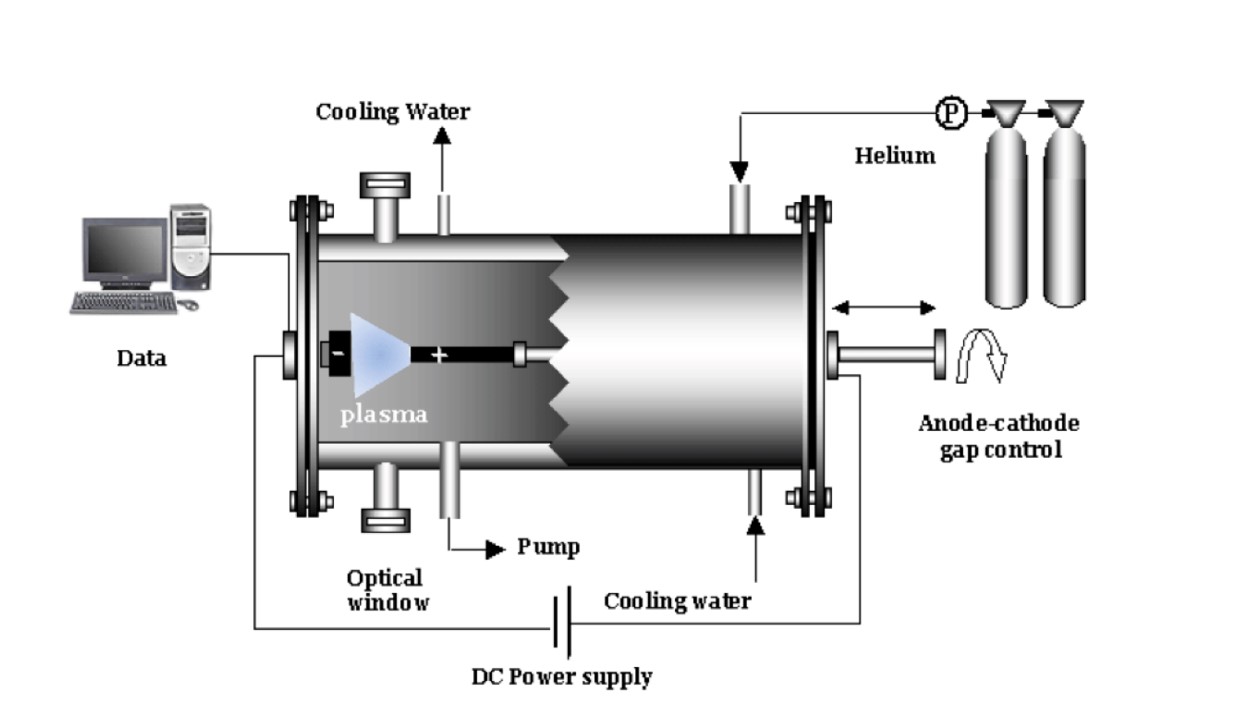
The essence of the electric arc method is the thermal evaporation of a graphite electrode in the plasma of an arc discharge burning in a helium atmosphere. In the interelectrode space in an arc discharge with a voltage of 20, 25 V; At a distance of 0.5, 2 mm, the stabilized arc current is 50, 100 A, the pressure is from 100 to 500 Torr (He), and intense dissipation of the anode material occurs (Buyantuev et al., 2013; Ermagambet et al., 2020).
Graphene's popularity among researchers and engineers is growing day by day as it has extraordinary optical, electrical, mechanical and thermal properties. Many experts predict in the near future the possible replacement of silicon transistors with more economical and fast-acting graphene transistors. It is known that graphene has excellent mechanical properties; the material is strong and rigid, and thanks to its flexibility it can evenly cover almost any surface. Many researchers and technology leaders view graphene as a useful material that could significantly accelerate the advent of flexible devices and paper-thin displays (Buyantuev et al., 2016).
Can be used as structural modifiers of structural materials based on carbon nanomaterials, hydrogen batteries, radio electronics elements, lubricating mixtures, varnishes and paints, highly efficient adsorbents, gas distribution layers of fuel rods. The use of carbon nanostructures in fine chemical synthesis, biology and medicine is widely discussed (Czajczyńska et al., 2017; Jekal et al., 2024; Kulikova and Tukacheva, 2017).
The purpose of the study is to examine ways to recycle plastic macaque waste and obtain carbon nanomaterials from plastic macaque waste.
The novelty of this study lies in the development of a sustainable and cost-effective method for transforming plastic waste into graphene-forming nanomaterials through a combination of pyrolysis and electric arc discharge. Unlike traditional methods of synthesizing graphene and carbon nanomaterials, which often involve high costs, complex procedures, or hazardous chemicals, the approach outlined in this research utilizes widely available plastic waste as a raw material, making it both environmentally and economically effective.
This article is structured as follows: after the introduction, the materials and methods section outlines the experimental procedures, followed by the results, which detail the characterization and analysis of the obtained nanomaterials. The discussion then interprets these findings, leading to a conclusion that highlights the environmental and scientific significance of the study.
2. Materials and Methods
2.1. Preparation of carbon nanomaterials
Carbon nanomaterials were obtained by the electric arc discharge method based on carbon material from Polyethylene terephthalate (PET) waste, obtained at 1173 K in an environment of nitrogen and water vapor.
2.2. The characterization technique
The moisture content, ash content and volatility of the samples were determined using a Thermoster Eltra thermogravimetric analyzer (according to ASTM D7582-12) (ASTM, 2012). The total pore volume, bulk density, pH of the aqueous extract, and adsorption activity for methyl orange were determined in accordance with the methods.
Chemical analysis and surface morphology were studied by energy-dispersive X-ray spectroscopy using an SEM instrument (Quanta 3D 200i) with an attachment for energy-dispersive analysis from EDAX. The type of carbon modification was studied using Raman spectroscopy using the Raman scattering (RS) method. Raman spectra of the samples were recorded on an Integra Spectra probe scanning microscope using a laser with a radiation wavelength of 473 nm. X-ray diffraction measurements were performed using a SmartLab X-ray diffractometer from Rigaku Corporation. The source of X-ray radiation is an X-ray tube Cu Kα radiation. The tube current and voltage are set to 30 mA and 40 kV, respectively.
Determination of electrical characteristics (dielectric constant ε, electrical resistance R) was carried out by measuring the electrical capacitance C of samples on a serial LCR-800 device (L, C, R meter) at an operating frequency of 1, 5, 10 kHz with a base error of 0.05-0.1% continuously, in dry air, in thermostatic mode with a holding time of 3 minutes at each fixed temperature. Plane-parallel samples were pre-fabricated in the form of disks with a diameter of 10 mm and a thickness of 5–6 mm with a binder additive (~ 1.5%). Pressing was carried out under a pressure of 20 kg/cm2. The resulting disks were fired in a silit furnace at 473 K for 6 hours. They were then thoroughly ground on both sides.
The dielectric constant was determined from the electrical capacity of the sample and the electrical capacity of the capacitor. To obtain the relationship between electrical induction D and electric field strength E, the Sawyer-Tower circuit was used. Visual observation of D (E-hysteresis loops) was carried out on an S1-83 oscilloscope with a voltage divider consisting of resistances of 6 mOhm and 700 kOhm, and a reference capacitor of 0.15 μF. Generator frequency 300 Hz. Plastic waste was crushed and carbonization and activation processes were carried out in a high-temperature rotary laboratory oven BR-12NRT at a temperature of 900 °C and a speed of 5 °C/min.
In all temperature studies, samples were placed in an oven, the temperature was measured with a chromel-alumel thermocouple connected to a B2-34 voltmeter with an error of ~0.1 mV. Temperature change rate ~5 K/min. The value of the dielectric constant at each temperature was determined by the formula ε = C/C0, where C0 is the capacitance of the capacitor without the test substance (air).
3. Results
As a result of heat treatment, a porous carbon material, a liquid product, a gas and a solid organic product were obtained (Figure 1).
Products formed as a result of (a) thermal treatment of plastic waste; (b) porous carbon material; (c) liquid product; (d) gas; (e) solid organic product.
During the process, the composition of gases at different temperatures was analyzed using gas chromatography. The results of the analysis are presented in Table 1. The material balance of the carbonization and activation process is presented in Table 2.
Gas composition resulting from the process of carbonization and activation of plastic waste.
Analyzing the gas composition, we noticed that during heat treatment, flammable gases (hydrogen, methane) are formed, and when we tried to burn the resulting gas, we noticed that it burned very intensely (Figure 1d). Consequently, it is possible to extract hydrogen from the resulting gas composition and use it in hydrogen energy, or purify the combustible gas mixture from CO2 gas and use it to reheat the furnace. This increases the economic efficiency of the waste recycling process.
If we focus on the material balance of the processes, then the yield of porous carbon formed as a result of the activation process (24.8%) is 3 times higher compared to the carbonization process (8.41%). The higher the yield of the main product, the more economically profitable it is.
In addition, yellow-white organic matter (Figure 1e) released as a result of thermal treatment of plastic waste was analyzed and studied by X-ray diffraction. The study revealed that terephthalic and sebacic acids were formed. Figure 2 presents the results of X-ray phase analysis of terephthalic and sebacic acids.
Terephthalic and sebacic acids have a wide range of applications. Terephthalic acid is mainly used to produce saturated polyesters. Polyethylene terephthalate accounts for 90% of the total production of these polymers.
Sebacic acid is used industrially to produce alkyd resins, polyhexamethylene sebacinamide as a stabilizer, polyester fibers and adhesives.
As a result, carbon nanomaterial was obtained from the obtained solid carbon material using the electric arc method, the current conductivity of the starting material and the resulting nanomaterial was measured and a conclusion was drawn. Figure 3 shows the process of producing carbon nanomaterials using the electric arc method. The current was 100 A at normal voltage (75 V), in an inert environment (nitrogen) (Figure 4).
Figure 5 shows current conductors made of carbon nanomaterial, obtained by the electric arc method after the activation process of plastic waste.
Measurement of electrical resistance of carbon materials: (a) heat-treated plastic waste; (b) heat-treated plastic waste using the electric arc method; (c) graphite.
As a result of the research, it is clear that the electrical resistance of the carbonized carbon material after the electric arc method (1.3 Ohm) decreased by ~3.6 times compared to the original (4.7 Ohm), which means that the electrical resistance decreased and the conductivity current increased, which indicates that a structural change in the structure of the carbon material occurred. The electrical resistance of carbonized and activated carbon material after the electric arc method (2.9 Ohm) decreased by 3.2 times compared to the original (9.3 Ohms). It can be noted that the electrical resistance of carbon nanomaterials after the electric arc method is lower than the electrical resistance of graphite (2.1 Ohm). An analysis of the technical, physicochemical properties of the resulting plastic waste after carbonization and activation processes was carried out (Table 3).
As a result, the pH value of plastic waste after carbonization and activation processes is close to neutral, and according to the analysis of its physicochemical properties, the characteristics of adsorption properties after the carbonization process have a slightly higher value compared to the activation process.
Figures 6 show the results of scanning electron microscopic analysis and the elemental composition of solid carbon material obtained by the carbonization process by the electric arc method. As a result, the formation of macroporous carbon materials can be observed.
Results of SEM analysis of carbonized porous carbon material: (a) x30; (b) x200; (c) x2000; (d) elemental composition.
According to the results, the amount of carbon in the porous carbon material was 95.52%, and the amount of oxygen was 4.48%.
Figure 7a-c presents the results of scanning electron microscope observations of solid carbon material obtained during activation by the electric arc method. The study found that nanoparticles ranging in size from 53.2 nm to 99.2 nm were produced, as seen in an SEM image of the porous carbon material magnified 25,000 times. Figure 7d presents the results of elemental analysis of a porous carbon material obtained by the activation process by the electric arc method.
Results of SEM analysis of carbonization and activated porous carbon material: (a) x30; (b) x200; (c) x25000; (d) carbonization and elemental composition.
Elemental composition of nanomaterial formed by the electric arc method from carbon material obtained after the activation process from plastic waste: C-93.39%, N-1.88%, O-3.89%, Al-0.11%, Si-0 .11%, S-0.23%, Fe-0.38%.
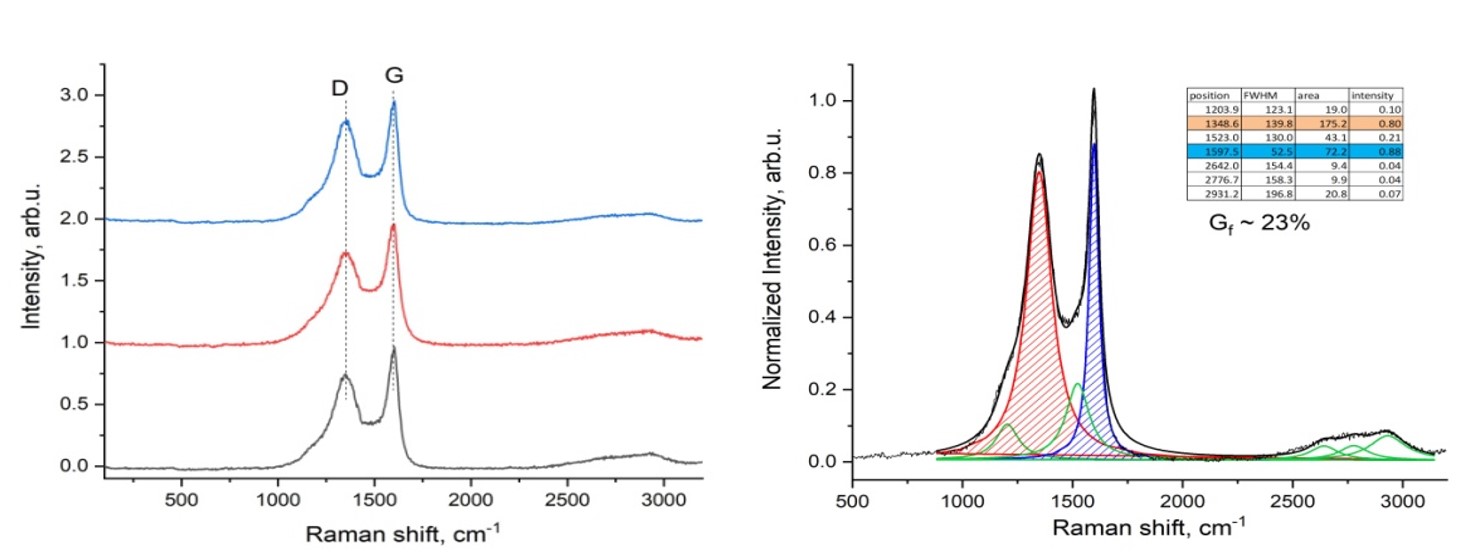
Figure 8 shows the results of Raman spectroscopic analysis of plastic waste after carbonization process. The spectrum of the sample is typical of amorphous carbon. The broad D-band peak indicates that the sample contains a relatively large amount of disordered structure and defects.
Figure 9 shows the results of Raman spectroscopic analysis of plastic waste obtained from the electric arc carbonization process.
As a result of Raman spectroscopic analysis of a porous carbon material obtained in the process of carbonization by the electric arc method (100 A, 75 V), it was found that it contains a spectrum of graphite-like carbon G (1593 cm-1). with varying degrees of crystallinity and order. The D band (1349 cm-1) is a disordered band associated with structural defects and amorphous carbon, while the G band corresponds to in-plane bonding of sp2 carbon atoms. The ratio of the intensities of the D and G peaks (ID/IG = 0.84) indicates a considerable number of defects in the material under consideration. As a result, the degree of graphitization of carbon materials was Gf-28%, which is 5% more than the original one. Thus, it can be seen that the quality of the material after the electric arc method has increased slightly compared to the material after the primary carbonization process.
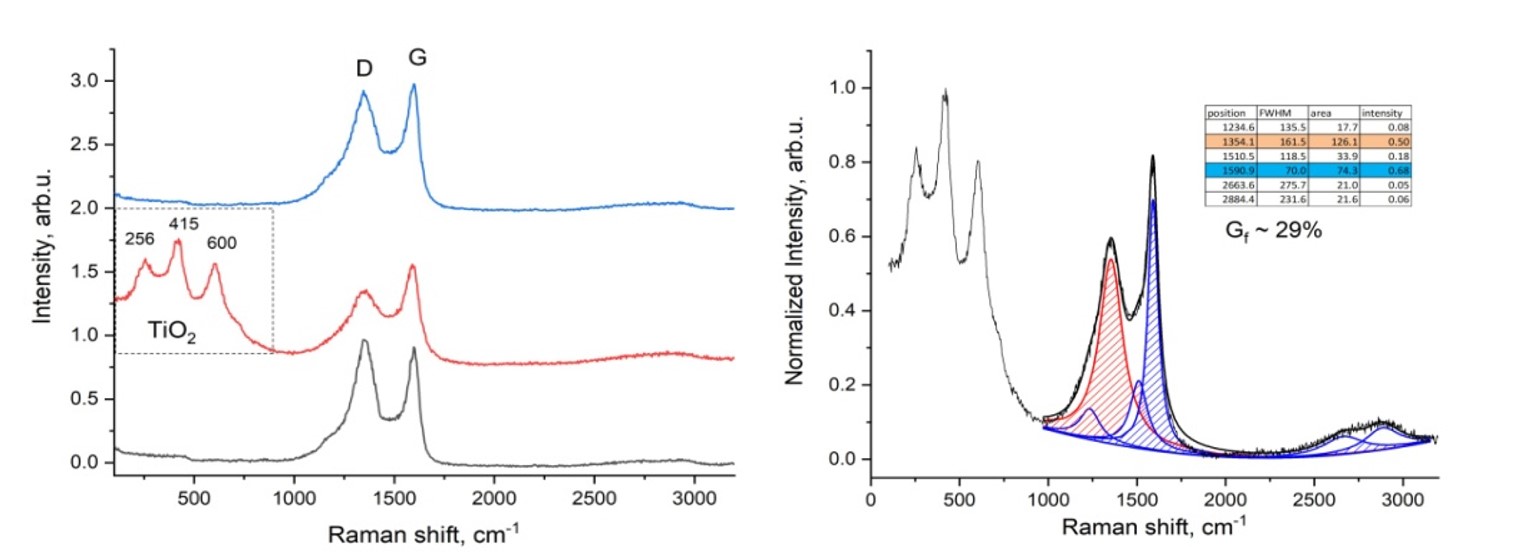
Figure 10 shows the results of Raman spectroscopic analysis of plastic waste after the activation process. Judging by the results, the sample consists of a mixture of amorphous carbon and titanium oxide. The degree of graphitization of the resulting material was Gf-29%.
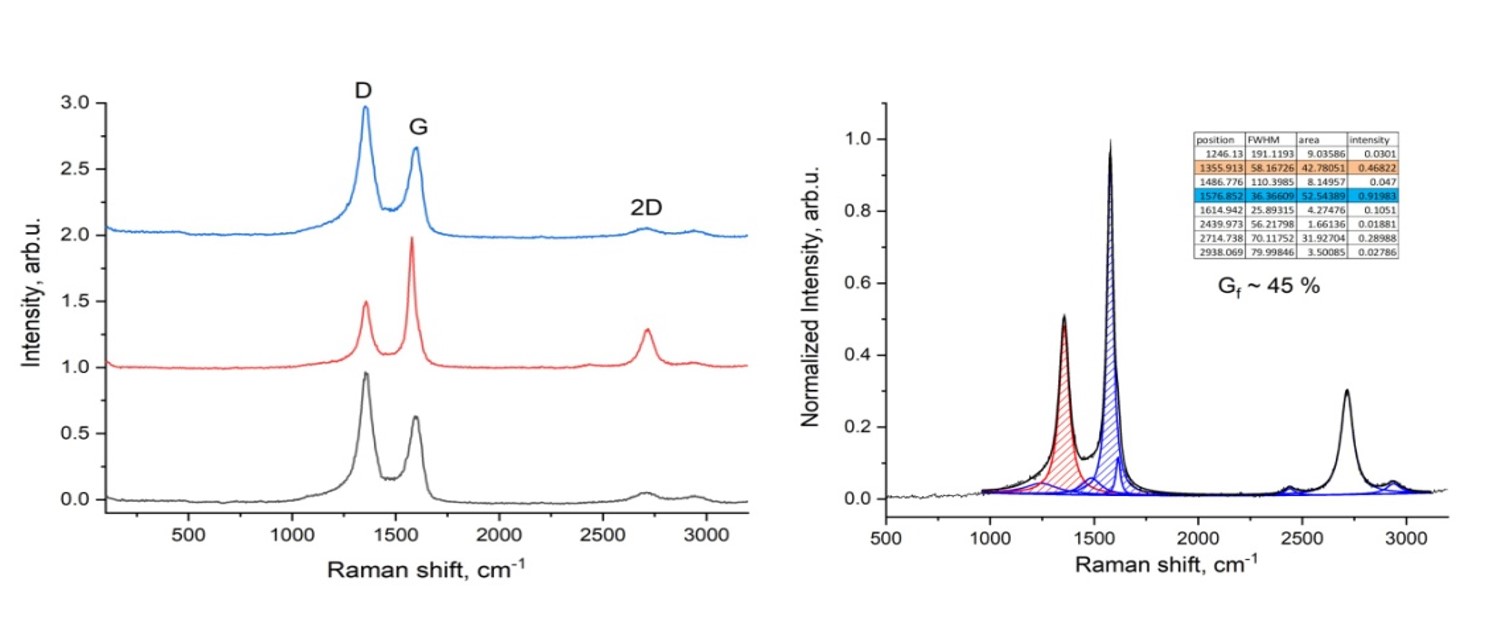
Figure 11 shows the results of Raman spectroscopic analysis of carbonized and activated plastic waste using the electric arc method. The spectrum of the sample is characteristic of the spectrum of graphite material.
The combined scattering spectra of carbonized and activated plastic waste after electric arc method correspond to peaks D (1355 cm-1), 2D (2715 cm-1) and G (1576 cm-1), respectively. The peak at 1576 cm-1 indicates the formation of graphitic structure, and the peak at 2715 m-1 indicates the formation of graphene as well as CNTs. The relative intensity ratio of the D band to the G band (ID/IG) can be used to accurately assess the quality of modern carbon materials. Based on the ratio of the intensities of the ID/IG bands, which is 0.85, it can be argued that there are a considerable number of material defects.
It is known that the 2D band of Raman spectra is more sensitive to the overlay of graphene sheets. For example, the 2D band position of single-layer graphene is ∼2679 cm-1, while for multilayer graphene (containing 2-4 layers), the 2D band position shifts toward higher wavenumbers along with peak broadening. In addition, the I2D/IG intensity ratios for single-, double-, triple-, and multilayer (>4) graphene layers are typically >1.6, ∼0.8, ∼0.30, and ∼0.07, respectively. In this sample, a 2D band located in the region of ∼2705-2718 cm-1 indicates the presence of low-layer graphene and graphene-containing materials in the sample. The intensity ratio I2D/IG is 1.72, which indicates the formation of single-layer graphene. The ratio of the intensities of G and 2D peaks characterizes single- and multilayer graphene. In our case, IG / I2D is 0.6, which confirms the formation of single-layer graphene (for single-layer graphene the ratio is 0.6-1). The degree of graphitization is Gf - 45%, which exceeds 1.5 times the original sample (Gf - 29%).
Table 4 presents the results of a study of solid carbon materials obtained after the processes of carbonization and activation of plastic waste by the electrophysical method. For comparison, BaTiO3 and graphite were studied. As a result of the research, it is clear that the properties of porous carbon materials obtained after the process of carbonization and activation of plastic waste are close to the properties of graphite.
Therefore, it is known that the production of graphite uses a very high temperature (>2000 °C), and in our experimental conditions it was shown that it is possible to obtain a nanomaterial with a graphite structure at a much lower temperature (<900 °C) and the use of the electric arc method in the second stage, that is, it is economically profitable.
4. Discussion
Our study demonstrates the successful recycling of plastic waste into carbon nanomaterials using the electric arc discharge method. This procedure is in line with the current research trends, which are focused on an ecological process of recycling plastic waste by producing carbon nanomaterials, as highlighted by Dai et al. (2023) in their review on carbon waste production from plastic waste.
Table 1 shows that usable Hydrogen and methane gas were obtained, which can be extracted, purified, and reused. These data correspond with the findings of Kabeyi and Olanrewaju (2023), who, in their review and Design Overview of Plastic Waste-to-Pyrolysis Oil Conversion with Implications on the Energy Transition, highlighted the promising plastic-to-green energy pathway that exists in producing hydrogen gas from plastic waste. Our study does not agree with their two-stage pyrolysis method–low-temperature plasma catalytic steam-reforming process at 2500С. It should also be noted that this research project obtained graphene-forming nanomaterials, which align with recent trends in plastic-waste derived nanoparticles. It agrees with the works of Panomsuwan et al. (2022), who investigated biomasses in pyrolysis as an effective fabrication route for nitrogen-doped carbons, illustrating the increased electrocatalytic activity of carbon source materials.
The observed increase in conductivity in carbon materials after the electric arc discharge method agrees with the work of Abdulhameed et al. (2021), who reviewed the method of enhancing conductivity using Carbon Nanotubes. From their work, we can also derive another agreeing statement: a limitation faced by carbon nanomaterials produced by the electric arc method, which is the inability to effectively control the reduction of electrical resistance and the increase of conductivity. This can also be said of the increase in quality observed in the material after the electric arc method, but there exists no control over this process, and this was also confirmed in the review of carbon nanotubes by Rahamathulla et al. (2021).
According to the review of Nayak et al. (2022) on the advances in plastic waste-derived carbon nanomaterial for supercapacitor applications, Ni improves the degree of graphitization and thermal conductivity of plastic waste-derived carbon nanomaterials; this agrees with our results and also explains the increase in graphitization after the electric arc method. It also creates room for more research on the relationship between the amount of Ni and the increase in graphitization in carbon nanoparticles.
From our research, we were able to produce graphite at a lower temperature of about (<900 °C); this goes against popular beliefs and agrees with the works of Liang et al. (2021), who explored an ecological and efficient approach to synthesizing graphite from carbon dioxide at ultralow temperatures (126 °C). Also, from our research, we notice the presence of a graphite structure from the Raman spectroscopy, which confirms the disadvantage of graphene as stated by Lim et al. (2021), who stated that graphene can aggregate with one another or even reform graphite through π-π stacking and van der Waals interaction. This presents a limitation in graphene nanomaterials and creates room for future research.
5. Conclusion
Among the many problems facing humanity today is environmental pollution from plastic waste. In this study, plastic waste was crushed and burned in a pyrolysis oven at 900 °C to produce a porous carbon nanomaterial. Using the resulting porous carbon material as an electrode, carbon nanomaterial was obtained by burning it with an electric arc method. A physicochemical study of the composition of carbon nanomaterial was carried out and it was proven that the samples formed on the reactor wall are graphene-forming nanomaterials. The composition of substances formed as a result of carbonization and activation, electric arc processes, was determined by the methods of elemental and microscopic analysis, combined scattering (Raman spectroscopy), X-ray diffraction, and gas chromatography. The possibility of obtaining a number of valuable products (nanomaterial, hydrogen, flammable gas, organic acids) through complex plastic waste processing has been shown.
The proposed method for producing graphene-containing materials is based on the electric arc discharge method, which is the most promising method for producing nanomaterials, offering high purity and minimal defects.
The electric arc discharge method for producing graphene and graphene-containing materials has many advantages, such as low production cost, high efficiency and the possibility of synthesis without using any catalyst, the method can be easily used in the laboratory and can be scaled up to an industrial process.
The use of graphene as electrodes for batteries, capacitors to replace lithium-ion batteries, as well as to obtain high-strength composite materials by adding small amounts of graphene in the electronics and construction industries will solve environmental problems.
The environmental effect of the proposed method of recycling plastic waste is to create an environmentally friendly technology based on the processing of carbon-containing waste to produce products with high added value. The creation of this technology will make it possible not to use different types of chemical compounds and reagents and solve the environmental aspect of an economically viable product like graphene. The proposed method is unique in that the raw material used for the synthesis of graphene and graphene-containing materials is plastic waste, the amount of which is sufficient throughout the country, compared to the technology for producing graphene and graphene-like materials from many other precursors (HNO3, HCl, H2SO4, etc.) and using various other methods (Hummers method, micromechanical decomposition, thermal decomposition of SiC). The promise of these studies lies in the possibility of large-scale production of graphene from waste materials, which will lead to the appearance of domestically produced materials and composites based on them on the Kazakhstan market.
Future research should focus on optimizing the electric arc discharge process to further improve the quality and consistency of the graphene nanomaterials, particularly reducing material defects and enhancing conductivity. Additionally, studies should explore the use of different types of plastic waste to assess the versatility of the applied method.
Acknowledgements
This research was funded by the Science Committee of the Ministry of Science and Higher Education of the Republic of Kazakhstan (Grant No. BR24992833, Grant No. AR19577512).
References
-
ABDULHAMEED, A., WAHAB, N.Z.A., MOHTAR, M.N., HAMIDON, M.N., SHAFIE, S. and HALIN, I.A., 2021. Methods and applications of electrical conductivity Enhancement of materials using carbon nanotubes. Journal of Electronic Materials, vol. 50, no. 6, pp. 3207-3221. http://doi.org/10.1007/s11664-021-08928-2
» http://doi.org/10.1007/s11664-021-08928-2 -
ADLA, K., DEJAN, K., NEIRA, D. and DRAGANA, Š., 2022. Degradation of ecosystems and loss of ecosystem services. In: J.C. PRATA, A.I. RIBEIRO and T. ROCHA-SANTOS, eds. One health London: Academic Press, pp. 281-327. http://doi.org/10.1016/B978-0-12-822794-7.00008-3
» http://doi.org/10.1016/B978-0-12-822794-7.00008-3 - AIPOVA, A.R. and DYUZHENKOVA, N.V., 2016. Main trends in the packaging market. Bulletin of Scientific Conferences, vol. 5, no. 9, pp. 8-10.
-
AL-RUMAIHI, A., SHAHBAZ, M., MCKAY, G., MACKEY, H. and AL-ANSARI, T., 2022. A review of pyrolysis technologies and feedstock: a blending approach for plastic and biomass towards optimum biochar yield. Renewable & Sustainable Energy Reviews, vol. 167, pp. 112715. http://doi.org/10.1016/j.rser.2022.112715
» http://doi.org/10.1016/j.rser.2022.112715 - AMERICAN SOCIETY FOR TESTING AND MATERIALS – ASTM, 2012. ASTM D7582-12: standard test methods for proximate analysis of coal and coke by macro thermogravimetric analysis. West Conshohocken: ASTM International.
- ARSENTIEV, V.A. and MIKHAILOVA, N.V., 2007. Pererabotka otkhodov: ispol’zovaniye resursnogo potentsiala [Waste processing: use of resource potential]. Tverdyye Bytovyye Otkhody, vol. 8, no. 14, pp. 60-63.
- BUYANTUEV, S.L., KONDRATENKO, A.S. and KHMELEV, A.B., 2013. Preparation of carbon nanomaterials by integrated plasma processing of coal. Vestnik VSGUTU, vol. 3, no. 42, pp. 21-25.
- BUYANTUEV, S.L., URKHANOVA, L.A., KHMELEV, A.B., LKHASARANOV, S.A., KONDRATENKO, A.S., VOLOKITIN, O.G. and TISHKOV, N.L., 2016. Concerning the use of carbon nanomaterials obtained by plasma method for producing the composite. Vestnik VSGUTU, vol. 6, no. 63, pp. 19-26.
-
CZAJCZYŃSKA, D., ANGUILANO, L., GHAZAL, H., KRZYŻYŃSKA, R., REYNOLDS, A., SPENCER, N. and JOUHARA, H., 2017. Potential of pyrolysis processes in the waste management sector. Thermal Science and Engineering Progress, vol. 3, pp. 171-197. http://doi.org/10.1016/j.tsep.2017.06.003
» http://doi.org/10.1016/j.tsep.2017.06.003 -
DAI, L., KARAKAS, O., CHENG, Y., COBB, K., CHEN, P. and RUAN, R., 2023. A review on carbon materials production from plastic wastes. Chemical Engineering Journal, vol. 453, pp. 139725. http://doi.org/10.1016/j.cej.2022.139725
» http://doi.org/10.1016/j.cej.2022.139725 - ERMAGAMBET, B.T., KAZANKAPOVA, M.K., AITMAGAMBETOVA, A.Z. and KASENOVA, Z.M., 2020. A method for producing graphene-containing nanomaterials from carbon products using the electric arc discharge method. Republic of Kazakhstan. Patent, nº 2020/0859.1. 15-12-2020.
-
ESCALANTE, J., CHEN, W., TABATABAEI, M., HOANG, A.T., KWON, E.E., LIN, K.A. and SARAVANAKUMAR, A., 2022. Pyrolysis of lignocellulosic, algal, plastic, and other biomass wastes for biofuel production and circular bioeconomy: a review of thermogravimetric analysis (TGA) approach. Renewable & Sustainable Energy Reviews, vol. 169, pp. 112914. http://doi.org/10.1016/j.rser.2022.112914
» http://doi.org/10.1016/j.rser.2022.112914 - GUDIM, Y.A. and GOLUBEV, A.A., 2009. Bezotkhodnaya tekhnologiya vysokotemperaturnoy utilizatsii nesortirovannykh tverdykh kommunal’nykh otkhodov [Non-waste technology for high-temperature recycling of unsorted solid municipal waste]. Ekologiia i promyshlennost' Rossii, vol. 2, pp. 4-7.
-
HASSAN, T., SRIVASTWA, A.K., SARKAR, S. and MAJUMDAR, G., 2022. Characterization of plastics and polymers: a comprehensive study. IOP Conference Series. Materials Science and Engineering, vol. 1225, no. 1, pp. 012033. http://doi.org/10.1088/1757-899X/1225/1/012033
» http://doi.org/10.1088/1757-899X/1225/1/012033 -
JEKAL, S., OTGONBAYAR, Z., NOH, J., SA, M., KIM, J., KIM, C., CHU, Y., KIM, H., SONG, S., CHOI, H., OH, W. and YOON, C., 2024. Designing novel LIDAR-detectable plate-type materials: synthesis, chemistry, and practical application for autonomous working environment. ACS Applied Materials & Interfaces, vol. 16, no. 15, pp. 19121-19136. http://doi.org/10.1021/acsami.4c00470 PMid:38588341.
» http://doi.org/10.1021/acsami.4c00470 -
KABEYI, M.J.B. and OLANREWAJU, O.A., 2023. Review and design overview of plastic waste-to-pyrolysis oil conversion with implications on the energy transition. Journal of Energy, vol. 2023, pp. 1821129. http://doi.org/10.1155/2023/1821129
» http://doi.org/10.1155/2023/1821129 -
KAEWTRAKULCHAI, N., CHANPEE, S., JADSADAJERM, S., WONGRERKDEE, S., MANATURA, K. and EIAD-UA, A., 2024. Co-hydrothermal carbonization of polystyrene waste and maize stover combined with KOH activation to develop nanoporous carbon as catalyst support for catalytic hydrotreating of palm oil. Carbon Resources Conversion, vol. 7, no. 4, pp. 100231. http://doi.org/10.1016/j.crcon.2024.100231
» http://doi.org/10.1016/j.crcon.2024.100231 -
KAEWTRAKULCHAI, N., PUTTA, A., PASEE, W., FUANGNAWAKIJ, K., PANOMSUWAN, G. and EIAD-UA, A., 2019. Magnetic carbon nanofibers from horse manure via hydrothermal carbonization for methylene blue adsorption. IOP Conference Series. Materials Science and Engineering, vol. 540, no. 1, pp. 012006. http://doi.org/10.1088/1757-899X/540/1/012006
» http://doi.org/10.1088/1757-899X/540/1/012006 - KASSENOVA, Z., ISKAKOV, Y., BOLAT, Y., BAUYRZHAN, K., MEZGIL, S., DINA, I., MAIRA, K. and DARIGA, N., 2024. Effectiveness of oil-contaminated soil reclamation with humic preparations. International Journal of Agriculture and Biosciences, vol. 13, no. 3, pp. 474-487.
-
KHOAELE, K.K., GBADEYAN, O.J., CHUNILALL, V. and SITHOLE, B., 2023. The devastation of waste plastic on the environment and remediation processes: a critical review. Sustainability, vol. 15, no. 6, pp. 5233. http://doi.org/10.3390/su15065233
» http://doi.org/10.3390/su15065233 -
KULIKOVA, Y.V. and TUKACHEVA, K.O., 2017. Analysis of recycling technologies of polymer composite materials. Transport. Transport Facilities. Ecology, vol. 4, pp. 103-120. http://doi.org/10.15593/24111678/2017.04.08
» http://doi.org/10.15593/24111678/2017.04.08 -
LIANG, C., CHEN, Y., WU, M., WANG, K., ZHANG, W., GAN, Y., HUANG, H., CHEN, J., XIA, Y., ZHANG, J., ZHENG, S. and PAN, H., 2021. Green synthesis of graphite from CO2 without graphitization process of amorphous carbon. Nature Communications, vol. 12, no. 1, pp. 119. http://doi.org/10.1038/s41467-020-20380-0 PMid:33402678.
» http://doi.org/10.1038/s41467-020-20380-0 -
LIM, J., BEE, S., SIN, L.T., RATNAM, C.T. and HAMID, Z.A.A., 2021. A review on the synthesis, properties, and utilities of functionalized carbon nanoparticles for polymer nanocomposites. Polymers, vol. 13, no. 20, pp. 3547. http://doi.org/10.3390/polym13203547 PMid:34685309.
» http://doi.org/10.3390/polym13203547 -
NAYAK, S.K., SAURABH, S., KAR, A., SAHOO, B.B., SAHOO, N.K. and SAHOO, P.K., 2022. Advances in plastic waste-derived carbon nanomaterial for supercapacitor applications: trends, challenges and prospective. Materials Today: Proceedings, vol. 67, pp. 1024-1032. http://doi.org/10.1016/j.matpr.2022.05.519
» http://doi.org/10.1016/j.matpr.2022.05.519 -
OEHLMANN, J., SCHULTE-OEHLMANN, U., KLOAS, W., JAGNYTSCH, O., LUTZ, I., KUSK, K.O., WOLLENBERGER, L., SANTOS, E.M., PAULL, G.C., VAN LOOK, K.J.W. and TYLER, C.R., 2009. A critical analysis of the biological impacts of plasticizers on wildlife. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, vol. 364, no. 1526, pp. 2047-2062. http://doi.org/10.1098/rstb.2008.0242 PMid:19528055.
» http://doi.org/10.1098/rstb.2008.0242 -
OYEWALE, J.A., TARTIBU, L.K. and OKOKPUJIE, I.P., 2023. A review and bibliometric analysis of sorting and recycling of plastic wastes. International Journal of Design & Nature and Ecodynamics, vol. 18, no. 1, pp. 63-74. http://doi.org/10.18280/ijdne.180107
» http://doi.org/10.18280/ijdne.180107 -
PAN, D., SU, F., LIU, C. and GUO, Z., 2020. Research progress for plastic waste management and manufacture of value-added products. Advanced Composites and Hybrid Materials, vol. 3, no. 4, pp. 443-461. http://doi.org/10.1007/s42114-020-00190-0
» http://doi.org/10.1007/s42114-020-00190-0 -
PANOMSUWAN, G., EIAD-UA, A., KAEWTRAKULCHAI, N., SEIZAWA, A. and ISHIZAKI, T., 2022. Cattail leaf-derived nitrogen-doped carbons via hydrothermal ammonia treatment for electrocatalytic oxygen reduction in an alkaline electrolyte. International Journal of Hydrogen Energy, vol. 47, no. 59, pp. 24738-24749. http://doi.org/10.1016/j.ijhydene.2022.05.213
» http://doi.org/10.1016/j.ijhydene.2022.05.213 - PETROV, A.V., DORIOMEDOV, M.S. and SKRIPACHEV, S.Y., 2015. Recycling technologies of polymer composite materials (review). Proceedings of VIAM, vol. 8, pp. 62-73.
-
RAHAMATHULLA, M., BHOSALE, R.R., OSMANI, R.A.M., MAHIMA, K.C., JOHNSON, A.P., HANI, U., GHAZWANI, M., BEGUM, M.Y., ALSHEHRI, S., GHONEIM, M.M., SHAKEEL, F. and GANGADHARAPPA, H.V., 2021. Carbon Nanotubes: current perspectives on diverse applications in targeted drug delivery and therapies. Materials, vol. 14, no. 21, pp. 6707. http://doi.org/10.3390/ma14216707 PMid:34772234.
» http://doi.org/10.3390/ma14216707 -
TORRES-AGULLO, A., KARANASIOU, A., MORENO, T. and LACORTE, S., 2021. Overview on the occurrence of microplastics in air and implications from the use of face masks during the COVID-19 pandemic. The Science of the Total Environment, vol. 800, pp. 149555. http://doi.org/10.1016/j.scitotenv.2021.149555 PMid:34426330.
» http://doi.org/10.1016/j.scitotenv.2021.149555
Publication Dates
-
Publication in this collection
07 Apr 2025 -
Date of issue
2024
History
-
Received
14 Aug 2024 -
Accepted
31 Oct 2024

 Plastic waste recycling for the production of graphene nanomaterials using electric arc discharge
Plastic waste recycling for the production of graphene nanomaterials using electric arc discharge