Abstract
Genyatremus luteus primarily inhabits estuarine regions and holds significant commercial value along the Brazilian Amazon coast. This study aimed to investigate the population structure and reproductive biology of G. luteus caught in the Paciência River estuary, in the state of Maranhão. Approximately 30 specimens were collected monthly from November 2015 to October 2017, totaling 475 individuals (295 females and 180 males). The sex ratio differed significantly over the study period, at 1.6F:1M (χ2 = 84.3; df = 10; p < 0.05). The mean length at first sexual maturity was 12.66 cm for females, 12.32 cm for males, and 12.56 cm for combined sexes, exhibiting negative allometric growth (b < 3) with peaks in the condition factor of 2.04 and 1.30 for females and males, respectively. Females showed a broader length range (23.16 ± 3.97 cm), possibly attributed to a reproductive strategy to ensure species perpetuation. These findings contribute to optimizing fisheries management strategies, ensuring that individuals can reproduce before being caught. Additionally, analyzing seasonal reproductive patterns relative to environmental variables, such as rainfall, offers deeper insights into how external factors influence reproductive cycles and population dynamics.
Keywords:
morphometry; condition factor; reproductive biology; gonad-somatic index; spawning
Resumo
A espécie Genyatremus luteus vive principalmente em regiões estuarinas e possuindo valor comercial significativo na Costa Amazônica Brasileira, este estudo teve como objetivo investigar a estrutura populacional e aspectos da biologia reprodutiva de G. luteus capturados no estuário do Rio Paciência, no Estado do Maranhão. Aproximadamente 30 espécimes foram capturados mensalmente entre novembro de 2015 e outubro de 2017, totalizando 475 indivíduos (295 fêmeas e 180 machos). A razão sexual diferil significativamente para o período estudado, 1,6F:1M. (χ2 = 84,3; g.l. = 10; p < 0,05). O comprimento médio de primeira maturação sexual foi de 12,66 cm para fêmeas, 12,32 cm para machos e 12,56 cm para os sexos combinados, exibindo crescimento alométrico negativo (b<3) e picos no fator de condição de 2,04 e 1,30 para fêmeas e machos, respectivamente. As fêmeas apresentaram maior amplitude de comprimento (23,16 ± 3,97 cm) possivelmente atribuída a uma estratégia de perpetuação da espécie (ou seja, reprodução). Essas informações facilitam a otimização das estratégias de manejo pesqueiro, garantindo que os indivíduos possam se reproduzir antes da captura. Além disso, a análise dos padrões sazonais de reprodução em relação às variações ambientais, como a precipitação, proporciona uma compreensão mais profunda de como fatores externos influenciam os ciclos reprodutivos e a dinâmica populacional.
Palavras-chave:
morfometria; fator de condição; biologia reprodutiva; índice gonadossomático; desova
1. Introduction
The Amazon Coast, encompassing the states of Amapá, Pará, and Maranhão, is characterized by significant marine fishing activities driven by the high nutrient availability in its waters. The interaction between the substantial fluvial discharge and tidal forces plays a crucial role in the distribution of both nutrients and sediments, thereby influencing the productivity of marine habitats and the dynamics of local fisheries (Bentes et al., 2012; Silva et al., 2023). Thus, having high biological productivity, sustaining high biomass, and having a complex food web positively influence local activity in fishing and adjacent regions (Wolff et al., 2000). These characteristics provide the development of many organisms and the exploitation of fisheries resources in these regions (Marceniuk et al., 2013 ).
In recent decades, fishing sustainability has been subject to intense international debate. Therefore, management measures must be promoted to develop a viable economic, social, and ecological fishing program to guarantee the perpetuation of fish stocks. Hence, it will be possible to ensure the monitoring and preservation of the diversity of stocks and fishing activity, which, in addition to constituting a cultural legacy, is the primary means of survival for coastal communities (Almeida et al., 2016; Cámara and Santero-Sanchez, 2019).
When evaluating the reproductive aspects of fish stocks, it is crucial to consider conservation measures for aquatic organisms. These evaluations provide valuable information on the fishing potential, spawning periods and locations, and the minimum legal size for capture (Brown-Peterson et al., 2011). Therefore, assessing reproductive parameters—such as sex ratio, length-weight relationships, reproductive cycles, and spawning seasons—of economically significant species is essential. This information is vital for establishing appropriate closed fishing seasons and minimum catch sizes, ensuring that regulations are based on the reproductive characteristics of species most affected by fishing pressure (Carvalho et al., 2021).
The Genyatremus luteus is a species that has preferences for estuarine and marine water environments, in addition to presenting opportunistic behavior; these conditions ease its development almost every year (Barletta et al., 2003; Carvalho-Neta et al., 2011; Jimenez et al., 2013; Bomfim et al., 2019). Thus, it can be configured as an economically significant stock, being a species of great importance to the State (Fernandes, 1982; Nunes et al., 2020) and to this factor, it is necessary to have a management plan for its exploration.
This study aims to provide detailed insights into the population structure and reproductive characteristics of G. luteus in the Paciência River estuary, located in Raposa, Maranhão. By enhancing our understanding of these aspects, the research seeks to contribute to effective fisheries management and the development of sustainable fishing regulations. These efforts are crucial for ensuring the long-term viability and conservation of G. luteus, a species of significant commercial value.
2. Material and Methods
2.1. Area description
Approximately 30 individuals were collected monthly in the Paciência River estuary in the metropolitan region of São Luís, Brazil. The Paciência River Basin covers an area of 150.5 km2, with a perimeter of 71.2 km. It includes the main channel, the most prominent watercourse of Maranhão Island, extending 27.82 km and exhibiting a drainage density (Dd) of 1.2 km/km2 (Figure 1). The estuarine system of the Paciência River is influenced by the Golfão Maranhense (São Marcos Bay and São José Bay). This basin is located within the Maranhão Island Hydrographic System, encompassing four municipalities: São Luís, Paço do Lumiar, São José de Ribamar, and Raposa (Lopes, 2016). The estuary is characterized by hydrodynamics governed by a semi-diurnal tidal system (two high tides and two low tides per lunar day, with proportional intervals of approximately 6 hours) and an average amplitude of 4.6 meters, which can reach up to 7.2 meters during spring tides. On average, tidal amplitudes are below 5.5 meters, generating tidal currents of up to 7 knots (Ferreira, 1989). According to meteorological conditions, the climate is classified as tropical and humid with two distinct seasons marked by precipitation and wind intensity: dry (August to November), and rainy (January to May). June, July, and December were considered a transitional season based on data from the National Institute of Meteorology – INMET.
Sampled area of specimens of Genyatremus luteus captured between the months of November 2015 to October 2017 of the Paciência River estuary – MA.
Sampling took place from November 2015 to October 2017, and the fish were later transported to the Fisheries Biology laboratory at the State University of Maranhão (UEMA). After obtaining the samples in the laboratory, the individuals were measured with the aid of a caliper (accuracy: 0.01) to get the lengths: total (TL) and standard (SL) in centimeters, and total weight (TW) in grams using a scale (accuracy: 0.001). Subsequently, the specimens were dissected to remove the ovaries, and then the viscera were removed, and the eviscerated weight (EW) in grams was determined. The accumulated rainfall (mm) and temperature (°C) data were extracted from the National Institute of Meteorology - INMET (2024) database.
2.2. Biological, numerical, and statistical treatment
For the length-weight relationship (LWR), the variables standard length (SL) and total weight (TW) of the individuals were analyzed using non-linear regression, described by Keys (1928) and von Bertalanffy (1938) (Equation 1):
where W (TW) and L (SL) are variables, parameter a is the intercept, and b is the allometric coefficient (Santos, 1978). To estimate the sex ratio, the number of females by the total number of males was calculated. The chi-square test (χ2) was used to determine statistical differences to assess the species’ reproductive potential (Seckendorff and Azevedo, 2007; Cavalcante et al., 2012).
The Gonadossomatic Index (GSI) was calculated to indicate the reproductive period, according to the Equation 2 proposed by Maddock and Burton (1998):
where Gw is the gonad weight in grams (g), and Ew is the weight of the gutted fish in grams (g). The GSI of the grouped sexes was correlated with the temperature and precipitation. Statistical analyses were performed using Past 3.14 and STATISTICA 10.0 software.
The condition factor was calculated using the Equation 3 suggested by Hardardottir et al. (2001) as follows:
where K is the Fulton's condition factor, Li is the observed length, Wi is the observed weight of the ith fish, and the constant is a scaling factor equal to 100,000 if metric units (grams and millimeters).
Gonad tissue analyses were also performed on images obtained in a camera coupled with an optical microscope and processed in Zen 2012 software. The stages of reproductive material development have been adapted from the methodology proposed by Brown-Peterson et al. (2011).
The average length of the first gonadal maturation (L50) was calculated from the cumulative curve of adult occurrence frequencies by Tl class as a function of the logistic function. The maturation stages were grouped into juveniles (stage A) and adults (stages B + C + D). The percentage of matures by length class was defined and considered a dependent variable (Y), and the total length as an independent variable (X). Subsequently, these values were fitted to a logistic curve, using the Statistical Program 10.0 according to the Formula 4:
where P is the proportion of mature owners, r is the slope of the curve, L is the length, and Lm is the average length of sexual maturity.
3. Results
Of the individuals of G. luteus, 51% are female (n = 295) and 38% male (n = 180) (Table 1). Therefore, it was possible to see that females had greater amplitude in length (23.16 ± 3.97 cm; 243.40 ± 113.32 g).
The result of the angular coefficient b for males and females demonstrates that there was negative allometry (b < 3); that is, the species develops more in length than in weight (Figure 2).
Relationship between total length and total weight of females (A) and males (B) of Genyatremus luteus captured between November 2015 to October 2017 in the estuary of the Paciência River – MA.
The sex ratio for the period studied was 1.6F: 1M. According to the chi-square test (χ2 = 84.3; g.l. = 10; p < 0.05), there was a significant difference in the sex ratio between the sexes. When comparing the number of individuals per length class (χ2 values), it was possible to observe a statistical difference in the classes from 18-22 to 30-33, standing out 24-26 cm (χ2 = 24.0; p<0.05) (Table 2).
Chi-square values (χ2) per class of total length (cm) of specimens of Genyatremus luteus acquired in the estuary of the Paciência River – MA.
The GSI showed the highest mean for females in July 2017 (8.4 ± 5.15 g) and the lowest in August 2017 with 0.34 ± 0.73 g. Concerning males, the highest average of the GSI occurred in April 2017 (4.1 ± 4.41 g) and the lowest in March 2017 (0.4 ± 0.22 g) (Figure 3). Thus, the highest means for females was found in April 2017 (2.04 ± 1.78 g). Regarding males, the highest mean of the K occurred in April 2016 (1.30 ± 0.26 g).
Monthly variation of the Gonadal Index (GI) and Condition Factor (K) of Genyatremus luteus for females (A) and males (B) captured between the months of November 2015 to October 2017 in the estuary of the Paciência River – MA.
However, males maintained consistency regarding the Fulton factor, with no variation in length or size. The mean value obtained for females was 1.17, showing peaks of optimal condition at 30 cm and 32 cm. (Figure 4).
Fulton’s condition factor (K) per fork length (cm) class on the length of females (grey line) and males (black line) of Genyatremus luteus captured between the months of November 2015 to October 2017 in the estuary of the Paciência River – MA.
The highest rainfall indices in the region occurred in the first semester. The maximum value was observed in April and May 2017 with 362 and 329.9 mm, respectively. Minimum rainfall records were observed, with no rainfall in December 2015 and September 2017. The incidence of rain possibly favored the increase in the GSI with spawning in the consecutive month. Regarding the second semester, which is characterized by little or no rain, the temperature rises, favoring the induction of spawning (Figure 5).
Variation of local precipitation and Gonadal Index of Genyatremus luteus captured between the months of November 2015 to October 2017 in the estuary of the Paciência River – MA.
When analyzing the relative frequency of gonadal development stages, the maturational stage A (83.33%) was presented at a high frequency during January 2017. Stages B (80%), C (75%), and D (25%) stood out in September 2016 and May 2017. Meanwhile, males in the immature stage (33.33%) were in greater proportion in November 2016. Males in the development stage (88.88%), mature (66.66%), and emptied (9.09%) were very representative in the periods equivalent to December 2016, November 2015 and February 2017 (Figure 6).
Distribution of maturational stages of females (A) and males (B) of Genyatremus luteus captured between the months of November 2015 to October 2017 in the estuary of the Paciência River – MA.
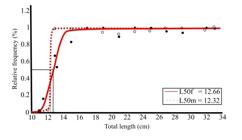
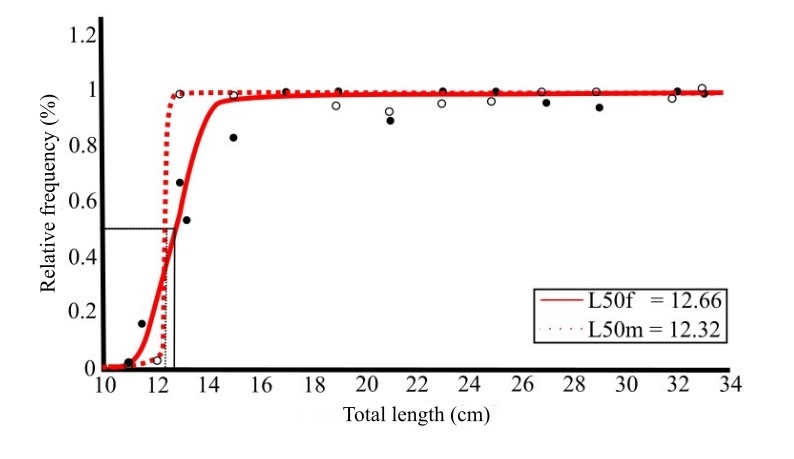
The average length at first sexual maturation of G. luteus was 12.66 cm for females and 12.32 cm for males (Figure 7). In the Paciência River estuary, males reached the first maturity at a smaller size than females. For the grouped L50 estimation, the calculated value was 12.56 cm.
Average length of the first sexual maturation of females (L50f) and males (L50m) of Genyatremus luteus captured between the months of November 2015 to October 2017 in the estuary of the Paciência River – MA. (%) Relative frequency
Geniatremus luteus is a gonochoristic species with no identifiable sexual dimorphism in body shape or coloration. Microscopic analysis of the ovaries allowed classification into six stages of gonadal development: A) immature - characterized by the presence of only primary growth oocytes (PG); B) developmental - characterized by the presence of oocytes in cortical alveolar (CA) phase; primary vitellogenesis (Vtg1), and secondary vitellogenesis (Vtg2); C) capable of spawning - the presence of oocytes in tertiary vitellogenesis (Vtg3); D) active spawning - the presence of oocytes in phases: migration of the germinal vesicle (GVM), hydrated oocytes (OH), and post-ovulatory follicles (POF); For the male (Figure 8E and F), the spermatogenesis is cystic, as developing germ cells are enclosed within germinal cysts formed by enveloping the Sertoli-cell process; within each cyst, the maturation of germ cells is synchronous.
Microscopic identification of maturation stages in 10x lens for females and male of Genyatremus luteus, captured in the Paciência River - MA estuary. (A) female immature; (B) female maturing; (C) female capable of spawning; (D) female regressing; (E) male immature; (F) male mature. PG = growth oocytes; OP = Primary Oocyte; OJ = young oocyte; CA = cortical alveolar; Vtg 1 = Oocyte in Vitellogenesis 1; Vtg 2 = Oocyte in Vitellogenesis 2; Vtg 3 = Oocyte in Vitellogenesis 3; GVM = migration of the germinal vesicle; OH = hydrated oocytes; FPo = post-ovulatory follicle; SS = Seminiferous tubules in the testes—cysts of spermatogenic cells in the secondary; SP = Cysts of spermatogenic cells in the primary testes. The bar indicates 100 µm.
4. Discussion
The individuals of G. luteus presented data of total length and total weight varying respectively from 12 to 33 cm and 40.15 to 663.99 g. In the Paciência River estuary, Pinheiro (2010) observed data for this species’ lengths ranging from 5.6 to 20.6 cm. These patterns are smaller than those found in the present study. Silva-Júnior et al. (2007) identified specimens of G. luteus in the Paciência River Basin (Ilha de São Luís – MA), and the minimum and maximum amplitude of the total length and total weight of the species ranged from 7.75 cm to 60.80 cm and 2.70 g to 248.91 g.
The growth of G. luteus was negative allometric (b < 3), in agreement with the study carried out by Noleto-Filho et al. (2012) (b = 2.25) for the same species captured in the estuary of São José de Ribamar, Maranhão. According to Cantanhêde et al. (2007), fish exhibiting negative allometric growth tend to develop a more elongated body shape. This type of growth is often linked to adaptive strategies, as suggested by Viana et al. (2006) and Carvalho et al. (2017). In the case of G. luteus, the negative allometric growth observed may result from low predation pressure from other organisms, which supports its role as a top predator in the food chain within the Paciência River estuary. Additionally, Teixeira et al. (2024) state that the reproductive period of the species may significantly influence the allometric coefficient (b), as G. luteus drastically reduces its feeding rate to allocate energy for reproduction.
The sex ratio showed the predominance of females (1.7F: 1M), not in all months, occurring with a higher incidence in April, August, and November 2016, whereas males were more found in January and September 2016. This result agrees with the study by Fernandes et al. (2017), in which they found a predominant proportion of females (1:3.89) in the region of Pará between 2002 and 2003. On the other hand, Sousa et al. (2017) identified a ratio of 1:1 in the Paciência River estuary.
Peaks of reproductive activity observed through the GSI and K occurred in months of high rainfall (April 2016 and 2017). According to Braga (2001), pluviometric activity with more intense rain can trigger the spawning process, causing greater reproductive activity of fish, especially when they find the most appropriate environmental conditions for maintaining their stocks (Lucas and Baras, 2001). Silva et al. (2018) observed that G. luteus captured in the Baia de São Marcos - MA region can tolerate seasonal variations, having a record of capture (species abundance) throughout the year. This factor is widely utilized in fish biology studies as it offers crucial insights into the physiological state of these animals, operating under the assumption that individuals with greater weight and length are in better condition (Lima-Junior et al., 2002; Queirós et al., 2024). In contrast, if the fished population does not show isometric growth, Fulton's K factor demonstrates a direct dependence on the length of the fish (Froese, 2006; Lloret et al., 2013). Notably, the Fulton factor was selected over another factor due to the minimal variation in the length of fish captured within the sample size.
According to Santos (1978), there is no specific size that determines when individuals initiate their sexual maturity; instead, it is a relative frequency of adults that increases accordingly with length. The values found in the present study for the L50 of males and females of the species G. luteus are consistent with those presented by Teixeira et al. (2024). On the other hand, the estimated value of L50 for the grouped sexes was 12.55 cm. By contrast, the research by Gómez et al. (2002) found a length of first maturity greater than that of the present study, equal to 34.5 cm in the Gulf of Paria region (Venezuela). According to Camargo and Lima Junior (2007), the decrease in the L50 could mean a potential danger of overfishing. This decrease may indicate that fish are reaching the minimum length of first maturation early as a need for stock renewal or that they may belong to another stock, and the L50 may vary between stocks (Ikeda, 2003).
Establishing a maturity scale is crucial for understanding and predicting the behavioral variations that the population undergoes throughout the year (Cavalcanti, 1994). The ovarian organization appeared to be asynchronous. Due to the species’ intermittent reproductive behavior, which spawns multiple times throughout the reproductive period, histological observations conducted in this study revealed oocytes at all stages of oocyte development, including oocytes in primary/young growth, secondary/pre-vitellogenic growth, in the cortical alveolus phase, and finally, vitellogenic. The presence of post-ovulatory follicles in fish ovarian tissue, according to Bazzoli and Godinho (1991), is indicative of recent spawning. The spawning of the species above occurs staggered over a long period, with the potential to spawn throughout the year under conditions of adequate water quality and nutrition. Consequently, upon maturity, the ovary exhibits oocytes in all stages of oocyte development (Reid and Holdway, 1995); a situation was observed in the present study, where oocytes at all stages of development could be observed on the same slide.
The results of this study provide essential data for the fisheries management of Geniatremus luteus. The negative allometry (b < 3) indicates that this species prioritizes length growth overweight, influencing the optimal size and sexual maturity for capture. The average length at first sexual maturation (L50) was 12.66 cm for females and 12.32 cm for males. This suggests a minimum catch size of 13 cm, ensuring that most individuals can reproduce before being harvested. The gonadosomatic index (GSI) revealed reproductive peaks in females in July and males in April, correlated with periods of higher rainfall. This pattern suggests intensified reproductive activity during the first half of the year. Consequently, a closed season between April and August is recommended to protect peak spawning periods and allow stock replenishment.
5. Conclusions
With mean sizes at first maturity of 12.66 cm for females and 13.32 cm for males, implementing a minimum capture size and an appropriate closed season, especially during the rainy season, ensures that individuals reproduce before being captured, thus contributing to the sustainability of Geniatremus luteus fisheries. Due to limited specific information on the conservation status of G. luteus, the findings of this study play a significant role in understanding reproductive processes and provide a foundation for the development of public policies for fisheries management and planning, aimed at stock preservation and population sustainability.
References
-
ALMEIDA, Z.S., SANTOS, N.B., SOUSA, H.L., CARVALHO NETA, R.N.F. and ANDRADE, T.S.O.M., 2016. Biologia reprodutiva da pescada amarela (Cynoscion acoupa) capturada na baía de São Marcos, Maranhão, Brasil. Biota Amazônia, vol. 6, no. 1, pp. 46-54. http://doi.org/10.18561/2179-5746/biotaamazonia.v6n1p46-54
» http://doi.org/10.18561/2179-5746/biotaamazonia.v6n1p46-54 -
BARLETTA, M., BARLETTA-BERGAN, A., SAINT-PAUL, U. and HUBOLD, G., 2003. Seasonal changes in density, biomass, and diversity of estuarine fishes in tidal mangrove creeks of the lower Caeté Estuary (northern Brazilian coast, east Amazon). Marine Ecology Progress Series, vol. 256, pp. 217-228. http://doi.org/10.3354/meps256217
» http://doi.org/10.3354/meps256217 - BAZZOLI, N. and GODINHO, H.P., 1991. Reproductive biology of the Acestrorhynchus lacustris (Reinhardt, 1874) (Pisces, Characidae) from Três Marias Reservoir, Brazil. Zoologischer Anzeiger, vol. 226, pp. 285-297.
-
BENTES, B., ISAAC, V.J., ESPÍRITO-SANTO, R.V., FRÉDOU, T., ALMEIDA, M.C., MOURÃO, K.R.M. and FRÉDOU, F.L., 2012. Abordagem multidisciplinar para a identificação dos sistemas de produção pesqueira na costa Norte do Brasil. Biota Neotropica, vol. 12, no. 1, pp. 81-92. http://doi.org/10.1590/S1676-06032012000100006
» http://doi.org/10.1590/S1676-06032012000100006 - BOMFIM, A.C., FARIAS, D.S.D., MORAIS, I.C.C., ROSSI, S., GAVILAN, S.A. and SILVA, F.J.L., 2019. The impact of shrimp trawl bycatch on fish reproduction in northeastern Brazil. Biota Amazônia, vol. 9, pp. 37-42. http://doi.org/10.18561/2179-5746/biotaamazonia.v9n1p37-42.
-
BRAGA, F.M.S., 2001. Reprodução de peixes (Osteichthyes) em afluentes do reservatório de Volta Grande, Rio Grande, sudeste do Brasil. Iheringia. Série Zoologia, vol. 91, no. 91, pp. 67-74. http://doi.org/10.1590/S0073-47212001000200009
» http://doi.org/10.1590/S0073-47212001000200009 -
BROWN-PETERSON, N.J., WYANSKI, D.M., SABORIDO-REY, F., MACEWICZ, B.J. and LOWERRE-BARBIERI, S.K., 2011. A standardized terminology for describing reproductive development in fishes. Marine and Coastal Fisheries, vol. 3, no. 1, pp. 52-70. http://doi.org/10.1080/19425120.2011.555724
» http://doi.org/10.1080/19425120.2011.555724 -
CÁMARA, A. and SANTERO-SÁNCHEZ, R., 2019. Economic, social, and environmental impact of a sustainable fisheries model in Spain. Sustainability, vol. 11, no. 22, pp. 6311. http://doi.org/10.3390/su11226311
» http://doi.org/10.3390/su11226311 -
CAMARGO, M. and LIMA JUNIOR, W.M.A., 2007. Aspectos da biologia reprodutiva de seis espécies de peixes de importância comercial do médio rio Xingu: bases para seu manejo. Uakari, vol. 3, no. 1, pp. 64-77. http://doi.org/10.31420/uakari.v3i1.20
» http://doi.org/10.31420/uakari.v3i1.20 -
CANTANHÊDE, G., CASTRO, A.C.L. and GUBIANI, E.A., 2007. Biologia reprodutiva de Hexanematichthys proops (Siluriformes, Ariidae) no litoral ocidental maranhense. Iheringia. Série Zoologia, vol. 97, no. 4, pp. 498-504. http://doi.org/10.1590/S0073-47212007000400021
» http://doi.org/10.1590/S0073-47212007000400021 -
CARVALHO, I.F.S., CANTANHÊDE, L.G., DINIZ, A.L.C., CARVALHO-NETA, R.N.F. and ALMEIDA, Z.S., 2021. Reproductive biology of seven fish species of commercial interest at the Ramsar site in the Baixada Maranhense, Legal Amazon, Brazil. Neotropical Ichthyology, vol. 19, no. 2, pp. e200067. http://doi.org/10.1590/1982-0224-2020-0067
» http://doi.org/10.1590/1982-0224-2020-0067 -
CARVALHO, I.F.S., CANTANHÊDE, L.G., SANTOS, N.B., CARVALHO-NETA, R.N.F. and ALMEIDA, Z.S., 2017. Biologia reprodutiva de Plagioscion squamosissimus (Pisces, Sciaenidae) em uma área de proteção ambiental do Nordeste do Brasil. Boletim do Instituto de Pesca, vol. 43, no. 2, pp. 243-256. http://doi.org/10.20950/1678-2305.2017v43n2p243
» http://doi.org/10.20950/1678-2305.2017v43n2p243 - CARVALHO-NETA, R.N.F., NUNES, J.L.S. and PIORSKY, N.M., 2011. Peixes estuarinos do Maranhão. In: J.L.S. NUNES and N.M. PIORSKI, eds. Peixes marinhos e estuarinos do Maranhão São Luís: Café & Lápis, 224 p.
-
CAVALCANTE, L.F.M., OLIVEIRA, M.R. and CHELLAPPA, S., 2012. Aspectos reprodutivos do Ariacó, Lutjanus synagris nas águas costeiras do Rio Grande do Norte. Biota Amazônia, vol. 2, no. 1, pp. 45-50. http://doi.org/10.18561/2179-5746/biotaamazonia.v2n1p45-50
» http://doi.org/10.18561/2179-5746/biotaamazonia.v2n1p45-50 - CAVALCANTI, D.G., 1994. Reprodução do cascudo cinza Liposarcus ansisti (Holberg, 1893) (Loricariidae, Siluriforme): histologia de gônadas e fatores abióticos Jaboticabal: Universidade Estadual Paulista, 124 p. Dissertação.
- FERNANDES, G.L., 1982. Sobre a alimentação do peixe-pedra, Genyatremus luteus (Bloch, 1795) Jordan & Feisler 1893 (Teleostei, Pomadasyidae). Boletim do Laboratório de Hidrobiologia, vol. 4, pp. 65-76.
-
FERNANDES, S.C.P., BENTES, A.B., PEREIRA, L.J.G., NASCIMENTO, M.S. and SILVA, B.B., 2017. Biologia populacional do peixe-pedra, Cenyatremus luteus (bloch, 1790), na costa amazônica brasileira. Boletim do Instituto de Pesca, vol. 43, no. 4, pp. 527-541. http://doi.org/10.20950/1678-2305.2017v43n4p527
» http://doi.org/10.20950/1678-2305.2017v43n4p527 - FERREIRA, H.O., 1989. Contribuição ao estudo das correntes de maré do estreitos de Coqueiros e Mosquitos São Luís: LABOHIDRO, 7 p.
-
FROESE, R., 2006. Cube law, condition factor and weight-length relationships: history, meta-analysis and recommendations. Journal of Applied Ichthyology, vol. 22, no. 4, pp. 241-253. http://doi.org/10.1111/j.1439-0426.2006.00805.x
» http://doi.org/10.1111/j.1439-0426.2006.00805.x - GOMÉZ, G., GUZMAN, R. and CHACON, R., 2002. Algunos aspectos de La biologia reproductiva y poblacional del torroto, Genyatremus luteus, (Block, 1797) (Pisces: Haemulidae) em el golfo de Parla, Venezuela. Zootecnia Tropical, vol. 20, pp. 223-234.
- HARDARDOTTIR, K., KJESBU, O.S. and MARTEINSDOTTIR, G., 2001. Relationship between atresia, fish size and condition in Icelandic cod (Gadus morhua L.). London: ICES CIEM. CM2001/J:19.
-
IKEDA, R.G.P., 2003 [viewed 10 May 2024]. Idade, crescimento e aspectos reprodutivos de Macrodon ancylodon (Bloch e Schneider, 1801), na Costa Norte do Brasil [online]. São Paulo: Universidade de São Paulo, 131 p. Dissertação de Mestrado em Ciências. Available from: http://www.teses.usp.br/teses/disponiveis/21/21131/tde-26062008-150356/
» http://www.teses.usp.br/teses/disponiveis/21/21131/tde-26062008-150356/ -
INSTITUTO NACIONAL DE METEOROLOGIA – INMET [online], 2024 [viewed 10 May 2024]. Available from: http//www.inmet.gov.br
» http//www.inmet.gov.br -
JIMENEZ, E.A., ASANO FILHO, M. and FRÉDOU, F.L., 2013. Fish bycatch of the laulao catfish Brachyplatystoma vaillantii (Valenciennes, 1840) trawl fishery in the Amazon Estuary. Brazilian Journal of Oceanography, vol. 61, no. 2, pp. 129-140. http://doi.org/10.1590/S1679-87592013000200005
» http://doi.org/10.1590/S1679-87592013000200005 -
KEYS, A.B., 1928. The weight-length relationship in fishes. Proceedings of the National Academy of Sciences of the United States of America, vol. 14, no. 12, pp. 922-925. http://doi.org/10.1073/pnas.14.12.922 PMid:16587425.
» http://doi.org/10.1073/pnas.14.12.922 - LIMA-JUNIOR, S.E., CARDONE, I.B. and GOITEIN, R., 2002. Determination of a method for calculation of allometric condition factor of fish. Acta Scientiarum: Biological and Health Sciences, vol. 24, pp. 397-400. http://doi.org/10.4025/actascibiolsci.v24i0.2311.
-
LLORET, J., SHULMAN, G. and LOVE, R.M., 2013. Condition and health indicators of exploited marine fishes Oxford: Willey Blackwell. http://doi.org/10.1002/9781118752777
» http://doi.org/10.1002/9781118752777 -
LOPES, J.R., 2016. Bacias hidrográficas e climatologia no Maranhão. São Luís: Universidade Estadual do Maranhão, vol. 1, pp. 1-28. http://doi.org/10.55905/rdelosv17.n59-037
» http://doi.org/10.55905/rdelosv17.n59-037 -
LUCAS, M.C. and BARAS, E., 2001. Migration of freshwater fishes Malden: Blackwell Science, 440 p. http://doi.org/10.1002/9780470999653
» http://doi.org/10.1002/9780470999653 -
MADDOCK, D.M. and BURTON, M.P., 1998. Gross and histological observations of ovarian development and related condition changes in American plaice. Journal of Fish Biology, vol. 53, no. 5, pp. 928-944. http://doi.org/10.1111/j.1095-8649.1998.tb00454.x
» http://doi.org/10.1111/j.1095-8649.1998.tb00454.x -
MARCENIUK, A.P., CAIRES, A.R., WOSIACKI, W.B. and DARIO, F.D.N., 2013. Conhecimento e conservação dos peixes marinhos e estuarinos (Chondrichthyes e Teleostei) da costa norte do Brasil. Biota Neotropica, vol. 13, no. 4, pp. 251-259. http://doi.org/10.1590/S1676-06032013000400022
» http://doi.org/10.1590/S1676-06032013000400022 - NOLETO-FILHO, E.M., YAURI, W.L.M. and SANTOS, R.L., 2012. Captura de reprodutores de peixe-pedra Genyatremus luteus (Block, 1797) (Pisces: Haemulidae) e manutenção em sistema fechado. Boletim do Laboratório de Hidrobiologia, vol. 25, pp. 55-60.
-
NUNES, Y.B.S., ARANHA, M.B., FREITAS, J., FERNANDES, J.F.F., SILVA, L.R. and BEZERRA, M.F., 2020. Length at first sexual maturity of economically important fishes in the Brazilian Northeast Coast. Ocean and Coastal Research, vol. 68, e20311. http://doi.org/10.1590/s2675-28242020068311
» http://doi.org/10.1590/s2675-28242020068311 - PINHEIRO, M.S.S. 2010. Ciclo de vida e estrutura de uma assembléia de peixes teleósteos em um manguezal da Raposa, Maranhão, Brasil. Rio Claro: Instituto de Biociências, Universidade Estadual Paulista, 180 p. Tese de Doutorado em Ciências Biológicas.
-
QUEIRÓS, K.B.N., SANTOS-ESPÍNOLA, N.B., RIBEIRO, E.B., COSTA, A.P., CARVALHO NETA, R.N.F. and ALMEIDA, Z.S., 2024. First sexual maturity and type of spawning of the fish Conodon nobilis (Linnaeus, 1758) from the Amazon coast – Brazil. Brazilian Journal of Biology = Revista Brasileira de Biologia, vol. 84, e284107. http://doi.org/10.1590/1519-6984.284107
» http://doi.org/10.1590/1519-6984.284107 -
REID, H.P. and HOLDWAY, D.A., 1995. Early development of the Australian crimsonspotted rainbowfish, Melanotaenia fluviatilis (Pisces: Melanotaeniidae). Marine and Freshwater Research, vol. 46, no. 2, pp. 475-480. http://doi.org/10.1071/MF9950475
» http://doi.org/10.1071/MF9950475 - SANTOS, E.P., 1978. Dinâmica de populações aplicada à pesca e piscicultura São Paulo: HUCITEC, 130 p.
- SECKENDORFF, R.W.V. and AZEVEDO, V.G., 2007. Abordagem histórica da pesca da tainha Mugil platanus e do parati Mugil curema (Perciformes: Mugilidae) no litoral norte do estado de São Paulo. Instituto de Pesca, vol. 28, pp. 1-8.
-
SILVA JÚNIOR, M.G., CASTRO, A.C.L., SOARES, L.S. and FRANÇA, V.L., 2007. Relação peso-comprimento de espécies de peixes do Estuário do Rio Paciência da Ilha do Maranhão, Brasil. Boletim do Laboratório de Hidrobiologia, vol. 20, pp. 31-38.
» https://doi.org/ -
SILVA, A.M.M., GLOVER, H.E., JOSTEN, M.E., GOMES, V.J.C., OGSTON, A.S. and ASP, N.E., 2023. Implications of a large river discharge on the dynamics of a tide-dominated Amazonian estuary. Water, vol. 15, no. 5, pp. 849. http://doi.org/10.3390/w15050849
» http://doi.org/10.3390/w15050849 -
SILVA, M.H.L., TORRES JÚNIOR, A.R., CASTRO, A.C.L., AZEVEDO, J.W.J., FERREIRA, C.F.C., CARDOSO, R.L., NUNES, J.L.S. and CARVALHO-NETA, R.N.F., 2018. Fish assemblage structure in a port region of the Amazonic coast. Iheringia. Série Zoologia, vol. 108, no. 0, pp. 1-11. http://doi.org/10.1590/1678-4766e2018018
» http://doi.org/10.1590/1678-4766e2018018 -
SOUSA, A.F.R., SANTOS, N.B., CARVALHO-NETA, R.N.F. and ALMEIDA, Z.S., 2017. Aspectos reprodutivos do peixe Lutjanus synagris (Perciformes, Lutjanidae) capturado na Costa Nordeste do Brasil. Revista Brasileira de Engenharia de Pesca, vol. 10, no. 1, pp. 106-120. http://doi.org/10.18817/repesca.v10i1.1369
» http://doi.org/10.18817/repesca.v10i1.1369 -
TEIXEIRA, M.J., SILVA, M.H.L., CASTRO, A.C.L., ÂNDRADE, M., LOPES, Y.V.A., MARINHO, Y.F., SILVA, E.P., ESCHRIQUE, S.A. and AZEVEDO, J.W.J., 2024. Aspects of population dynamics for two species of perciforms (Genyatremus luteus and Macrodon ancylodon), from the stopping fishery in a Macro-Tidal environment belonging to the Amazon coast. DELOS Desarrollo Local Sostenible, vol. 17, no. 51, pp. 49-73. http://doi.org/10.55905/rdelosv17.n51-004
» http://doi.org/10.55905/rdelosv17.n51-004 - VIANA, A.P., FRÉDOU, T. and LUCENA, F., 2006. Aplicações de técnicas morfométricas no estudo da morfometria de pescada branca, Plagioscion squamosissimus, Heckel (1940), Perciformes, Sciaenidae, desembarcada na Ilha de Mosqueiro-PA. Boletim do Laboratório de Hidrobiologia, vol. 19, pp. 1-12.
- VON BERTALANFFY, L., 1938. A quantitative theory of organic growth (inquiries on growth laws II). Human Biology, vol. 10, pp. 181-213.
-
WOLFF, M., KOCH, V. and ISAAC, V., 2000. A trophic flow model of the Caeté mangrove estuary (north Brazil) with considerations for the sustainable use of its resources. Estuarine, Coastal and Shelf Science, vol. 50, no. 6, pp. 789-803. http://doi.org/10.1006/ecss.2000.0611
» http://doi.org/10.1006/ecss.2000.0611
Publication Dates
-
Publication in this collection
07 Feb 2025 -
Date of issue
2024
History
-
Received
10 May 2024 -
Accepted
15 Nov 2024

 Population structure and reproductive indicators of Genyatremus luteus (Pisces: Haemulidae) in the Brazilian Amazon coast
Population structure and reproductive indicators of Genyatremus luteus (Pisces: Haemulidae) in the Brazilian Amazon coast