Abstract
Research work was designed to investigate the density and diversity of pelagic rotifers in a Lake near Marala Headworks. The physico-chemical parameters of water such as pH, dissolved oxygen, temperature, electrical conductivity, transparency and turbidity were evaluated. Correlation between rotifers and these parameters was also studied. Plankton sampling was done on monthly basis in order to check the population density of rotifers. In total, 18 species of rotifers were identified which belonged to 11 genera. The highest number of rotifers and their diversity was shown by genera namely Brachionus, Keratella, and Filinia. The Brachionus calyciflorus was dominant species in all the samples with mean population density (41%). Analysis of variance of physico-chemical parameters presented that the air and water temperature, electrical conductivity, transparency, dissolved oxygen and oxygen saturation were statistically significant in all the months. While pH was statistically non-significant (p≥0.05. Pearson correlation showed that oxygen and transparency were negatively correlated with rotifers density and diversity. Air and water temperature, concentration of hydrogen ions (pH), electrical conductivity and salinity showed positive relationship with density and diversity of rotifers.
Keywords:
pelagic rotifers; biodiversity; Marala Headworks; river Chenab; physical-chemical parameters
Resumo
O trabalho de pesquisa foi projetado para investigar a densidade e diversidade de rotíferos pelágicos em um lago perto de Marala Headworks. Foram avaliados os parâmetros físico-químicos da água como pH, oxigênio dissolvido, temperatura, condutividade elétrica, transparência e turbidez. A correlação entre rotíferos e esses parâmetros também foi estudada. A amostragem do plâncton foi realizada mensalmente para verificar a densidade populacional dos rotíferos. No total, foram identificadas 18 espécies de rotíferos pertencentes a 11 gêneros. O maior número de rotíferos e sua diversidade foi demonstrado pelos gêneros Brachionus, Keratella e Filinia. O Brachionus calyciflorus foi a espécie dominante em todas as amostras, com densidade populacional média (41%). A análise de variância dos parâmetros físico-químicos mostrou que a temperatura do ar e da água, condutividade elétrica, transparência, oxigênio dissolvido e saturação de oxigênio foram estatisticamente significantes em todos os meses. Enquanto o pH foi estatisticamente não significativo (p≥0,05), a correlação de Pearson mostrou que o oxigênio e a transparência foram negativamente correlacionados com a densidade e diversidade dos rotíferos. A temperatura do ar e da água, a concentração de íons de hidrogênio (pH), a condutividade elétrica e a salinidade mostraram relação positiva com a densidade e diversidade de rotíferos.
Palavras-chave:
rotíferos pelágicos; biodiversidade; Marala Headworks; rio Chenab; parâmetros físico-químicos
1. Introduction
Rotifera is a phylum of freshwater microscopic metazoans. The word “rotifer” procured from a Latin word meaning “wheel-bearer”, because of ciliated corona around the mouth, rotifers show wheel like motion. Rotifers are triploblastic, pseudocoelomate and eutelic. Their size varies from 200 to 500 µm. Rotifers are distinctly important group of littoral and limnetic micro-invertebrate (Wallace and Snell, 2010WALLACE, R.L. and SNELL, T.W., 2010. Rotifera. In: J.H. THORP and A.P. COVICH, eds., Ecology and classification of North American freshwater invertebrates. Oxford: Elsevier, chap. 8, pp. 173-235. http://dx.doi.org/10.1016/B978-0-12-374855-3.00008-X.
http://dx.doi.org/10.1016/B978-0-12-3748...
), with almost 95% of the familiar rotifer species are present in freshwater which are brought to be their native habitat. Rotifers adjust themselves to different types of environmental conditions and planktonic forms are usual in surface water (Pejler, 1995PEJLER, B., 1995. Relation to habitat in rotifers. Hydrobiologia, vol. 313, no. 1, pp. 267-278. http://dx.doi.org/10.1007/BF00025959.
http://dx.doi.org/10.1007/BF00025959...
; Wallace and Snell, 2010WALLACE, R.L. and SNELL, T.W., 2010. Rotifera. In: J.H. THORP and A.P. COVICH, eds., Ecology and classification of North American freshwater invertebrates. Oxford: Elsevier, chap. 8, pp. 173-235. http://dx.doi.org/10.1016/B978-0-12-374855-3.00008-X.
http://dx.doi.org/10.1016/B978-0-12-3748...
) with a high density and population diversity, with enormous capacity to tolerate environmental conditions (Neves et al., 2003NEVES, I.F., ROCHA, O., ROCHE, K.F. and PINTO, A.A., 2003. Zooplankton community structure of two marginal lakes of the River Cuiabá (Mato Grosso, Brazil) with analysis of Rotifera and Cladocera diversity. Brazilian Journal of Biology = Revista Brasileira de Biologia, vol. 63, no. 2, pp. 329-343. http://dx.doi.org/10.1590/S1519-69842003000200018. PMid:14509855.
http://dx.doi.org/10.1590/S1519-69842003...
).
Their density may occur up to 1000 organisms per liter and are prime filter feeders on bacteria and algae (Wallace et al., 2006WALLACE, R.L., SNELL, T.W., RICCI, C. and NOGRADY, T., 2006. Rotifera. In: H. SEGERS, ed. Guides to the identification of the microinvertebrates of the continental waters of the world. Leiden: Kenobi Productions, Ghent and Backhuys Publishers, vol. 1, Biology, ecology and systematics.). Rotifers play an important part in freshwater ecosystem. Rotifers are opportunistic and pervasive organism, occuring almost in all types of freshwater habitats. Some parasitic species are also found. The phylum rotifera consist of three classes, 120 genera and 2150 species (Barnes et al., 2001BARNES, R.S.K., CALOW, P., OLIVE, P.J.W., GOLDING, D.W. and SPICERS, J.I., 2001. The invertebrates: a synthesis. Oxford: Blackwell, 98 p.). The three classes are Seisonidae, Bdelloidea and Monogononta. The class Seisonidae contains only 3 species, there are 461 species of class Bedelloidea and only one species among these is marine. Monogononta have 1,570 species in which 1,488 species are free living and freshwater taxa (Segers, 2008SEGERS, H., 2008. Global diversity of rotifers (Rotifera) in freshwater. Hydrobiologia, vol. 595, no. 1, pp. 49-59. http://dx.doi.org/10.1007/s10750-007-9003-7.
http://dx.doi.org/10.1007/s10750-007-900...
). Exact figure of this group may be higher, considering the prevalence of cryptic phylogeny in Monogononta alongwith contradictory type of taxonomic information.
Water quality and biological characteristics are linked with diversity and density of living organisms (Dhanasekaran et al., 2017DHANASEKARAN, M., BHAVAN, P.S., MANICKAM, N. and KALPANA, R., 2017. Physico-chemical characteristics and zooplankton diversity in a perennial lake at Dharmapuri (Tamil Nadu, India). Journal of Entomology and Zoology Studies, vol. 5, no. 1, pp. 285-292.). Variety of organisms can give a clear sign of human interruption in a natural ecosystem (Chughtai et al., 2011CHUGHTAI, M.I., MAHMOOD, K. and AWAN, A.R., 2011. Assessment of planktonic diversity in River Chenab as affected by sewage of Multan city. Pakistan Journal of Botany, vol. 43, no. 5, pp. 2551-2555.). They transfer the energy from producers like algae and bacteria to consumers like small fish, insects and crustacean (Baloch et al., 2005BALOCH, W.A., JAFRI, S.I.H. and SOOMRO, A.N., 2005. Spring zooplankton composition of Rawal Lake, Islamabad. Sindh University Research Journal, vol. 37, no. 2, pp. 41-46.). Rotifers are a rich source of lipids, minerals and proteins. Instead of fishmeal, they can be used as a cheap source of food for cultured fish (Fernando, 1994FERNANDO, C.H., 1994. Zooplanktons, fish and fisheries in Tropical freshwaters. Hydrobiologia, vol. 272, no. 1-3, pp. 105-123. http://dx.doi.org/10.1007/BF00006516.
http://dx.doi.org/10.1007/BF00006516...
; Kibria et al., 1997KIBRIA, G., NUGEOGODA, D., FAIRCLOUGH, R., LAM, P. and BRADLY, A., 1997. Zooplanktons: its biochemistry and significance in Aquaculture. Naga, vol. 20, no. 2, pp. 8-14.). Under favorable conditions like photoperiod, availability of suitable temperature and food, the life cycle of rotifers is very short. Temperature and abundance of food are related to the rate of reproduction and survival of rotifers. In 1702, Leeuwenhoek observed that rotifers have capability to tolerate desiccation for many months. Rotifers undergo a period of dormancy without water. This state is called cryptobiosis. Rotifers are found in three forms mictic female, amictic female and male. They reproduce sexually however parthenogenesis do occur (Glime, 2013GLIME, J.M., 2013. Invertebrates: Rotifers. In: J.M. GLIME, ed. Bryophyte ecology. vol. 2. Bryological Interaction, chapt. 4-5. Ebook sponsored by Michigan Technological University and the International Association of Bryologists.).
Rotifers are abundant in lake having ample food resources. Certain important factors such as food resources, competitors, predators and physico-chemical parameters have great effect on the succession of rotifers (Sugumaran and Amsath, 2015SUGUMARAN, J. and AMSATH, A., 2015. Seasonal diversity of rotifers from agniyar estuary, Thanjavur District, Tamil Nadu, India. International Journal of Pure and Applied Zoology, vol. 3, no. 4, pp. 287-292.). Environmental factors, e.g., pH and temperature also affect rotifer community. Since various factors shape up rotifer population in a lake, so it is necessary to include diverse factors into research at one time by making a comparison of their contribution in formation of rotifer community. Many water bodies such as river, lake, flood plains etc. have been studied for rotifer diversity. In standing fresh waters, there is slow water exchange. Therefore, these are particularly sensitive to inputs like pollutants, toxins, nutrients and fertilizers. Effects of pollution and human interference on water reservoirs have reported serious concerns. Little information exists about rotifer fauna of Pakistan. Therefore the objectives of current study were to (i) check the physico-chemical parameters of the lake, (ii) collect rotifers from Marala Headworks (Pakistan) (iii) identify and determine the seasonal variation in rotifer density with respect to physicochemical nature of lake water.
2. Material and Methods
2.1. Research area
Marala is a hydro-engineering project and its important role to control the flood and flow of water in the River Chenab. The Marala Headworks is situated at the River Chenab near Sialkot city, Punjab, Pakistan. The river Chenab is 1,086 km long with latitude 32.6733 °N, 74.4636 °E. It starts from Kulu and Kangra Districts of Himachal Pradesh in India. It take its way to occupied Jammu & Kashmir. After passing PirPanjal Mountains, it arrives in the Sialkot District of Pakistan. The Marala Headworks was built in 1968 across the river.
2.2. Sites selection and time period
The lake which was selected for sampling is 900 m long in North-East side and 125 m wide in South-West side. It was divided into four sampling sites. Each site was 125 m apart. These sites were named as MS1, MS2, MS3, and MS4. The total length of the sampling site was 500 m. Each site was further divided into three sub-sites named as A, B and C (Figure 1). Water sampling was done from September to August by checking monthly variations with the purpose to check the existing condition of water. Sampling was done between 11 A.M. to 2 P.M, mostly in the 2nd but sometimes in the 3rd week of each month.
2.3. Measurement of physico-chemical parameters
Water physicochemical parameters such as dissolved oxygen, concentration of hydrogen ions (pH), temperature, oxygen saturation, salinity, transparency and electrical conductivity of water were measured.
2.4. Sampling of pelagic rotifers
Sampling was done with the help of zooplankton net from water following procedures by Koste (1978)KOSTE, W., 1978. Rotatoria. Die Rädertiere Mitteleuropas, begründet von Max Voigt. Überordnung Monogononta. Berlin: Gebrüder Borntraeger, I. Text U. II. Tafelbed. (T. 234), 673 p. and Sulehria and Malik (2012)SULEHRIA, A.Q.K. and MALIK, M.A., 2012. Population dynamics of planktonic rotifers in Balloki headworks. Pakistan Journal of Zoology, vol. 44, no. 3, pp. 663-669.. The mesh size of the net was 37 µm and about 599.12 m3 capacity by volume. The length of zooplankton net was 85cm and its base had a diameter of 30cm. The sampling was done by towing the net horizontally so that approximately, 40 litres of water had passed through it. The water samples were preserved in 50 ml plastic bottles having 5% buffered formalin. In order to study live organisms, some planktonic samples were kept without formalin.
2.5. Identification of rotifers
Samples of preserved rotifers were examined and identified by using OLYMPUS compound microscope method following Segers (2007)SEGERS, H., 2007. Annotated checklist of the rotifers (Phylum Rotifera), with notes on nomenclature, taxonomy and distribution. Zootaxa, vol. 1564, no. 1, pp. 1-104. http://dx.doi.org/10.11646/zootaxa.1564.1.1.
http://dx.doi.org/10.11646/zootaxa.1564....
. Vital stain i.e., 1% neutral red were used to stain the live specimens to study their internal features. Rotifers were identified up to species level on the basis of their morphological characteristics. The rotifers images were taken with the help microscope having 5.0 megapixel camera.
2.6. Counting of rotifers
Rotifers were counted with the help of Sedgewick-Rafter chamber by using an OLYMPUS microscope. In short, Sedgewick- Rafter chamber was filled with sample by using a pipette. It was covered with cover slip carefully to avoid air bubble. After 2 to 4 minutes, the sample settled and counting was started. The rotifers density was checked by applying the Formula 1:
where, C = Counted specimens, L = Strip length, D = Depth of the strip, W = Strip width in mm, S = Number of counted strips, V1 = (50) (1) (W) = mm3.
2.7. Statistical survey
All experiments were performed in triplicate. Pearson’s correlation was used to determine the relationship between rotifer species and physico-chemical parameters. Dendrogram was plotted for comparative study of major rotifers species. Relative abundance of species in different months was noted by drawing species abundance curve. Analysis of variance (ANOVA) was used to determine the level of significance at p ≤ 0.05. Means and standard error were calculated using Microsoft Excel 2019.
3. Results
3.1. Cluster analysis (Dendrogram)
Dendrogram (cluster analysis) showed 7 major clusters of 18 rotifers species at eucladian distance 3. Cluster 1 was represented by one species Brachionus calyciflorus, cluster 2 consisted of Keratella valga, Epiphanes branchionus, B. angularis, cluster 3 was composed of Philodina roseola, B. quadridentatus, Euclanis dilatata, B. falcatus, cluster 4 had Polyarthra vulgaris, Lecane lunaris, K. cochlearis, Filinia longiseta, cluster 5 comprised of Asplanchna priodonta, Filinia minuta, Keratella quadrata, Trichocerca longiseta, cluster 6 consisted of one species Lecane quadridentata, cluster 7 have also one species i.e. Trochosphaera solstitialis. At eucladian distance 22, all these clusters aggregated into single cluster (Figure 2).
3.1. Physico-chemical parameters
The relationship between rotifer species and physico-chemical parameters as determined by Pearson’s correlation is shown in Table 1. The highest air temperature was observed in June (38 ± 0.06 °C) and lowest in February (22 ± 0.07 °C). The water temperature was high in April (32 ± 0.33 °C) and low in February (15 ± 0.75 °C). Dissolved oxygen (mg/l) was observed to be high in December (11 ± 0.04 mg/l) and low in July (6 ± 0.02 mg/l). Conductivity (µs/cm) was found high in October (329 ± 1.69 µs/cm). Oxygen saturation (mgL)-1 was high in September (1.8 ± 0.23 mgL-1) and low in April (1.1 ± 0.37 mgL-1). pH value observed in all months, ranged from 6.5 to 7. The value of transparency was observed in all months from September to August and was ranged from 0.2 to 1 inch (Table 2).
3.2. Analysis of variance (ANOVA)
ANOVA values (F=49.32, P=0.000) for water temperatures, (F=75.87, P=0.000) for air temperature, (F=6.25, P=0.000) for dissolved oxygen, (F=0.66, P=0.762) for pH, (F=5.00, P=0.000) for oxygen saturation, (F=220, P=0.000) for electrical conductivity and (F = 20.73, P = 0.000) for transparency showed statistically significant difference among different rotifer species in different months (September-August). However no such variations were observed when aforementioned parameters were compared among species at different sites (Data not shown).
3.3. Population dynamics of rotifers
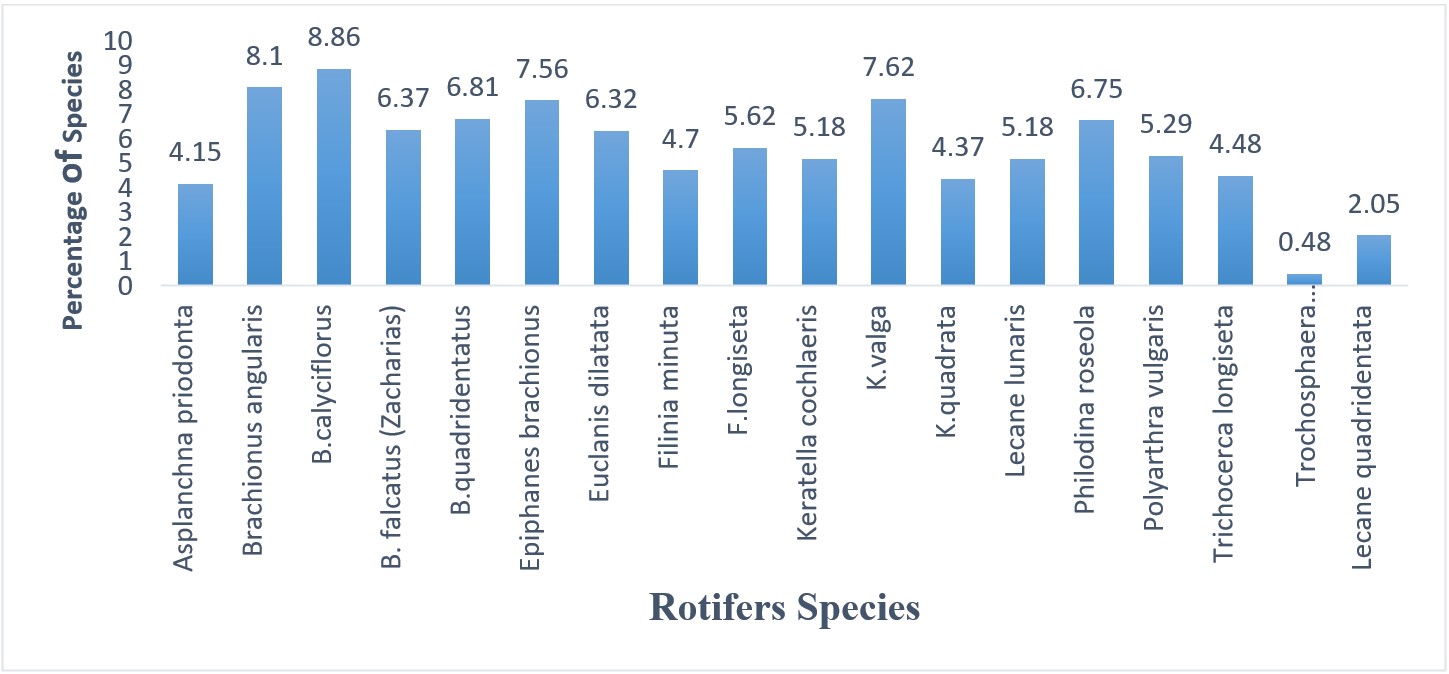
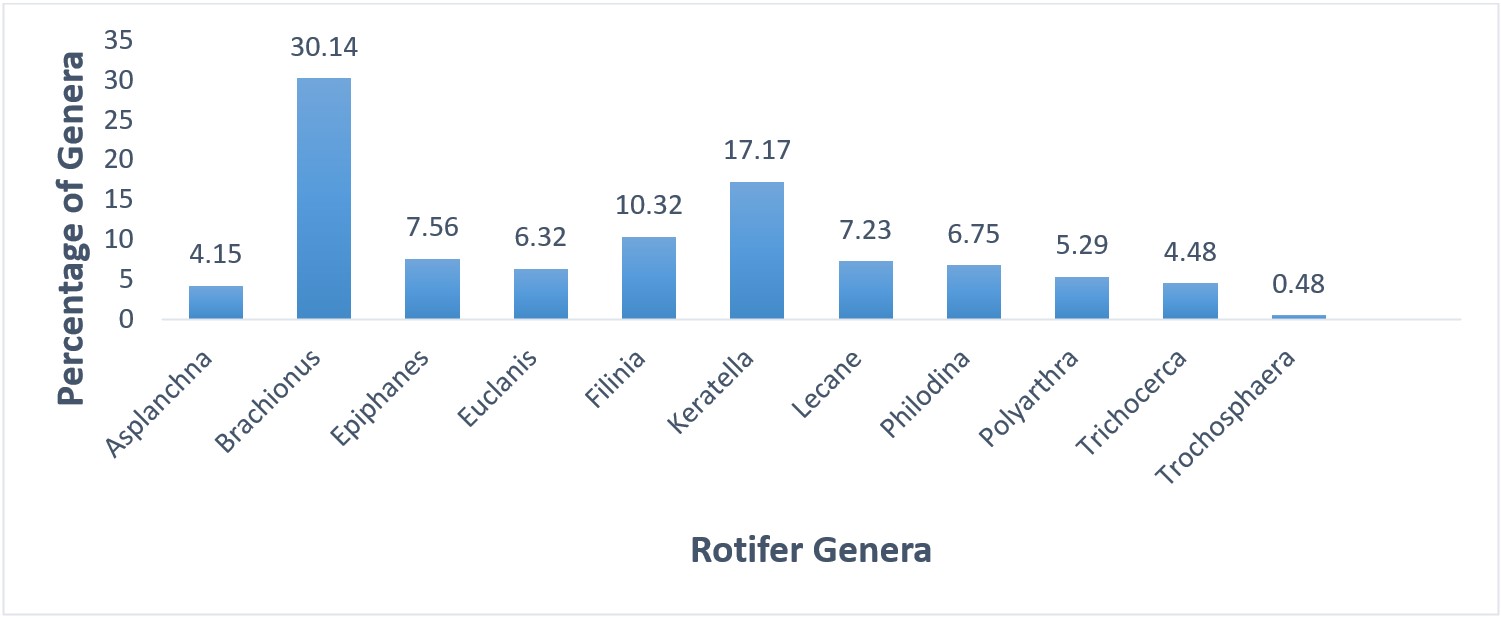
18 rotifer species belonging to 11 genera were identified in this study. Relative representation of rotifer species and genera is shown in Figures 3 and 4. Rotifer density was high in June (39.21ind/ml) and low in February (10.37 ind/ml). The comparative (%) presentation of rotifers species and their mean population density was in sequence of B. calyciflorus (8.86%) >B. angularis (8.1%) >Keratella valga (7.62%) >Epiphanes branchious (7.56%) >B. quadridentatus (6.81% (Figure 3). The comparative (%) representation of rotifer genera showed that dominant genera were in sequence of Brachionus (30.14%) >Keratella (17.17%) >Filinia (10.32% (Figure 4). In september, the dominant genus with mean percentage (29.26%) was Brachionus. In October, the dominant genus with mean percentage (30.95%) was Brachionus. Keratella was dominant in December (24.17%), Brachionus was dominant in January with mean percentage of 19.31%, Brachionus was dominant from February to August with mean percentage (26.08%, 29.9%, 28.4%, 34.68%, 37.12%, 28.75%, 30.25%, respectively (Data not shown).
3.4. Species abundance curve
Abundance curve of species was plotted between the months and the species of rotifers found in each month. The species abundance curve showed that the B. calyciflorus was found at the peak with abundance (41%). It showed its maximum abundance throughout the year. The T. solititialis was found at the end of the curve with least abundance (2.25%) in the whole year. The remaining species abundance lied between these two above mentioned values (Figure 5).
3.5. Impact of physico-chemical parameters on rotifers diversity and density
It was observed that air, water temperature, conductivity, oxygen saturation, salinity, and pH showed positive correlation with rotifers density and diversity. On the other hand, the dissolved oxygen and transparency were negatively correlated with rotifers (Figure 6).
4. Discussion
In the present research analysis, it was observed that population dynamics of rotifers was strongly correlated with physico-chemical parameters of the lake either in positive or negative way. This indicated that environmental factors had great impact on rotifers population (Zarfdjian, et al., 2000ZARFDJIAN, M.-H., MICHALOUDI, E., BOBORI, D.C. and MOURELATOS, S., 2000. Zooplankton abundance in the Aliakmon River, Greece. Belgian Journal of Zoology, vol. 130, no. 1, pp. 29-33.; Chittapun et al., 2007CHITTAPUN, S., PHOLPUNTHIN, P. and SEGERS, H., 2007. Diversity of rotifer fauna from five coastal peat swamps on Phuket Island, Southern Thailand. South Asia, vol. 33, pp. 383-387. http://dx.doi.org/10.2306/scienceasia1513-1874.2007.33.383.
http://dx.doi.org/10.2306/scienceasia151...
). Rotifers have great ability to adjust themselves in the changing environment (Allan, 1976ALLAN, J.D., 1976. Life history patterns in zooplankton. American Naturalist, vol. 110, no. 971, pp. 165-180. http://dx.doi.org/10.1086/283056.
http://dx.doi.org/10.1086/283056...
). Air and water temperature have great influence on the rotifer’s population. Air temperature of the lake near Marala Headworks varied from 20 °C to 38 °C and water temperature from 15 °C to 32 °C throughout the year. Positive correlation between temperature and population dynamics of rotifers was recorded in this study and correlate with findings by Scholl and Kiss (2008)SCHOLL, K. and KISS, A., 2008. Spatial and temporal distribution patterns of zooplankton assemblages (Rotifera, Cladocera, Copepoda) in the water bodies of the Gemenc floodplain (Duna-Dráva National Park, Hungary). Opuscula Zoologica, vol. 39, pp. 65-76., Sulehria et al. (2009aSULEHRIA, A.Q.K., QAMAR, M.F., ANJUM, R.F., EJAZ, M. and HUSSAIN, A., 2009a. Seasonal fluctuations of Rotifers in a fish pond at district Bahawalnagar, Pakistan. Biologia, vol. 55, no. 1-2, pp. 21-28., bSULEHRIA, A.Q.K., QAMAR, M.F., HAIDER, S., EJAZ, M. and HUSSAIN, A., 2009b. Water quality and Rotifer diversity in the fish pond at district Mianwali, Pakistan. Biologia, vol. 55, no. 1-2, pp. 79-85.) and Sulehria and Malik (2012)SULEHRIA, A.Q.K. and MALIK, M.A., 2012. Population dynamics of planktonic rotifers in Balloki headworks. Pakistan Journal of Zoology, vol. 44, no. 3, pp. 663-669..
Oxygen also had great impact on the rotifer’s abundance in water. The oxygen concentration varied from 6mg/L to 11mg/L throughout the year. It was observed that the dissolved oxygen concentration was high in winter while low in summer. Dissolved oxygen showed negative correlation with rotifers population. Similar findings were presented by Sulehria and Malik (2012)SULEHRIA, A.Q.K. and MALIK, M.A., 2012. Population dynamics of planktonic rotifers in Balloki headworks. Pakistan Journal of Zoology, vol. 44, no. 3, pp. 663-669.. Though, findings were entirely contradictory with the studies observed in the River Ravi (Lahore) by Sulehria et al. (2009a)SULEHRIA, A.Q.K., QAMAR, M.F., ANJUM, R.F., EJAZ, M. and HUSSAIN, A., 2009a. Seasonal fluctuations of Rotifers in a fish pond at district Bahawalnagar, Pakistan. Biologia, vol. 55, no. 1-2, pp. 21-28. & 2009b) where rotifers showed positive relation with dissolved oxygen. It was observed that the oxygen saturation was less in winter and more in summer, while varied from 1.1mg/L to 1.8mg/L throughout the year. Oxygen saturation had positive correlation with the rotifers population. Our findings are in agreement with the result of Javed and Hayat (1996)JAVED, M. and HAYAT, S., 1996. Planktonic productivity of river water as a bioindicator of freshwater contamination by metals. In: Proceedings of Pakistan Congress of Zoology, 1996, Pakistan. Punjab: Zoological Society of Pakistan, vol. 16, pp. 283-298.; and Baloch et al. (2008)BALOCH, W.A., SOOMRO, A.N. and BULEDI, G.H., 2008. Zooplankton, especially Rotifer and Cladoceran Communities of the spring and rainwater streams Nai) in Kirthar range, Sindh, Pakistan. Sindh University Research Journal, vol. 40, no. 1, pp. 17-22..
The pH value interfered with rotifer population and showed great impact on the density of rotifers. The suitable pH of rotifers varied from 6.5 to 8.5 (Barnes, 1974BARNES, R., 1974. Invertebrate zoology. 3rd ed. Philadelphia: W. B. Saunders Company, 870 p.; Berziņs and Pejler, 1987BERZIŅS, B. and PEJLER, B., 1987. Rotifer occurrence in relation to pH. Hydrobiologia, vol. 147, no. 1, pp. 107-116. http://dx.doi.org/10.1007/BF00025733.
http://dx.doi.org/10.1007/BF00025733...
; Neschuk et al., 2002NESCHUK, N., CLAPS, M. and GABELLONE, N., 2002. Planktonic rotifers of a saline-lowland river: the Salado River (Argentina). Ann. Limnol.-. International Journal of Limnology, vol. 38, no. 3, pp. 191-198. http://dx.doi.org/10.1051/limn/2002017.
http://dx.doi.org/10.1051/limn/2002017...
). The current research had a value ranged from 6.5 to 7. pH showed positive effect on the rotifer’s population. Similar findings were presented by Amsha Devi AND Suresh Kumar (2014), Sulehria et al. (2009a)SULEHRIA, A.Q.K., QAMAR, M.F., ANJUM, R.F., EJAZ, M. and HUSSAIN, A., 2009a. Seasonal fluctuations of Rotifers in a fish pond at district Bahawalnagar, Pakistan. Biologia, vol. 55, no. 1-2, pp. 21-28. and Sulehria and Malik (2012)SULEHRIA, A.Q.K. and MALIK, M.A., 2012. Population dynamics of planktonic rotifers in Balloki headworks. Pakistan Journal of Zoology, vol. 44, no. 3, pp. 663-669.. Our findings was contradictory to the past study by Sulehria et al. (2009b)SULEHRIA, A.Q.K., QAMAR, M.F., HAIDER, S., EJAZ, M. and HUSSAIN, A., 2009b. Water quality and Rotifer diversity in the fish pond at district Mianwali, Pakistan. Biologia, vol. 55, no. 1-2, pp. 79-85.. Transparency also showed negative correlation with rotifers population. Koli and Muley (2012)KOLI, B.K. and MULEY, V.D., 2012. Study of zooplankton diversity and seasonal variation with special reference to physicochemical parameters in Tulshi reservoir of Kolhapur District (M.S) India. International Scientific Research Journal, vol. 4, no. 1, pp. 38-49. also reported similar data. Salinity had also effect on rotifers abundance. It was observed that salinity was more in summer and less in winter ranged and showed positive correlation with rotifers population
Rotifers are considered as opportunistic and most abundant organisms among the major zooplankton groups (Allan, 1976ALLAN, J.D., 1976. Life history patterns in zooplankton. American Naturalist, vol. 110, no. 971, pp. 165-180. http://dx.doi.org/10.1086/283056.
http://dx.doi.org/10.1086/283056...
). The pelagic rotifers showed response very rapidly to the environmental fluctuation than other zooplankton (Gannon and Stemberger, 1978GANNON, J.E. and STEMBERGER, R.S., 1978. Zooplankton (especially crustaceans and rotifers) as indicator of water quality. Transactions of the American Microscopical Society, vol. 97, no. 1, pp. 16-35. http://dx.doi.org/10.2307/3225681.
http://dx.doi.org/10.2307/3225681...
). In present research analysis, 18 species of rotifers belongings to 11 genera were observed from four different sites. The most abundant genera were Brachionus>Keratella>Filinia. The relative (%) presentation of rotifers species and their mean population density was in sequence B. calyciflorus (41%) >B. angularis (37.5%) >Keratella valga (35.2%). Out of 18 species of rotifers, 4 species of genus Brachionus were present. Brachionus genus is considered as eutrophic and usually appears at higher status of eutrophication. Majority of the Brachionus species including B. angularis and B. calyciflorus were present in eutrophic condition. Presence of B. calyciflorus and B. quadridentatus is an indication of eutrophic state of the water body.
Brachionus calyciflorus was most prevalent species and its abundance was high during the summer season. There was an increase in composition and abundance of rotifers with temperature increase of the lake. Similar findings have also been observed in another study pond near Gujranwala. Though, this result was contradictory to other studies of water bodies around Aurangabad region (India) where species richness of rotifers was lowest in June.
5. Conclusion
From the current study, it is concluded that rotifers are important representatives of the structure and function of freshwater bodies such as lake and ecology. Rotifers may be considered as one of the rapidly flourishing metazoans and pioneer species. Density and diversity of rotifer species increases with the increase in food, temperature and pollution. This study also indicated relative abundance of B. calyciflorus, a representative of eutrophic conditions.
References
- ALLAN, J.D., 1976. Life history patterns in zooplankton. American Naturalist, vol. 110, no. 971, pp. 165-180. http://dx.doi.org/10.1086/283056
» http://dx.doi.org/10.1086/283056 - AMSHA DEVI, V. and SURESH KUMAR, R., 2014. Diversity of Rotifer (Rotifera) With Special Reference to Physico-Chemical Parameters from a Tropical Reservoir, Kullurchandai, Virudhunagar District, India. International Research Journal of Environmental Sciences, vol. 3, no. 5, pp. 80-85.
- BALOCH, W.A., JAFRI, S.I.H. and SOOMRO, A.N., 2005. Spring zooplankton composition of Rawal Lake, Islamabad. Sindh University Research Journal, vol. 37, no. 2, pp. 41-46.
- BALOCH, W.A., SOOMRO, A.N. and BULEDI, G.H., 2008. Zooplankton, especially Rotifer and Cladoceran Communities of the spring and rainwater streams Nai) in Kirthar range, Sindh, Pakistan. Sindh University Research Journal, vol. 40, no. 1, pp. 17-22.
- BARNES, R., 1974. Invertebrate zoology 3rd ed. Philadelphia: W. B. Saunders Company, 870 p.
- BARNES, R.S.K., CALOW, P., OLIVE, P.J.W., GOLDING, D.W. and SPICERS, J.I., 2001. The invertebrates: a synthesis Oxford: Blackwell, 98 p.
- BERZIŅS, B. and PEJLER, B., 1987. Rotifer occurrence in relation to pH. Hydrobiologia, vol. 147, no. 1, pp. 107-116. http://dx.doi.org/10.1007/BF00025733
» http://dx.doi.org/10.1007/BF00025733 - CHITTAPUN, S., PHOLPUNTHIN, P. and SEGERS, H., 2007. Diversity of rotifer fauna from five coastal peat swamps on Phuket Island, Southern Thailand. South Asia, vol. 33, pp. 383-387. http://dx.doi.org/10.2306/scienceasia1513-1874.2007.33.383
» http://dx.doi.org/10.2306/scienceasia1513-1874.2007.33.383 - CHUGHTAI, M.I., MAHMOOD, K. and AWAN, A.R., 2011. Assessment of planktonic diversity in River Chenab as affected by sewage of Multan city. Pakistan Journal of Botany, vol. 43, no. 5, pp. 2551-2555.
- DHANASEKARAN, M., BHAVAN, P.S., MANICKAM, N. and KALPANA, R., 2017. Physico-chemical characteristics and zooplankton diversity in a perennial lake at Dharmapuri (Tamil Nadu, India). Journal of Entomology and Zoology Studies, vol. 5, no. 1, pp. 285-292.
- FERNANDO, C.H., 1994. Zooplanktons, fish and fisheries in Tropical freshwaters. Hydrobiologia, vol. 272, no. 1-3, pp. 105-123. http://dx.doi.org/10.1007/BF00006516
» http://dx.doi.org/10.1007/BF00006516 - GANNON, J.E. and STEMBERGER, R.S., 1978. Zooplankton (especially crustaceans and rotifers) as indicator of water quality. Transactions of the American Microscopical Society, vol. 97, no. 1, pp. 16-35. http://dx.doi.org/10.2307/3225681
» http://dx.doi.org/10.2307/3225681 - GLIME, J.M., 2013. Invertebrates: Rotifers. In: J.M. GLIME, ed. Bryophyte ecology. vol. 2. Bryological Interaction, chapt. 4-5. Ebook sponsored by Michigan Technological University and the International Association of Bryologists.
- JAVED, M. and HAYAT, S., 1996. Planktonic productivity of river water as a bioindicator of freshwater contamination by metals. In: Proceedings of Pakistan Congress of Zoology, 1996, Pakistan. Punjab: Zoological Society of Pakistan, vol. 16, pp. 283-298.
- KIBRIA, G., NUGEOGODA, D., FAIRCLOUGH, R., LAM, P. and BRADLY, A., 1997. Zooplanktons: its biochemistry and significance in Aquaculture. Naga, vol. 20, no. 2, pp. 8-14.
- KOLI, B.K. and MULEY, V.D., 2012. Study of zooplankton diversity and seasonal variation with special reference to physicochemical parameters in Tulshi reservoir of Kolhapur District (M.S) India. International Scientific Research Journal, vol. 4, no. 1, pp. 38-49.
- KOSTE, W., 1978. Rotatoria. Die Rädertiere Mitteleuropas, begründet von Max Voigt. Überordnung Monogononta. Berlin: Gebrüder Borntraeger, I. Text U. II. Tafelbed. (T. 234), 673 p.
- NESCHUK, N., CLAPS, M. and GABELLONE, N., 2002. Planktonic rotifers of a saline-lowland river: the Salado River (Argentina). Ann. Limnol.-. International Journal of Limnology, vol. 38, no. 3, pp. 191-198. http://dx.doi.org/10.1051/limn/2002017
» http://dx.doi.org/10.1051/limn/2002017 - NEVES, I.F., ROCHA, O., ROCHE, K.F. and PINTO, A.A., 2003. Zooplankton community structure of two marginal lakes of the River Cuiabá (Mato Grosso, Brazil) with analysis of Rotifera and Cladocera diversity. Brazilian Journal of Biology = Revista Brasileira de Biologia, vol. 63, no. 2, pp. 329-343. http://dx.doi.org/10.1590/S1519-69842003000200018 PMid:14509855.
» http://dx.doi.org/10.1590/S1519-69842003000200018 - PEJLER, B., 1995. Relation to habitat in rotifers. Hydrobiologia, vol. 313, no. 1, pp. 267-278. http://dx.doi.org/10.1007/BF00025959
» http://dx.doi.org/10.1007/BF00025959 - SCHOLL, K. and KISS, A., 2008. Spatial and temporal distribution patterns of zooplankton assemblages (Rotifera, Cladocera, Copepoda) in the water bodies of the Gemenc floodplain (Duna-Dráva National Park, Hungary). Opuscula Zoologica, vol. 39, pp. 65-76.
- SEGERS, H., 2007. Annotated checklist of the rotifers (Phylum Rotifera), with notes on nomenclature, taxonomy and distribution. Zootaxa, vol. 1564, no. 1, pp. 1-104. http://dx.doi.org/10.11646/zootaxa.1564.1.1
» http://dx.doi.org/10.11646/zootaxa.1564.1.1 - SEGERS, H., 2008. Global diversity of rotifers (Rotifera) in freshwater. Hydrobiologia, vol. 595, no. 1, pp. 49-59. http://dx.doi.org/10.1007/s10750-007-9003-7
» http://dx.doi.org/10.1007/s10750-007-9003-7 - SUGUMARAN, J. and AMSATH, A., 2015. Seasonal diversity of rotifers from agniyar estuary, Thanjavur District, Tamil Nadu, India. International Journal of Pure and Applied Zoology, vol. 3, no. 4, pp. 287-292.
- SULEHRIA, A.Q.K. and MALIK, M.A., 2012. Population dynamics of planktonic rotifers in Balloki headworks. Pakistan Journal of Zoology, vol. 44, no. 3, pp. 663-669.
- SULEHRIA, A.Q.K., QAMAR, M.F., ANJUM, R.F., EJAZ, M. and HUSSAIN, A., 2009a. Seasonal fluctuations of Rotifers in a fish pond at district Bahawalnagar, Pakistan. Biologia, vol. 55, no. 1-2, pp. 21-28.
- SULEHRIA, A.Q.K., QAMAR, M.F., HAIDER, S., EJAZ, M. and HUSSAIN, A., 2009b. Water quality and Rotifer diversity in the fish pond at district Mianwali, Pakistan. Biologia, vol. 55, no. 1-2, pp. 79-85.
- WALLACE, R.L. and SNELL, T.W., 2010. Rotifera. In: J.H. THORP and A.P. COVICH, eds., Ecology and classification of North American freshwater invertebrates Oxford: Elsevier, chap. 8, pp. 173-235. http://dx.doi.org/10.1016/B978-0-12-374855-3.00008-X
» http://dx.doi.org/10.1016/B978-0-12-374855-3.00008-X - WALLACE, R.L., SNELL, T.W., RICCI, C. and NOGRADY, T., 2006. Rotifera. In: H. SEGERS, ed. Guides to the identification of the microinvertebrates of the continental waters of the world Leiden: Kenobi Productions, Ghent and Backhuys Publishers, vol. 1, Biology, ecology and systematics.
- ZARFDJIAN, M.-H., MICHALOUDI, E., BOBORI, D.C. and MOURELATOS, S., 2000. Zooplankton abundance in the Aliakmon River, Greece. Belgian Journal of Zoology, vol. 130, no. 1, pp. 29-33.
Publication Dates
-
Publication in this collection
29 Apr 2022 -
Date of issue
2024
History
-
Received
22 Mar 2021 -
Accepted
04 June 2021