Abstract
Ozone gas is considered as a safe antimicrobial agent in food industries. Here, we evaluated the antifungal and antiaflatoxigenic activities of ozone against fungal contamination in nuts. The most predominant fungal genera in nuts were Aspergillus, Penicillium, Fusarium, and Rhizopus. Ozone (4 ppm) significantly reduced the fungal sporulation of A. flavus and their aflatoxin production. Interestingly, ozone treatment of nuts reduced the total fungal count and increased aflatoxins degradation by approximately 95% and 85%, respectively. Ozone displayed high efficiency to increase the permeability of cell membrane and injury of cell wall of fungi. Increasing the exposure time of ozone in nuts up to 180 minutes showed to reduce the total lipid, carbohydrates, and protein by around 41.2%, 42.7% and 38.4% respectively, in pistachio, almond and peanuts. In conclusion, ozonation is a suitable decontaminating approach for reducing the microbial load in nuts, when used with suitable exposure time.
Keywords:
Aspergillus; aflatoxins; antifungal; antiaflatoxigenic; ozone; nuts; nutritional quality
Resumo
O gás ozônio é considerado um agente antimicrobiano seguro em indústrias alimentícias. Aqui, avaliamos as atividades antifúngicas e antiaflatoxigênicas do ozônio contra a contaminação fúngica em nozes. Os gêneros fúngicos mais predominantes em nozes foram Aspergillus, Penicillium, Fusarium e Rhizopus. O ozônio (4 ppm) reduziu significativamente a esporulação fúngica de A. flavus e sua produção de aflatoxinas. Curiosamente, o tratamento de nozes com ozônio reduziu a contagem total de fungos e aumentou a degradação de aflatoxinas em aproximadamente 95% e 85%, respectivamente. O ozônio apresentou alta eficiência para aumentar a permeabilidade da membrana celular e a lesão da parede celular dos fungos. O aumento do tempo de exposição do ozônio em nozes em até 180 minutos levou à redução do total de lipídios, carboidratos e proteínas em 41,2%, 42,7% e 38,4%, respectivamente, em pistache, amêndoa e amendoim. Em conclusão, a ozonização é uma abordagem de descontaminação adequada para reduzir a carga microbiana em nozes, quando usada com tempo de exposição adequado.
Palavras-chave:
Aspergillus; aflatoxinas; antifúngico; antiaflatoxigênico; ozônio; nozes; qualidade nutricional
1. Introduction
Nuts are rich in nutrients and thus they can be easily contaminated with toxigenic fungi during harvesting, storage and transition processing, where temperature and humidity conditions are suitable for the growth of fungi (Lien et al., 2019LIEN, K.W., WANG, X., PAN, M.H. and LING, M.P., 2019. Assessing aflatoxin exposure risk from peanuts and peanut products imported to Taiwan. Toxins, vol. 11, no. 2, p. 80. http://dx.doi.org/10.3390/toxins11020080. PMid:30717134.
http://dx.doi.org/10.3390/toxins11020080...
; Wu et al., 2018WU, Q., XIE, L. and XU, H., 2018. Determination of toxigenic fungi and aflatoxins in nuts and dried fruits using imaging and spectroscopic techniques. Food Chemistry, vol. 252, pp. 228-242. http://dx.doi.org/10.1016/j.foodchem.2018.01.076. PMid:29478536.
http://dx.doi.org/10.1016/j.foodchem.201...
). Nuts were reported to be contaminated with different species of Aspergillus, Rhizopus, and Penicillium that are toxic to human and cause a reduction in nutritional value and a decrease of market significance of food products (Mir et al., 2022MIR, S.A., SHAH, M.A., MIR, M.M., SIDIQ, T., SUNOOJ, K.V., SIDDIQUI, M.W., MARSZAŁEK, K. and KHANEGHAH, A.M., 2022. Recent developments for controlling microbial contamination of nuts. Critical Reviews in Food Science and Nutrition. In press. http://dx.doi.org/10.1080/10408398.2022.2038077. PMid:35170397.
http://dx.doi.org/10.1080/10408398.2022....
; Norlia et al., 2019NORLIA, M., JINAP, S., NOR-KHAIZURA, M.A.R., RADU, S., CHIN, C.K., SAMSUDIN, N.I.P. and FARAWAHIDA, A.H., 2019. Molecular characterisation of aflatoxigenic and non-aflatoxigenic strains of Aspergillus section Flavi isolated from imported peanuts along the supply chain in Malaysia. Toxins, vol. 11, no. 9, p. 501. http://dx.doi.org/10.3390/toxins11090501. PMid:31470527.
http://dx.doi.org/10.3390/toxins11090501...
; Rahimi et al., 2021RAHIMI, A., SASANI, E., REZAIE, S., DALLAL, M.M.S., MAHMOUDI, S., AHMADI, A., GHAFFARI, M., AALA, F. and KHODAVAISY, S., 2021. Molecular identification of aflatoxigenic Aspergillus species in dried nuts and grains collected from Tehran, Iran. Journal of Environmental Health Science & Engineering, vol. 19, no. 2, pp. 1795-1799. http://dx.doi.org/10.1007/s40201-021-00734-6. PMid:34900308.
http://dx.doi.org/10.1007/s40201-021-007...
).
Mycotoxins are toxic secondary metabolites of fungi that can infect nuts and seeds during harvest/post-harvest and storage. Aflatoxins are the main reported mycotoxins in nuts and seeds that produced by different species of Aspergillus such as A. flavus, A. parasiticus and A. niger at low concentration they cause acute aflatoxicosis symptoms in both humans and animals (Battilani, 2010BATTILANI, P., 2010. Mycotoxins in nuts. Options Méditerranéennes A, vol. 94, pp. 167-193.; Ono et al., 2011ONO, E., HIROOKA, E., ROSSI, C. and ONO, M., 2011. Mycotoxins in seeds and nuts. In: V.R. PREEDY, R.R. WATSON and V.B. PATEL, eds. Nuts and seeds in health and disease prevention. Amsterdam: Elsevier, pp. 121-127.; Pickova et al., 2021PICKOVA, D., OSTRY, V. and MALIR, F., 2021. A recent overview of producers and important dietary sources of aflatoxins. Toxins, vol. 13, no. 3, p. 186. http://dx.doi.org/10.3390/toxins13030186. PMid:33802572.
http://dx.doi.org/10.3390/toxins13030186...
). In this context, chemical control was applied to protect seeds and nuts against aflatoxigenic fungi (Davies et al., 2021DAVIES, C.R., WOHLGEMUTH, F., YOUNG, T., VIOLET, J., DICKINSON, M., SANDERS, J.W., VALLIERES, C. and AVERY, S.V., 2021. Evolving challenges and strategies for fungal control in the food supply chain. Fungal Biology Reviews, vol. 36, pp. 15-26. http://dx.doi.org/10.1016/j.fbr.2021.01.003. PMid:34084209.
http://dx.doi.org/10.1016/j.fbr.2021.01....
). However, due to the toxicity effect of chemically synthesized fungicides and preservatives to human and animal, crucial needs should be taken to develop safe and natural alternative strategy to prevent growth of pathogenic fungi and aflatoxins production in nuts (Davies et al., 2021DAVIES, C.R., WOHLGEMUTH, F., YOUNG, T., VIOLET, J., DICKINSON, M., SANDERS, J.W., VALLIERES, C. and AVERY, S.V., 2021. Evolving challenges and strategies for fungal control in the food supply chain. Fungal Biology Reviews, vol. 36, pp. 15-26. http://dx.doi.org/10.1016/j.fbr.2021.01.003. PMid:34084209.
http://dx.doi.org/10.1016/j.fbr.2021.01....
; Lien et al., 2019LIEN, K.W., WANG, X., PAN, M.H. and LING, M.P., 2019. Assessing aflatoxin exposure risk from peanuts and peanut products imported to Taiwan. Toxins, vol. 11, no. 2, p. 80. http://dx.doi.org/10.3390/toxins11020080. PMid:30717134.
http://dx.doi.org/10.3390/toxins11020080...
).
Ozone was used for the purpose of microbial decontamination against viruses, Gram-negative and Gram-positive bacteria, spores, and fungi (Manousaridis et al., 2005MANOUSARIDIS, G., NERANTZAKI, A., PALEOLOGOS, E., TSIOTSIAS, A., SAVVAIDIS, I.N. and KONTOMINAS, M., 2005. Effect of ozone on microbial, chemical and sensory attributes of shucked mussels. Food Microbiology, vol. 22, no. 1, pp. 1-9. http://dx.doi.org/10.1016/j.fm.2004.06.003.
http://dx.doi.org/10.1016/j.fm.2004.06.0...
). Ozone has short life span and displayed minimal hazard effects on food constituents (Botondi et al., 2021BOTONDI, R., BARONE, M. and GRASSO, C., 2021. A review into the effectiveness of ozone technology for improving the safety and preserving the quality of fresh-cut fruits and vegetables. Foods, vol. 10, no. 4, p. 748. http://dx.doi.org/10.3390/foods10040748. PMid:33915979.
http://dx.doi.org/10.3390/foods10040748...
; Mir et al., 2022MIR, S.A., SHAH, M.A., MIR, M.M., SIDIQ, T., SUNOOJ, K.V., SIDDIQUI, M.W., MARSZAŁEK, K. and KHANEGHAH, A.M., 2022. Recent developments for controlling microbial contamination of nuts. Critical Reviews in Food Science and Nutrition. In press. http://dx.doi.org/10.1080/10408398.2022.2038077. PMid:35170397.
http://dx.doi.org/10.1080/10408398.2022....
; Rahmati et al., 2022RAHMATI, E., KHOSHTAGHAZA, M.H., BANAKAR, A. and EBADI, M.T., 2022. Decontamination technologies for medicinal and aromatic plants: a review. Food Science & Nutrition, vol. 10, no. 3, pp. 784-799. http://dx.doi.org/10.1002/fsn3.2707. PMid:35311169.
http://dx.doi.org/10.1002/fsn3.2707...
). Thus, ozone in both gaseous and water‐soluble forms was approved by the US, FDA for use as an antimicrobial agent for food chains and is recognized as a “green technology (Khadre et al., 2001KHADRE, M.A., YOUSEF, A.E. and KIM, J.-G., 2001. Microbiological aspects of ozone applications in food: a review. Journal of Food Science, vol. 66, no. 9, pp. 1242-1252. http://dx.doi.org/10.1111/j.1365-2621.2001.tb15196.x.
http://dx.doi.org/10.1111/j.1365-2621.20...
). Several studies reported the safety of using ozone as a disinfecting agent for dried foods, spices, pistachios, meat production and processing plants (Akbas and Ozdemir, 2006AKBAS, M. and OZDEMIR, M., 2006. Effect of different ozone treatments on aflatoxin degradation and physicochemical properties of pistachio. Journal of the Science of Food and Agriculture, vol. 86, no. 13, pp. 2099-2104. http://dx.doi.org/10.1002/jsfa.2579.
http://dx.doi.org/10.1002/jsfa.2579...
; Vallone and Stella, 2014VALLONE, L. and STELLA, S., 2014. Evaluation of antifungal effect of gaseous ozone in a meat processing plant. Italian Journal of Food Safety, vol. 3, no. 2, p. 1680. http://dx.doi.org/10.4081/ijfs.2014.1680. PMid:27800339.
http://dx.doi.org/10.4081/ijfs.2014.1680...
), against Escherichia coli, Bacillus spp and Micrococcus. In relation to fungi, ozone was found to suppress sporulation and increase mycelia leakage of sugar and nutrients of dermatophytes related to Microsporum gypseum and Microsporum canis (Ouf et al., 2016), and ozonated water suppress the growth of Candida albicans and Zygosaccharomyces bailii cells (Restaino et al., 1995). In addition, ozone was effective to inhibit the formation of germ tubes in the pathogenic fungi C. albicans and A. flavus (Ouf et al., 2019; Zargaran et al., 2017). Additionally, the ozonation process is also suggested as a novel technology able to degrade mycotoxins, including aflatoxins, fumonisins, ochratoxin, patulin, deoxynivalenol and zearalenone (Afsah-Hejri et al., 2020AFSAH-HEJRI, L., HAJEB, P. and EHSANI, R.J., 2020. Application of ozone for degradation of mycotoxins in food: a review. Comprehensive Reviews in Food Science and Food Safety, vol. 19, no. 4, pp. 1777-1808. http://dx.doi.org/10.1111/1541-4337.12594. PMid:33337096.
http://dx.doi.org/10.1111/1541-4337.1259...
).
We aimed in this study, to explore the antifungal and detoxifying actions of ozone gas against aflatoxins in nut samples, as well as to determine the effect of ozone on the nutritional quality of the investigated nuts.
2. Materials and Methods
2.1. Sampling
Seven samples, representing various types of pure (unsalted) and unshelled nut products were collected randomly from different markets in Al-Hassa, Saudi Arabia during the months of January - March 2022. The nuts include pistachio (Pistacia vera), hazelnuts (Corylus colurna), cashews (Anacardium occidentale), peanut (Arachis hypogaea), almonds (Prunus dulcis), chestnut (Castanea), and walnut (Juglans regia). In the laboratory, samples were individually finely ground in a common household blender. The blender’s cup was rinsed in 85% alcohol between samples. The powder kept tightly packed in a new study bags and stored at 4°C for further analysis.
2.2. Isolation of fungi
For fungal analysis, dilution method was used to determine total fungal counts in nut samples. One gram of each composite sample (fine powder) were transferred into screw-capped medicinal bottle containing 9 mL of sterile distilled water and were mechanically homogenized at constant speed for 15 min. The sample-water suspension was allowed to stand for 10 min with intermittent shaking before being plated. Appropriate tenfold serial dilutions (1:10) were prepared and one mL portions of suitable dilutions of the resulting samples suspension were used to inoculate Petri dishes each containing 15 mL Potato Dextrose Agar (PDA). Plates were then incubated for 7 days at 28 °C. Three replicates plates per medium were used for each sample and the developing fungi were counted. After incubation, the results were expressed in Colony-Forming Units (CFU) /g of samples; all plates were examined visually, directly and with a microscope (Suleiman et al., 2008SULEIMAN, M.N., EMUA, S.A. and TAIGA, A., 2008. Effect of aques leaf extracts on a spot fungus (Fusarium sp) isolated from Compea. American-Eurasian Journal of Sustainable Agriculture, vol. 2, pp. 261-263.).
2.3. Fungal identification
The developing fungal colonies were purified and identified up to the species level by microscopic examination, and this was made through the help of the following references: Alsohaili and Bani-Hasan, 2018ALSOHAILI, S. and BANI-HASAN, B.M., 2018. Morphological and molecular identification of fungi isolated from different environmental sources in northern eastern Jordan deseret. Jordan Journal of Biological Sciences, vol. 11, pp. 329-337.; Blaize et al., 2021BLAIZE, M., NORMAND, A.C., IMBERT, S., AL-HATMI, A.M.S., CHRYSSANTHOU, E., CASSAING, S., SCHUTTLER, C., HASSEINE, L., MAHINC, C., COSTA, D., BONNAL, C., RANQUE, S., SAUTOUR, M., RUBIO, E., DELHAES, L., RIAT, A., SENDID, B., KRISTENSEN, L., BRANDENBERGER, M., STUBBE, D., BRUN, S., PIARROUX, R. and FEKKAR, A., 2021. Antifungal susceptibility of 182 Fusarium species isolates from 20 European centers: comparison between EUCAST and gradient concentration strip methods. Antimicrobial Agents and Chemotherapy, vol. 65, no. 12, p. e0149521. http://dx.doi.org/10.1128/AAC.01495-21. PMid:34543091.
http://dx.doi.org/10.1128/AAC.01495-21...
; Visagie et al., 2014VISAGIE, C.M., HOUBRAKEN, J., FRISVAD, J.C., HONG, S.-B., KLAASSEN, C.H.W., PERRONE, G., SEIFERT, K.A., VARGA, J., YAGUCHI, T. and SAMSON, R.A., 2014. Identification and nomenclature of the genus Penicillium. Studies in Mycology, vol. 78, no. 1, pp. 343-371. http://dx.doi.org/10.1016/j.simyco.2014.09.001. PMid:25505353.
http://dx.doi.org/10.1016/j.simyco.2014....
.
2.4. Ozone production
Ozone was produced by an ozone generator through a controlled flow of oxygen by a corona discharge as previously described by our group (Ouf and Ali, 2021OUF, S.A. and ALI, E.M., 2021. Does the treatment of dried herbs with ozone as a fungal decontaminating agent affect the active constituents? Environmental Pollution, vol. 277, p. 116715. http://dx.doi.org/10.1016/j.envpol.2021.116715. PMid:33652183.
http://dx.doi.org/10.1016/j.envpol.2021....
). Briefly, the process of ozone sanitization was as follows: ozonation system made up of an oxygen tank, an ozone generator, and a gastight glass chamber. Three samples of 50 g from each nut samples were placed inside mesh sieves. Sieves were placed inside the chamber. Ozone was generated by providing dry oxygen to the ozone generator. The ozone concentration inside the chamber was adjusted to 4±0.2 ppm for four different periods (30, 60, 90, and 180 min). The airflow rate in the tube connected to inlet port was adjusted to 2.6 L min–1 using a flow meter. Relative humidity and temperature inside the chamber were monitored. To avoid the accumulation of ozone on the upper side of the chamber, a ventilator was placed inside the chamber.
2.5. Sporulation
The sporicidal activity of ozone was carried out by spore germination assay following the method of Das et al. (2010)DAS, K., TIWARI, R., SHRIVASTAVA, D. and BILASPUR, B.C., 2010. Techniques for evaluation of medicinal plant products as antimicrobial agent: current methods and future trends. Journal of Medicinal Plants Research, vol. 4, pp. 104-111.. The selected fungal species were exposed to 4 ppm ozone for 0 (control), 30, 60, 90, and 180 min. Spores were allowed to grow on Czapek-Dox medium (Sigma-Aldrich, Missouri, USA), then were harvested when the cultures were fully sporulated. Spores were collected by adding 5 mL of sterile water to each Petri dish and rubbing the surface with a sterile spreader. The suspension was collected and then centrifuged at 2000 rpm/min. A haemocytometer slide was used to count spore production. The germinated spores were observed using a light microscope. The degree of spore germination was measured by observing germ tubes.
2.6. In vitro test of mycelial growth inhibition
A. flavus was exposed to 4 ppm ozone for 0 (control), 30, 60, 90, and 180 min as described previously. Mycelial disks (5 mm in diameter) from the edges of a 7-day old culture were transferred to plates filled with potato dextrose agar media (Sigma-Aldrich, Missouri, USA) (3 plates per each treatment). Radial mycelial growth was determined by the calculation of the average of two perpendicular colony diameters for each replicate. Mycelial growth inhibition for each treatment and concentration after 7 days of incubation, at 25 ◦C in the dark, was calculated according to the following formula: (dc − dt)/dc) × 100, where dc is the average diameter of the fungal colony in the control, and dt is the average diameter of the ozonated fungal colony.
2.7. Leakage of proteins and DNA
Spore suspension solutions of A. flavus were prepared in saline solution (NaCl, 0.9% w/v). The fungal spore suspensions were exposed to ozone gas at 4 ppm for 0, 30, 60, and 90 minutes. Spore suspension in each case was centrifuged for 15 min at 4000 rpm at 4 ºC. The optical density (OD) of the supernatant was read at 280 and 260 nm to estimate the proteins and DNA leaked from the fungal cells (Khalil et al., 2019KHALIL, N.M., EL-GHANY, M.N. and RODRÍGUEZ-COUTO, S., 2019. Antifungal and anti-mycotoxin efficacy of biogenic silver nanoparticles produced by Fusarium chlamydosporum and Penicillium chrysogenum at non-cytotoxic doses. Chemosphere, vol. 218, pp. 477-486. http://dx.doi.org/10.1016/j.chemosphere.2018.11.129. PMid:30497030.
http://dx.doi.org/10.1016/j.chemosphere....
).
2.8. Determination of β-1,3-glucan
The fluorescence dye-binding microassay for β-1,3-glucan was applied following the method of Ko and Lin (2004)KO, Y.T. and LIN, Y.L., 2004. 1,3-beta-glucan quantification by a fluorescence microassay and analysis of its distribution in foods. Journal of Agricultural and Food Chemistry, vol. 52, no. 11, pp. 3313-3318. http://dx.doi.org/10.1021/jf0354085. PMid:15161189.
http://dx.doi.org/10.1021/jf0354085...
. 5 mg of lyophilized mycelial powder was suspended in 300 μL of NaOH (1 M) and 30 μL of NaOH (6 M), and then the mixture was incubated at 80°C for 30 min. Then, samples were centrifuged at 4000g and the supernatant was added to 630 μL of methyl blue mixture (pH 9.5) and incubated for 30 min. The samples were decolorized. The fluorescence intensity was measured with a fluorescence spectrophotometer (F-7100, Hitachi Ltd., Tokyo, Japan) at an excitation wavelength of 398 nm and an emission wavelength of 502 nm.
2.9. Determination of the chitin content
The content of chitin was determined according to the method of OuYang et al. (2019)OUYANG, Q., DUAN, X., LI, L. and TAO, N., 2019. Cinnamaldehyde exerts its antifungal activity by disrupting the cell wall integrity of Geotrichum citri-aurantii. Frontiers in Microbiology, vol. 10, no. 55, pp. 1-9. http://dx.doi.org/10.3389/fmicb.2019.00055. PMid:30761105.
http://dx.doi.org/10.3389/fmicb.2019.000...
. Lyophilized mycelia were added to saturate KOH and heated at 160°C for 15 min. The samples were then dehydrated with 95% ethanol and 96.6% absolute ethanol successively. The chitin content was calculated as: Chitin content (%) =Wf/Wi×1.26×100, where Wf is weight of lyophilized mycelia, Wi is weight of the sample after dehydration, and 1.26 represents the conversion factor of the relative molecular mass of N-acetamino-glucose/relative molecular mass of glucosamine.
2.10. Scanning Electron Microscopy (SEM)
A small pieces (2 mm × 2 mm) colonized by mycelia of ozonated and non ozonated A. flavus were sampled from the center of each colony and fixed at 4 ºC for 12 h in 2% (w/v) glutaraldehyde in 0.1 M phosphate buffer (pH 7.0). The mycelial specimens were dehydrated in graded ethanol. After drying in a critical point dryer (Model 13200-AB, SPI SUPPLIES, PA, United States) and gold-coating in a sputter coater (Model JFC-1600, NTC, Japan), the mycelial specimens were examined under a scanning electron microscope (Model JSM-6390/LV, NTC, Japan).
2.11. Transmission Electron Microscopy (TEM)
Treated mycelia with ozone were fixed in 2.5% glutaraldehyde for 4 h at 4°C. Subsequently, mycelia were washed with phosphate buffer for 10 min followed by post fixation in 1.0% osmium tetraoxide for 2 h at 4°C. Samples were dehydrated in a graded ethanol series and 100% acetone. They were soaked in a mixture of epoxy resin and acetone (1:1) and then in epoxy resin overnight. The specimens were embedded in Spurr’s resin (SPI Supplies, West Chester, PA, USA). Ultrathin sections were prepared by and mounted on copper grids and stained with saturated uranyl acetate and lead citrate. The samples were examined by a transmission electron microscope (JEM-14000, JEOL Ltd., Tokyo, Japan).
2.12. Aflatoxins production
A. flavus was inoculated in 500 μl of yeast extract sucrose in an Eppendorf tube and incubated for seven days. Briefly, to each Eppendorf tube, 500 μl chloroform was added and vortexed. The chloroform extract was transferred to a new vial and dried under air. Dry film was derivatized, and then analyzed quantitatively using HPLC. A 200 μl stock solution of Aflatoxins mix standard in methanol (Supelco, Bellefonte, Pa., USA), containing 200 ng B1, 60 ng B2, 200 ng G1, and 60 ng G2, was dried under nitrogen gas and derivatized. Four different concentrations of working standard solution were used for calibration curve preparation of each aflatoxin type.
2.13. HPLC conditions
The HPLC system used for aflatoxins quantification was an Agilent 1200 series system (Agilent, Berks., UK) with a fluorescence detector (FLD G1321A), an autosampler ALS G1329A, FC/ALS therm G1330B, Degasser G1379B, Bin Bump G1312A, and a C18 (Phenomonex, Luna 5 micron, 150 × 4.6 mm) column joined to a pre-column (security guard, 4 × 3-mm cartridge, Phenomenex Luna). The mobile phase was water /methanol /acetonitrile (60: 30:10, v/v/v) using an isocratic flow rate of 1 mL min-1 at 360 nm excitation and 440 nm emission wavelengths and a 25-min run time for aflatoxin quantification. Under these conditions, the LOD levels were 0.042, 0.015, 0.023 and 0.012, whereas LQD levels were 0.131, 0.045, 0.072 and 0.032 (ng injection-1) for AFG1, AFB1, AFG2 and AFB2, respectively.
2.14. Aflatoxin degradation in ground nut samples
Ozonated (100 g) and non-ozonated (100 g) ground nut samples were homogenized with sodium chloride and methanol–water (2:3) mixture and extracted. An aliquot of each suspension was passed through an immune-affinity column (Rhone Diagnostics Technologies, Glasgow, UK). Aflatoxins were quantified using an HPLC system as previously described. The percentage degradation of total aflatoxins was calculated depending on the difference between non-ozonated and ozonated samples.
2.15. Determination of protein, lipid, and sugars contents in ozonated nuts
The ground samples were exposed to ozone according to the method described by Paes et al. (2017)PAES, J., FARONI, L., MARTINS, M., CECON, P. and HELENO, F., 2017. Reaction kinetics of ozone gas in wheat flour. Engenharia Agrícola, vol. 37, no. 3, pp. 520-528. http://dx.doi.org/10.1590/1809-4430-eng.agric.v37n3p520-528/2017.
http://dx.doi.org/10.1590/1809-4430-eng....
. Nut samples of 500 g were packed in glass containers with a capacity of 3.0 L and screw-on lids, with connections for gas inlet and outlet. After passing ozone through the sample, the gas was directed to a thermal destroyer manufacturing on demand. Ozone gas concentrations of 4 ppm applied for 90 and 180 minutes. Ozone was used with a flow rate of 3.0 L min−1, at a temperature of 25 °C with three replicates. The samples were evaluated for protein, lipid, and sugars contents. Total protein was measured by total protein assay kit was from G-Biosciences (St. Louis, MO) according to the method of Jablonski et al. (2010)JABLONSKI, J.E., FU, T.J., JACKSON, L.S. and GENDEL, S.M., 2010. Determination of protein levels in soy and peanut oils by colorimetric assay and ELISA. Journal of AOAC International, vol. 93, no. 1, pp. 213-220. PMid:20334183..
For Lipids, A known weight of each sample was defatted in a Soxhlet apparatus using petroleum ether as the solvent for 8 h. Defatted samples were dried overnight in a fume hood to remove residual traces of petroleum ether, after which the samples were weighed to calculate lipid content (Venkatachalam and Sathe, 2006VENKATACHALAM, M. and SATHE, S.K., 2006. Chemical composition of selected edible nut seeds. Journal of Agricultural and Food Chemistry, vol. 54, no. 13, pp. 4705-4714. http://dx.doi.org/10.1021/jf0606959. PMid:16787018.
http://dx.doi.org/10.1021/jf0606959...
).
Total sugars were determined following the method of Dubois et al. (1951)DUBOIS, M., GILLES, K., HAMILTON, J.K., REBERS, P.A. and SMITH, F., 1951. A colorimetric method for the determination of sugars. Nature, vol. 168, no. 4265, p. 167. http://dx.doi.org/10.1038/168167a0. PMid:14875032.
http://dx.doi.org/10.1038/168167a0...
. Briefly, a known weight of the sample was extracted with 1 mL of distilled deionized water containing 1 mM NaN3 and centrifuged and the supernatant was collected. 100 µL of distilled deionized water was added to the supernatant followed by 200 µL of lead acetate (20% w/v), and the sample was vortexed to mix the contents. 200 µL of Na2SO4 (20% w/v) was added to the previous mixture and the sample was vortexed and centrifuged. A known volume of the resulting supernatant was made up to 400 µL with distilled deionized water, and 10 µL of 80% (w/v) phenol and 1 mL of concentrated H2SO4 were added; the contents were mixed using a vortex mixer. Samples were allowed to cool to room temperature, and the absorbance was read at 490 nm (DuBois et al., 1956DUBOIS, M., GILLES, K.A., HAMILTON, J.K., REBERS, P.A. and SMITH, F., 1956. Colorimetric method for determination of sugars and related substances. Analytical Chemistry, vol. 28, no. 3, pp. 350-356. http://dx.doi.org/10.1021/ac60111a017.
http://dx.doi.org/10.1021/ac60111a017...
).
2.16. Determination of the lipid profile by gas chromatography
The lipid profile of raw oil extracted from non-ozonated (0 ppm) and ozonated nuts at the concentration 4 ppm for 90 and 180 min was analyzed. The fatty acids were esterified from the oil extracted by the method of Bligh and Dyer (1959)BLIGH, E.G. and DYER, W.J., 1959. A rapid method of total lipid extraction and purification. Canadian Journal of Biochemistry and Physiology, vol. 37, no. 8, pp. 911-917. http://dx.doi.org/10.1139/o59-099. PMid:13671378.
http://dx.doi.org/10.1139/o59-099...
, according to Mendonça et al. (2017)MENDONÇA, M.A., ARAÚJO, W.M.C., BORGO, L.A. and ALENCAR, E.R., 2017. Lipid profile of different infant formulas for infants. PLoS One, vol. 12, no. 6, p. e0177812. http://dx.doi.org/10.1371/journal.pone.0177812. PMid:28570611.
http://dx.doi.org/10.1371/journal.pone.0...
. Briefly, Gas Chromatograph (Shimadzu Corp., Japan) with AOC 20i autoinjector, flame ionization detector (FID-2014) and Rt-2560 chromatographic column (Restek, Bellefonte, PA, USA), measuring 100 m long, with 0.25 mm internal diameter and 0.20 μm film thickness, was used in the chromatographic analysis. The chromatographic conditions were: Split type injection mode with injection temperature of 200 °C; the column heating ramp was programmed to start at 140 °C until reaching 200 °C. Gas Chromatograph temperature was 220 °C and helium was used as the carrier gas, with flow in the column of 1.2 mL min−1. The chromatographic run was equivalent to 45 min. Oil samples were diluted in 2 mL hexane before injection. The volume injected was 1 μL. Identification of each fatty acid was performed by comparison with the retention time of the fatty acid standard Supelco 37 component FAME mix (Supelco, USA). The results were expressed in g of each fatty acid.
2.17. Statistical analysis
The results of all the experiments were expressed as the mean value of three independent replicates. Statistical analysis of the significance differences between the mean values of the results was identified by one-way analysis of variance (ANOVA) followed by Duncan’s test at the 5% level of significance (P < 0.05) using the Statistical Analysis Software (SAS)(Version:SAS9.4,SASInstituteInc.,Cary,NC,USA).
3. Results
3.1. Isolation of fungi from nut samples
The isolation and identification of fungi from different nut samples demonstrated, that the highest population density was observed in pistachio (5899 colonies/g) followed by peanut (4846 colonies/g) and almond (3796 colonies/g) (Table S1). The most predominant fungal genera were Aspergillus (13832 colonies/g), Penicillium (2286 colonies/g), Fusarium (580 colonies/g) and Rhizopus (490 colonies/g) (Table S1). Aspergillus represented the highest population density of fungi and isolated from approximately all test nuts. Aspergillus was represented by 12 species of which A. flavus was the highest in population density and counted 5401 colonies/g representing 30.08% of the total fungal population. Additionally, A. flavus was the most dominant species of the highest frequency of occurrence (7 cases of isolation) recovered from all nut samples (Table S1).
3.2. Effect of ozone on sporulation of some fungi isolated from nut samples
Based on the results of our previous work on herbs and spices, it was found that exposure of fungi to 4 ppm ozone gas for 280 minutes resulted in complete inhibition of all tested fungal species (Ouf and Ali, 2021OUF, S.A. and ALI, E.M., 2021. Does the treatment of dried herbs with ozone as a fungal decontaminating agent affect the active constituents? Environmental Pollution, vol. 277, p. 116715. http://dx.doi.org/10.1016/j.envpol.2021.116715. PMid:33652183.
http://dx.doi.org/10.1016/j.envpol.2021....
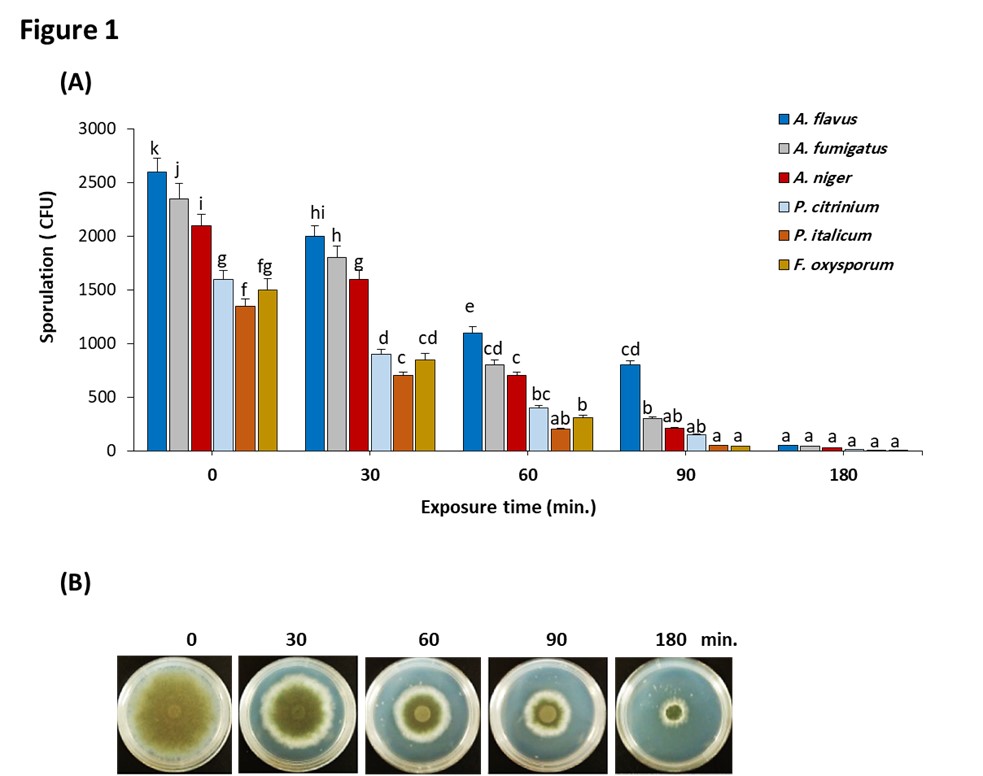
). Therefore, in this study we use ozone gas at a dose of 4 ppm applied at different exposure times (less than 280 minutes) to assess their role on inhibition of growth of most prominent fungal species in nuts. Our results revealed that ozone gas inhibit ability of fungal mycelium grown on CDA medium for 7 days at 28ºC to produce spores (Figure 1A). The extension of exposure time from 30 to 180 minutes resulted in a progressive reduction in spores' production of ozone-treated inoculum. The inoculum of P. citrinium, P. italicum, and F. oxysporum failed to form spores when treated with 4 ppm ozone for 180 minutes. A. flavus, A. fumigatus and A. niger were still capable of producing a very low yield of spores accounting 50, 43, and 30 cfu/mL, respectively when exposed to ozone gas for 180 minutes.
Effect of ozone gas on selected fungal species isolated from nut samples. (A) Effect of ozone gas on the sporulation of some selected fungal species isolated from nut samples. The sporicidal activity of ozone was carried out by spore germination assay. The selected fungal species were exposed to 4 ppm ozone for 0 (control), 30, 60, 90, and 180 min. Spores were allowed to grow on CDA medium, then were harvested when the cultures were fully sporulated. Columns labeled with the same letters are not significantly different at p < 0.05 by Duncan’s test. All values presented as the average of three replicates. Error bars represent the standard deviation. (B) Effect of ozone gas on radial growth of A. flavus. A. flavus was exposed to 4 ppm ozone for 0 (control), 30, 60, 90, and 180 min. Mycelial disks (5 mm in diameter) were transferred to plates filled with PDA media. Radial mycelial growth was determined after 5 days by the calculation of the average of two perpendicular colony diameters for each replicate.
3.3. Effect of ozone gas on the radial growth rate of A. flavus
Data presented in Table S1, revealed that A. flavus is the leading species depending on the population density, frequency of occurrence, and cases of isolation in all tested nut samples. Therefore, A. flavus was used as a model species to study the inhibitory mechanism of gaseous ozone on fungal growth. The mycelial growth inhibition of A. flavus obtained for each of the exposure time after 7 days was shown in Figure 1B. The results showed the exposure time dependent inhibitory effect of ozone on the radial growth of the mycelium. Full inhibition was obtained at the highest exposure time (180 minutes).
3.4. Effect of ozone on the cell membrane leakage and the components of cell wall of A. flavus
To gain a further insight into antifungal mechanism of ozone, the leakage of proteins and DNA was studied. Figure 2A-2B showed the stimulatory effect of ozone on the leakage of proteins and DNA from A. flavus in exposure times dependent manner. To quantify the effect of ozone gas on the two main components of cell walls, β-1, 3-glucan and chitin, their contents were measured. Ozone treatment decrease the content of β-1, 3-glucan significantly with increasing the exposure time (Figure 2C). The decreasing percentage of β-1, 3-glucan at 180 minutes reached approximately 73.33%. However, ozone did not affect the content of chitin (Figure 2D).
Effect of ozone on cell membrane leakage and components of cell wall. (A) Effect of ozone gas on Leakage of DNA. A. flavus was exposed to 4 ppm ozone for 0 (control), 30, 60, 90, and 180 min. Spore suspension in each case was centrifuged for 15 min at 4000 rpm at 4 ºC. The optical density (OD) of the supernatant was read at 260. (B) Leakage of proteins. A. flavus was exposed to 4 ppm ozone for 0 (control), 30, 60, 90, and 180 min. Spore suspension in each case was centrifuged for 15 min at 4000 rpm at 4 ºC. The optical density (OD) of the supernatant was read at 280. (C) The content of β-1,3-glucan. A. flavus was exposed to 4 ppm ozone for 0 (control), 30, 60, 90, and 180 min. The fluorescence dye-binding microassay for β-1,3-glucan was applied. The fluorescence intensity was measured with at an excitation wavelength of 398 nm and an emission wavelength of 502 nm. (D) The content of chitin. A. flavus was exposed to 4 ppm ozone for 0 (control), 30, 60, 90, and 180 min. Lyophilized mycelia were dehydrated with 95% ethanol. Columns labeled with the same letters are not significantly different at p < 0.05 by Duncan’s test. All values are presented as the average of three replicates. Error bars represent the standard deviation.
3.5. Ultrastructural analysis of the interaction between ozone gas and A. flavus cells using SEM and TEM
The effect of ozone gas on morphology of mycelia and conidiophore was examined under SEM and TEM. In untreated controls (0 min), development of mycelium and conidiophore was normal with abundant conidia (Figure 3A) (a,b). While in ozone treated culture (180 min), the growth of A. flavus was inhibited. Conidiophore development was clearly abnormal, where mycelia and conidiophores were shriveled compared to untreated controls (Figure 3B) (c,d). TEM also showed an amorphous layer on the surface of the control group (Figure 3B) (a,b); however, this layer was severely injured on the ozone- treated mycelia (Figure 3B) (c,d). Quantification analysis revealed that the cell wall thickness of the control was 170.12 ± 22.18 nm and that of the ozone- treated group was 121.58 ± 17.33 nm, which was significantly lower than that of the control group (P < 0.05).
Ultrastructure modifications of A. flavus following ozone treatment (4ppm, 180 min.). (A) Scanning electron micrographs showing untreated control (a, b) where development of mycelium and conidiophore was normal with abundant conidia and ozonated conidiophores (c, d) where conidiophore development was clearly abnormal and mycelia were shriveled compared to untreated controls (The arrows represent hyphae or conidiophore). (B) Transmission electron micrographs of mycelial morphology of untreated control (a, b) and ozonated (c, d) A. flavus. Amorphous layer on the surface of the control group however, this layer was injured on the ozonated mycelia. Quantification analysis displayed that the cell wall thickness of the control was 170.12 ± 22.18 nm and that of the ozonated group was 121.58 ± 17.33 nm.
3.6. Count of fungal species isolated from nut samples after ozonation
Data obtained from Table 1 showed, that the highest population density among nut samples was observed in pistachio, peanut, and almond. Thus, these three nuts were used for studying the antifungal effect of ozone on nuts. Ozone treatment (at 4 ppm for 180 minutes) significantly reduced the total fungal contamination by 95,6%, 95.1% and 96.5% in pistachio, peanut, and almond, respectively (Table 1). At this ozone dose, there was complete decontamination for A. wentii, A. ochraceous, P. oxalicum, P. comembeti, P. funiculosum, P. crustosum, P. fellutanum, Cochliobolus lunatus, Botryotrichum piluliferum, Trichoderma hamatum, Bispora antennata, and R. stolonifera.
Count of fungal species isolated from three selected nut samples, after the treatment with 4 ppm ozone gas for 90 and 180 minutes.
3.7. Effect of ozone on the aflatoxins production by A. flavus and the degradation of aflatoxins in nut samples
As shown in Figure 4A, the production of aflatoxins AFB1, AFB2, and AFG1 by A. flavus decreased significantly with increasing the exposure time of ozone. We further investigated the efficiency of ozone treatment to degrade aflatoxins on different nut samples. As shown in Figure 4B, upon exposure of different nuts to ozone, increasing the exposure time from 30 to 180 minutes resulted in a progressive reduction of total aflatoxins concentration (Figure 4B). The highest level of degradation was achieved in peanut, pistachio, and almond by 90%, 81,8%, and 84.4% respectively (Figure 4B).
Effect of ozone on the aflatoxins production. (A) Effect of ozone gas on production of aflatoxins (µg/g dry mass). A. flavus was exposed to 4 ppm ozone for 0 (control), 30, 60, 90, and 180 min. A. flavus was inoculated on yeast extract sucrose and incubated for seven days. Aflatoxins were extracted and then analyzed quantitatively using HPLC. (B) Effect of ozone gas on total aflatoxins concentration recovered from the investigated nut samples determined as µg/kg dry mass. 100 g of ozonated and non-ozonated nut samples homogenized with sodium chloride and methanol–water (2:3) mixture and extracted. Aflatoxins were quantified using an HPLC system. Columns labeled with the same letters are not significantly different at p < 0.05 by Duncan’s test. All values are presented as the average of three replicates. Error bars represent the standard deviation.
3.8. Effect of ozone gas on the stability of major ingredients of some selected nuts
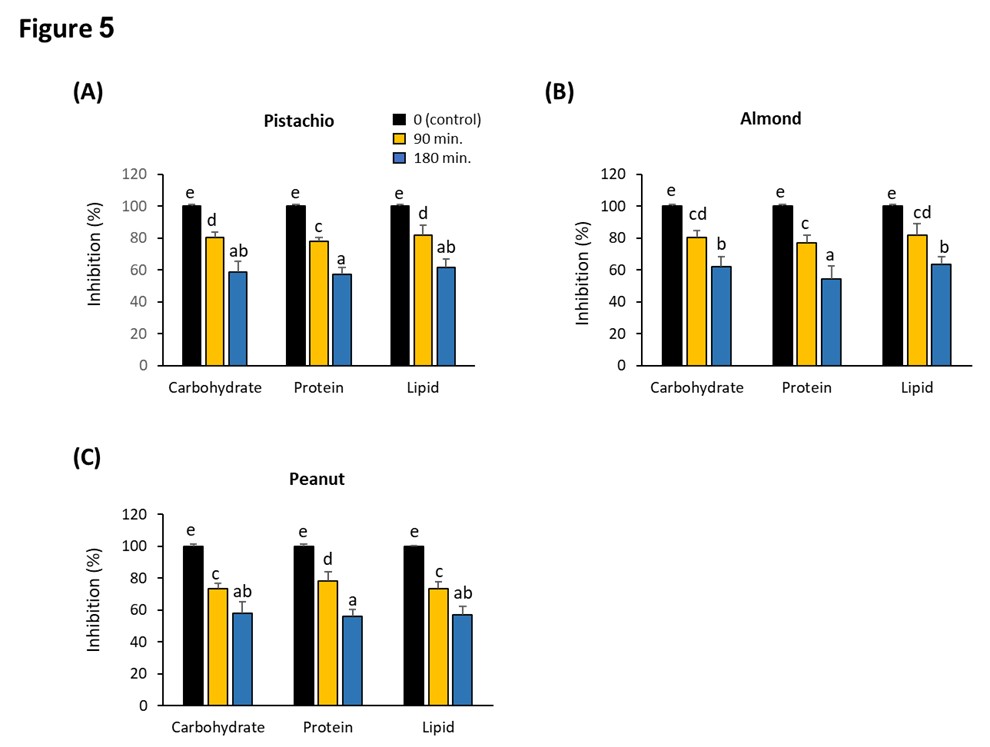
We measured the major ingredients in pistachio, peanut, and almond upon treatment with 4 ppm ozone for 90 and 180 minutes. Data revealed that there was significant reduction in total carbohydrate, lipid, and protein contents due to the interaction of ozone with nuts. The reduction in carbohydrate contents reaching 41.19%, 41.91%, and 38.09% in pistachio, peanut, and almond, respectively (Figure 5A-5C). The reduction in lipid contents was 38.45%, 43.10%, and 36.24% in pistachio, peanut, and almond, respectively (Figure 5A-5C). The variation in protein contents was 42.7, 43.82, and 45.67% in pistachio, peanut, and almond, respectively (Figure 5A-5C). Studying the lipid profile of ozonated pistachio, peanut, and almond, revealed significant reduction in different fatty acid contents (Table 2). For example, the most affected fatty acids in pistachio, peanut, and almond were stearic (C18:0) and palmitic (C16:0) with inhibition ranged from 51-60%, while arachidic (C20:0) was highly reduced in both pistachio and almond only, by 55% and 60% respectively (Table 2). Interestingly, peanut showed the most affected lipid profile with ozone among the three examined nuts.
Effect of ozone gas on nuts components. Effect of 4 ppm of ozone on the total contents of carbohydrate, protein and lipid in pistachio (A), Almond (B) and peanut (C). 500 g of each ground samples were exposed to ozone concentration of 4 ppm applied for 0 (control), 90, and 180 minutes. Values are mean ± SD of three independent experiments. Columns labeled with the same letters are not significantly different at p < 0.05 by Duncan’s test. All values are presented as the average of three replicates. Error bars represent the standard deviation.
Effect of 4 ppm of ozone gas applied for 90 and 180 minutes on the fatty acids composition of some selected nut samples
4. Discussion
Ozone is a potent oxidizing agent that can be used at low concentration and for short contact time as disinfecting agent in the food industry. This study investigated the potential use of ozone as a disinfectant agent against fungal contamination in nuts.
Our results identified peanut, pistachio and almond as the most contaminated nuts with fungi, while chestnut and walnut had the lowest. The most predominant fungal species were Aspergillus, Penicillium, Fusarium, and Rhizopus. Studies from US, Europe, Australia, and South America reported, that nuts can be contaminated with several fungi including Aspergillus, Rhizopus, and Penicillium. A. niger, A. flavus, A. parasiticus, and A. nomius that are known to produce mycotoxins and toxic to humans and animals (Bayman et al., 2002BAYMAN, P., BAKER, J.L. and MAHONEY, N.E., 2002. Aspergillus on tree nuts: incidence and associations. Mycopathologia, vol. 155, no. 3, pp. 161-169. http://dx.doi.org/10.1023/A:1020419226146. PMid:12617503.
http://dx.doi.org/10.1023/A:102041922614...
; Jørgensen, 2005JØRGENSEN, K., 2005. Occurrence of ochratoxin A in commodities and processed food--a review of EU occurrence data. Food Additives and Contaminants, vol. 22, suppl. 1, pp. 26-30. http://dx.doi.org/10.1080/02652030500344811. PMid:16332618.
http://dx.doi.org/10.1080/02652030500344...
; Kluczkovski, 2019KLUCZKOVSKI, A.M., 2019. Fungal and mycotoxin problems in the nut industry. Current Opinion in Food Science, vol. 29, pp. 56-63. http://dx.doi.org/10.1016/j.cofs.2019.07.009.
http://dx.doi.org/10.1016/j.cofs.2019.07...
; Tournas et al., 2015TOURNAS, V.H., NIAZI, N.S. and KOHN, J.S., 2015. Fungal presence in selected tree nuts and dried fruits. Microbiology Insights, vol. 8, pp. 1-6. http://dx.doi.org/10.4137/MBI.S24308. PMid:26056470.
http://dx.doi.org/10.4137/MBI.S24308...
). In this context, survey including almonds, pistachios, walnuts, and Brazil nuts found that the most common fungi were Aspergillus, Penicillium, and Rhizopus (Bayman et al., 2002BAYMAN, P., BAKER, J.L. and MAHONEY, N.E., 2002. Aspergillus on tree nuts: incidence and associations. Mycopathologia, vol. 155, no. 3, pp. 161-169. http://dx.doi.org/10.1023/A:1020419226146. PMid:12617503.
http://dx.doi.org/10.1023/A:102041922614...
). Rodrigues et al. (2012)RODRIGUES, P., VENÂNCIO, A. and LIMA, N., 2012. Aflatoxigenic fungi and aflatoxins in Portuguese almonds. The Scientific World Journal, vol. 2012, p. 471926. http://dx.doi.org/10.1100/2012/471926. PMid:22666128.
http://dx.doi.org/10.1100/2012/471926...
reported that the most frequent Aspergillus species in almonds were A. parasiticus 56%, A. flavus 36% and A. tamarii 8% (Rodrigues et al., 2012RODRIGUES, P., VENÂNCIO, A. and LIMA, N., 2012. Aflatoxigenic fungi and aflatoxins in Portuguese almonds. The Scientific World Journal, vol. 2012, p. 471926. http://dx.doi.org/10.1100/2012/471926. PMid:22666128.
http://dx.doi.org/10.1100/2012/471926...
). In addition, the most frequent fungi in walnuts were penicillium, A. tubingensis, A. niger, and A. flavus (Tournas et al., 2015TOURNAS, V.H., NIAZI, N.S. and KOHN, J.S., 2015. Fungal presence in selected tree nuts and dried fruits. Microbiology Insights, vol. 8, pp. 1-6. http://dx.doi.org/10.4137/MBI.S24308. PMid:26056470.
http://dx.doi.org/10.4137/MBI.S24308...
).
Inhibition of fungal growth with ozone treatment is one of the most efficient methods to control fungal contamination. This work focused on the decontamination of nuts using ozone concentrations of 4 ppm applied at different exposure times. Our data showed, that the spore production of all tested fungi were decreased with increasing the exposure time to ozone. The difference in the activity of ozone against different fungal species could result from the difference in their organic matter content, that might accelerate or decrease the toxic action of ozone (Hoigné and Bader, 1983HOIGNÉ, J. and BADER, H., 1983. Rate constants of reactions of ozone with organic and inorganic compounds in water—I: non-dissociating organic compounds. Water Research, vol. 17, no. 2, pp. 173-183. http://dx.doi.org/10.1016/0043-1354(83)90098-2.
http://dx.doi.org/10.1016/0043-1354(83)9...
). Ozone can suppress spore germination of fungal cells by oxidizing sulfhydryl and amino acid groups of enzymes of vital cellular components and causing rapid cell death (Afsah-Hejri et al., 2020AFSAH-HEJRI, L., HAJEB, P. and EHSANI, R.J., 2020. Application of ozone for degradation of mycotoxins in food: a review. Comprehensive Reviews in Food Science and Food Safety, vol. 19, no. 4, pp. 1777-1808. http://dx.doi.org/10.1111/1541-4337.12594. PMid:33337096.
http://dx.doi.org/10.1111/1541-4337.1259...
). In addition, ozonation showed to degrade RNA and DNA, and to induce mutagenic that affect spore germination and cause cell inactivation (Dillon et al., 1992DILLON, D., COMBES, R., MCCONVILLE, M. and ZEIGER, E., 1992. Ozone is mutagenic in Salmonella. Environmental and Molecular Mutagenesis, vol. 19, no. 4, pp. 331-337. http://dx.doi.org/10.1002/em.2850190412. PMid:1600961.
http://dx.doi.org/10.1002/em.2850190412...
; Ishizaki et al., 1981ISHIZAKI, K., SHINRIKI, N., IKEHATA, A. and UEDA, T., 1981. Degradation of nucleic acids with ozone. I. Degradation of nucleobases, ribonucleosides and ribonucleoside-5′-monophosphates. Chemical & Pharmaceutical Bulletin, vol. 29, no. 3, pp. 868-872. http://dx.doi.org/10.1248/cpb.29.868. PMid:7249159.
http://dx.doi.org/10.1248/cpb.29.868...
).
Our results showed that the increase in the ozone exposure time from 30 to 180 minutes resulted in a significant reduction in the radial growth of A. flavus. In consistent, El-Desouky et. al., 2012 showed, that ozonation of A. flavus at 40 ppm resulted in inhibition of mycelial growth by 65.6 to 95.6% for 5 to 20 min respectively (El-Desouky et al., 2012EL-DESOUKY, T.A., SHAROBA, A.M.A., EL-DESOUKY, A.I., EL-MANSY, H.A. and NAGUIB, K., 2012. Effect of ozone gas on degradation of aflatoxin B1 and Aspergillus Flavus fungal. Journal of Environmental & Analytical Toxicology, vol. 2, no. 2, p. 1000128. http://dx.doi.org/10.4172/2161-0525.1000128.
http://dx.doi.org/10.4172/2161-0525.1000...
). In addition, A. flavus growth was significantly reduced by 80.7 and 87.8% after 30 min of ozone exposure at concentrations of 40 and 60 μmol/mol, respectively (Savi et al., 2015SAVI, G., PIACENTINI, K. and SCUSSEL, V., 2015. Ozone treatment efficiency in aspergillus and penicillium growth inhibition and mycotoxin degradation of stored wheat grains (Triticum aestivum L.). Journal of Food Processing and Preservation, vol. 39, no. 6, pp. 940-948. http://dx.doi.org/10.1111/jfpp.12307.
http://dx.doi.org/10.1111/jfpp.12307...
). The mode of action of ozone against fungi was reported to be due to the oxidation of the constituents of the cell wall, cell membrane, and elements of cellular content. Ozone affect the polyunsaturated fatty acids in the fungal cell envelope to cause membrane permeability impairment and leakage of electrolytes and sugar cell contents (Guzel-Seydim et al., 2004GUZEL-SEYDIM, Z.B., GREENE, A.K. and SEYDIM, A.C., 2004. Use of ozone in the food industry. Lebensmittel-Wissenschaft + Technologie, vol. 37, no. 4, pp. 453-460. http://dx.doi.org/10.1016/j.lwt.2003.10.014.
http://dx.doi.org/10.1016/j.lwt.2003.10....
; Ouf and Ali, 2021OUF, S.A. and ALI, E.M., 2021. Does the treatment of dried herbs with ozone as a fungal decontaminating agent affect the active constituents? Environmental Pollution, vol. 277, p. 116715. http://dx.doi.org/10.1016/j.envpol.2021.116715. PMid:33652183.
http://dx.doi.org/10.1016/j.envpol.2021....
). Among the compounds that can also be oxidized by ozone are polysaccharides, enzymes and nucleic materials such as thymine and cytosine (Khadre et al., 2001KHADRE, M.A., YOUSEF, A.E. and KIM, J.-G., 2001. Microbiological aspects of ozone applications in food: a review. Journal of Food Science, vol. 66, no. 9, pp. 1242-1252. http://dx.doi.org/10.1111/j.1365-2621.2001.tb15196.x.
http://dx.doi.org/10.1111/j.1365-2621.20...
). Additionally, ozone might induce mutagenic effects leading to cell inactivation (Dillon et al., 1992DILLON, D., COMBES, R., MCCONVILLE, M. and ZEIGER, E., 1992. Ozone is mutagenic in Salmonella. Environmental and Molecular Mutagenesis, vol. 19, no. 4, pp. 331-337. http://dx.doi.org/10.1002/em.2850190412. PMid:1600961.
http://dx.doi.org/10.1002/em.2850190412...
).
Our results demonstrated the efficiency of ozone to make leakage of proteins and DNA from the cell membrane A. flavus. In consistent, Ouf et al. (2016)OUF, S.A., MOUSSA, T.A., ABD-ELMEGEED, A.M. and ELTAHLAWY, S.R., 2016. Anti-fungal potential of ozone against some dermatophytes. Brazilian Journal of Microbiology, vol. 47, no. 3, pp. 697-702. http://dx.doi.org/10.1016/j.bjm.2016.04.014. PMid:27287337.
http://dx.doi.org/10.1016/j.bjm.2016.04....
reported the effect of ozone in increasing the leakage of electrolytes and sugar in case of of M. gypseum, M. canis, T. interdigitale, T. mentagrophytes and T. rubrum (Ouf et al., 2016OUF, S.A., MOUSSA, T.A., ABD-ELMEGEED, A.M. and ELTAHLAWY, S.R., 2016. Anti-fungal potential of ozone against some dermatophytes. Brazilian Journal of Microbiology, vol. 47, no. 3, pp. 697-702. http://dx.doi.org/10.1016/j.bjm.2016.04.014. PMid:27287337.
http://dx.doi.org/10.1016/j.bjm.2016.04....
). The high leakage upon exposure to ozone might attributed to the damage of the membrane permeability, which greatly affect the normal physiological functioning of the cells (Pagés et al., 2020PAGÉS, M., KLEIBER, D. and VIOLLEAU, F., 2020. Ozonation of three different fungal conidia associated with apple disease: importance of spore surface and membrane phospholipid oxidation. Food Science & Nutrition, vol. 8, no. 10, pp. 5292-5297. http://dx.doi.org/10.1002/fsn3.1618. PMid:33133532.
http://dx.doi.org/10.1002/fsn3.1618...
). Our results showed, that ozone destroyed the inner layer of the cell wall of A. flavus by reducing the content of β-1,3-glucan rather than chitin. In this context, our SEM and TEM images showed significant morphological alterations in A. flavus upon exposure to ozone. These data are in consistent with the reported effects of ozone on A. flavus to cause changes in the components of the filamentous structure of the microorganism, such as conidiophore, vesicle and conidia, because of ozone oxidation (Guzel-Seydim et al., 2004GUZEL-SEYDIM, Z.B., GREENE, A.K. and SEYDIM, A.C., 2004. Use of ozone in the food industry. Lebensmittel-Wissenschaft + Technologie, vol. 37, no. 4, pp. 453-460. http://dx.doi.org/10.1016/j.lwt.2003.10.014.
http://dx.doi.org/10.1016/j.lwt.2003.10....
; Ouf et al., 2016OUF, S.A., MOUSSA, T.A., ABD-ELMEGEED, A.M. and ELTAHLAWY, S.R., 2016. Anti-fungal potential of ozone against some dermatophytes. Brazilian Journal of Microbiology, vol. 47, no. 3, pp. 697-702. http://dx.doi.org/10.1016/j.bjm.2016.04.014. PMid:27287337.
http://dx.doi.org/10.1016/j.bjm.2016.04....
).
Ozone gas has been proposed as an agent for the degradation of mycotoxins, among which are the aflatoxins (Prudente Junior and King, 2002PRUDENTE JUNIOR, A.D. and KING, J.M., 2002. Efficacy and safety evaluation of ozonation to degrade aflatoxin in corn. Journal of Food Science, vol. 67, no. 8, pp. 2866-2872. http://dx.doi.org/10.1111/j.1365-2621.2002.tb08830.x.
http://dx.doi.org/10.1111/j.1365-2621.20...
; Zorlugenç et al., 2008ZORLUGENÇ, B., ZORLUGENÇ, F.K., OZTEKIN, S. and EVLIYA, I.B., 2008. The influence of gaseous ozone and ozonated water on microbial flora and degradation of aflatoxin B(1) in dried figs. Food and Chemical Toxicology, vol. 46, no. 12, pp. 3593-3597. http://dx.doi.org/10.1016/j.fct.2008.09.003. PMid:18824207.
http://dx.doi.org/10.1016/j.fct.2008.09....
). Our results showed the high efficiency of ozone to reduce total aflatoxins in almost all examined nuts. In consistent several studies, support this finding. Proctor et al., 2004 showed a reduction in aflatoxin B1 of approximately 70% in peanuts after ozone application of 60 mg L−1 for 15 min (Proctor et al., 2004PROCTOR, A.D., AHMEDNA, M., KUMAR, J.V. and GOKTEPE, I., 2004. Degradation of aflatoxins in peanut kernels/flour by gaseous ozonation and mild heat treatment. Food Additives and Contaminants, vol. 21, no. 8, pp. 786-793. http://dx.doi.org/10.1080/02652030410001713898. PMid:15370830.
http://dx.doi.org/10.1080/02652030410001...
). Although the mode of action of detoxification with ozone is not clear for some mycotoxins, it is described that the oxidizing agents react with the functional groups in the mycotoxin molecules, change their molecular structures, and form products with lower molecular weight, less double bonds, and less toxicity (Wang et al., 2016WANG, L., SHAO, H., LUO, X., WANG, R., LI, Y., LI, Y., LUO, Y. and CHEN, Z., 2016. Effect of ozone treatment on deoxynivalenol and wheat quality. PLoS One, vol. 11, no. 1, p. e0147613. http://dx.doi.org/10.1371/journal.pone.0147613. PMid:26812055.
http://dx.doi.org/10.1371/journal.pone.0...
). McKenzie, et al., 1998, presented the mechanism of aflatoxin B1 degradation by ozone, the final products of the reaction being aldehydes, ketones, acids and carbon dioxide (McKenzie et al., 1998MCKENZIE, K.S., KUBENA, L.F., DENVIR, A.J., ROGERS, T.D., HITCHENS, G.D., BAILEY, R.H., HARVEY, R.B., BUCKLEY, S.A. and PHILLIPS, T.D., 1998. Aflatoxicosis in turkey poults is prevented by treatment of naturally contaminated corn with ozone generated by electrolysis. Poultry Science, vol. 77, no. 8, pp. 1094-1102. http://dx.doi.org/10.1093/ps/77.8.1094. PMid:9706072.
http://dx.doi.org/10.1093/ps/77.8.1094...
). Ozone reacts with the double C8-C9 units of the furan terminal of aflatoxin B1 forming the vinyl ether at the terminal furan ring in their structures (Proctor et al., 2004PROCTOR, A.D., AHMEDNA, M., KUMAR, J.V. and GOKTEPE, I., 2004. Degradation of aflatoxins in peanut kernels/flour by gaseous ozonation and mild heat treatment. Food Additives and Contaminants, vol. 21, no. 8, pp. 786-793. http://dx.doi.org/10.1080/02652030410001713898. PMid:15370830.
http://dx.doi.org/10.1080/02652030410001...
).
The Joint FAO/WHO Expert Committee On Food Additives (2006)JOINT FAO/WHO EXPERT COMMITTEE ON FOOD ADDITIVES, 2006. Evaluation of certain food contaminants. Geneva: World Health Organization. Sixty-fourth report of the Joint FAO/WHO Expert Committee on Food Additives. WHO technical report series, 930. has set international standards for maximum regulatory levels that provide a basis for food safety management. The Codex specifies a maximum limit of 15 µg/kg, which is 15 parts per billion (15 µg/kg = 15 ppb) for total aflatoxins (sum of AFB1, AFB2, AFG1, and AFG2) in peanuts, Brazil nuts, hazelnuts, pistachios, and almonds for further processing. A maximum limit of 10 µg/kg is also set for ready-to-eat Brazil nuts, dried figs, hazelnuts, pistachios, and almonds (Joint FAO/WHO Expert Committee on Food Additives, 2006JOINT FAO/WHO EXPERT COMMITTEE ON FOOD ADDITIVES, 2006. Evaluation of certain food contaminants. Geneva: World Health Organization. Sixty-fourth report of the Joint FAO/WHO Expert Committee on Food Additives. WHO technical report series, 930.). Our results revealed that ozonation process reduced aflatoxins level to approximately 2 to 10 µg/kg which is lower than the maximum allowed level for consumption.
Several studies showed that ozone can be used safely for reducing pathogenic fungi of nuts without affecting their nutritional properties (Afsah-Hejri et al., 2020AFSAH-HEJRI, L., HAJEB, P. and EHSANI, R.J., 2020. Application of ozone for degradation of mycotoxins in food: a review. Comprehensive Reviews in Food Science and Food Safety, vol. 19, no. 4, pp. 1777-1808. http://dx.doi.org/10.1111/1541-4337.12594. PMid:33337096.
http://dx.doi.org/10.1111/1541-4337.1259...
). For example, ozone increased the quality of chestnut shelf life without affecting its polyphenol content (Vettraino et al., 2019VETTRAINO, A.M., BIANCHINI, L., CARADONNA, V., FORNITI, R., GOFFI, V., ZAMBELLI, M., TESTA, A., VINCIGUERRA, V. and BOTONDI, R., 2019. Ozone gas as a storage treatment to control Gnomoniopsis castanea, preserving chestnut quality. Journal of the Science of Food and Agriculture, vol. 99, no. 13, pp. 6060-6065. http://dx.doi.org/10.1002/jsfa.9883. PMid:31226223.
http://dx.doi.org/10.1002/jsfa.9883...
), and ozone was effective in the degradation of aflatoxins in pistachios without affecting its fatty acid compositions or quality properties (Akbas and Ozdemir, 2006AKBAS, M. and OZDEMIR, M., 2006. Effect of different ozone treatments on aflatoxin degradation and physicochemical properties of pistachio. Journal of the Science of Food and Agriculture, vol. 86, no. 13, pp. 2099-2104. http://dx.doi.org/10.1002/jsfa.2579.
http://dx.doi.org/10.1002/jsfa.2579...
). Furthermore, ozonation was more efficient in the inactivation of A. flavus in Brazil nuts without affecting its fatty acid profile of the crude oil and other (Oliveira et al., 2020OLIVEIRA, J.M., ALENCAR, E.R., BLUM, L.E.B., FERREIRA, W.F.S., BOTELHO, S.C.C., RACANICCI, A.M.C., LEANDRO, E.S., MENDONÇA, M.A., MOSCON, E.S., BIZERRA, L.V.A.S. and SILVA, C.R., 2020. Ozonation of Brazil nuts: decomposition kinetics, control of Aspergillus flavus and the effect on color and on raw oil quality. LWT, vol. 123, p. 109106. http://dx.doi.org/10.1016/j.lwt.2020.109106.
http://dx.doi.org/10.1016/j.lwt.2020.109...
). In contrast, our results demonstrated the inhibitory effect of ozone on total carbohydrate, lipid, and protein contents of nuts when applied with exposure time over 90 min. Despite ozone is safe green technology to be used as a powerful oxidant disinfectants against fungi in food, caution should be consider, as ozone was reported to cause alteration in food ingredients (Trombete et al., 2016TROMBETE, F., FREITAS-SILVA, O., SALDANHA, T., VENÂNCIO, A. and FRAGA, M., 2016. Ozone against mycotoxins and pesticide residues in food: current applications and perspectives. International Food Research Journal, vol. 23, pp. 2545-2556.). These include the effect of ozone on color change, lipid oxidation, and the degradation of proteins and vitamins (Alexandre et al., 2017ALEXANDRE, A.P.S., CASTANHA, N., CALORI-DOMINGUES, M.A. and AUGUSTO, P.E.D., 2017. Ozonation of whole wheat flour and wet milling effluent: degradation of deoxynivalenol (DON) and rheological properties. Journal of Environmental Science and Health, Part B, vol. 52, no. 7, pp. 516-524. http://dx.doi.org/10.1080/03601234.2017.1303325. PMid:28541097.
http://dx.doi.org/10.1080/03601234.2017....
; Zhu, 2018ZHU, F., 2018. Effect of ozone treatment on the quality of grain products. Food Chemistry, vol. 264, pp. 358-366. http://dx.doi.org/10.1016/j.foodchem.2018.05.047. PMid:29853388.
http://dx.doi.org/10.1016/j.foodchem.201...
). Similarly, Ozone effectively reduced Aflatoxin B1 and total aflatoxins from pistachios kernels at 9.0 g m−3 concentrations for 420 min, while changes were observed in the organoleptic properties of ground pistachios, after 5.0 mg L−1 ozone treatment for 140 min (Akbas and Ozdemir, 2006AKBAS, M. and OZDEMIR, M., 2006. Effect of different ozone treatments on aflatoxin degradation and physicochemical properties of pistachio. Journal of the Science of Food and Agriculture, vol. 86, no. 13, pp. 2099-2104. http://dx.doi.org/10.1002/jsfa.2579.
http://dx.doi.org/10.1002/jsfa.2579...
).
Our data showed the adverse effect of ozone on the lipid profile of nuts with exposure time over 90 min. In supporting to this finding, ozone can oxidize lipids by increasing the oxygen reactive species (ROS) production by reacting with water inside the cells, and ROS can also interact with fatty acid in the cell membrane to cause lipid peroxidation (Heath, 2008HEATH, R.L., 2008. Modification of the biochemical pathways of plants induced by ozone: what are the varied routes to change? Environmental Pollution, vol. 155, no. 3, pp. 453-463. http://dx.doi.org/10.1016/j.envpol.2008.03.010. PMid:18456378.
http://dx.doi.org/10.1016/j.envpol.2008....
). In addition, ozone oxidizes unsaturated bonds of lipids to generate lipid oxidation (Khadre et al., 2001KHADRE, M.A., YOUSEF, A.E. and KIM, J.-G., 2001. Microbiological aspects of ozone applications in food: a review. Journal of Food Science, vol. 66, no. 9, pp. 1242-1252. http://dx.doi.org/10.1111/j.1365-2621.2001.tb15196.x.
http://dx.doi.org/10.1111/j.1365-2621.20...
). In consistent, ozonation of wheat flour showed to oxidised the lipids in treated (1500 ppm, up to 45 min) (Sandhu et al., 2011SANDHU, H.P., MANTHEY, F.A. and SIMSEK, S., 2011. Quality of bread made from ozonated wheat (Triticum aestivum L.) flour. Journal of the Science of Food and Agriculture, vol. 91, no. 9, pp. 1576-1584. http://dx.doi.org/10.1002/jsfa.4350. PMid:21445841.
http://dx.doi.org/10.1002/jsfa.4350...
), and to reduce the fatty acid value of volatile compounds related to lipid oxidation in whole grain flour (5 g/h, up to 45 min) (Obadi et al., 2018OBADI, M., ZHU, K.-X., PENG, W., NOMAN, A., MOHAMMED, K. and ZHOU, H.-M., 2018. Characterization of oil extracted from whole grain flour treated with ozone gas. Journal of Cereal Science, vol. 79, pp. 527-533. http://dx.doi.org/10.1016/j.jcs.2017.12.007.
http://dx.doi.org/10.1016/j.jcs.2017.12....
) and to increase the acid value of lipids in maize (Kells et al., 2001KELLS, S.A., MASON, L.J., MAIER, D.E. and WOLOSHUK, C.P., 2001. Efficacy and fumigation characteristics of ozone in stored maize. Journal of Stored Products Research, vol. 37, no. 4, pp. 371-382. http://dx.doi.org/10.1016/S0022-474X(00)00040-0. PMid:11463399.
http://dx.doi.org/10.1016/S0022-474X(00)...
). Furthermore, recently, we demonstrated the effect of ozone on reducing the total volume of essential oil in herbs and spices, despite the efficient use of ozone as an antifungal agent (Ouf and Ali, 2021OUF, S.A. and ALI, E.M., 2021. Does the treatment of dried herbs with ozone as a fungal decontaminating agent affect the active constituents? Environmental Pollution, vol. 277, p. 116715. http://dx.doi.org/10.1016/j.envpol.2021.116715. PMid:33652183.
http://dx.doi.org/10.1016/j.envpol.2021....
).
Ozone is one of the most promising disinfectant agent in food industry to ensure safety and quality. Despite, the costs of the equipment for ozone treatment are higher than the costs for the traditional approaches of fumigation or sanitization; the higher cost is compensated by long-term applications of the ozone (Botondi et al., 2021BOTONDI, R., BARONE, M. and GRASSO, C., 2021. A review into the effectiveness of ozone technology for improving the safety and preserving the quality of fresh-cut fruits and vegetables. Foods, vol. 10, no. 4, p. 748. http://dx.doi.org/10.3390/foods10040748. PMid:33915979.
http://dx.doi.org/10.3390/foods10040748...
). The electricity consumption of the ozone generators depends on both the amount of the oxygen flow and flow output of ozone. In this context, the aqueous ozone generators showed to be more cost effective than gaseous ones ozone generators (Aslam et al., 2020ASLAM, R., ALAM, M.S. and SAEED, P.A., 2020. Sanitization potential of ozone and its role in postharvest quality management of fruits and vegetables. Food Engineering Reviews, vol. 12, no. 1, pp. 48-67. http://dx.doi.org/10.1007/s12393-019-09204-0.
http://dx.doi.org/10.1007/s12393-019-092...
).
5. Conclusion
Ozone treatment can be an effective method for control of nut deterioration and toxin decontamination in the market. In this study, we demonstrated the efficiency of ozone gas (4 ppm) to significantly reduce the total fungal count and to degrade aflatoxins in pistachio, peanut, and almond. Ozone showed to increase the leakage of DNA and protein from fungal cell and to decrease the content of β-1, 3-glucan in fungal cell wall in an exposure time dependent manner. However, increasing the exposure time of ozone in nuts up to 180 minutes showed to reduce the total lipid, carbohydrates, and protein and to alter the fatty acid profile in pistachio, almond and peanuts. Thus, ozonation is a suitable green decontaminating approach for nuts, but the negative impact of the long exposure time of ozone on the nutritional quality of nuts should be considered.
Supplementary Material
Supplementary material accompanies this paper.
Table S1 Total count (TC) per gram dry weight, cases of isolations (CI), and occurrence (OC) of different fungal species isolated from different nut samples on Dichloran Rose-Bengal Chloramphenicol agar at 25°C.This material is available as part of the online article from https://www.scielo.br/j/bjb
Acknowledgements
This work was supported by the Deanship of Scientific Research, Vice Presidency for Graduate Studies and Scientific Research, King Faisal University, Saudi Arabia [Project No. GRANT711 (AN00080)]
References
- AFSAH-HEJRI, L., HAJEB, P. and EHSANI, R.J., 2020. Application of ozone for degradation of mycotoxins in food: a review. Comprehensive Reviews in Food Science and Food Safety, vol. 19, no. 4, pp. 1777-1808. http://dx.doi.org/10.1111/1541-4337.12594 PMid:33337096.
» http://dx.doi.org/10.1111/1541-4337.12594 - AKBAS, M. and OZDEMIR, M., 2006. Effect of different ozone treatments on aflatoxin degradation and physicochemical properties of pistachio. Journal of the Science of Food and Agriculture, vol. 86, no. 13, pp. 2099-2104. http://dx.doi.org/10.1002/jsfa.2579
» http://dx.doi.org/10.1002/jsfa.2579 - ALEXANDRE, A.P.S., CASTANHA, N., CALORI-DOMINGUES, M.A. and AUGUSTO, P.E.D., 2017. Ozonation of whole wheat flour and wet milling effluent: degradation of deoxynivalenol (DON) and rheological properties. Journal of Environmental Science and Health, Part B, vol. 52, no. 7, pp. 516-524. http://dx.doi.org/10.1080/03601234.2017.1303325 PMid:28541097.
» http://dx.doi.org/10.1080/03601234.2017.1303325 - ALSOHAILI, S. and BANI-HASAN, B.M., 2018. Morphological and molecular identification of fungi isolated from different environmental sources in northern eastern Jordan deseret. Jordan Journal of Biological Sciences, vol. 11, pp. 329-337.
- ASLAM, R., ALAM, M.S. and SAEED, P.A., 2020. Sanitization potential of ozone and its role in postharvest quality management of fruits and vegetables. Food Engineering Reviews, vol. 12, no. 1, pp. 48-67. http://dx.doi.org/10.1007/s12393-019-09204-0
» http://dx.doi.org/10.1007/s12393-019-09204-0 - BATTILANI, P., 2010. Mycotoxins in nuts. Options Méditerranéennes A, vol. 94, pp. 167-193.
- BAYMAN, P., BAKER, J.L. and MAHONEY, N.E., 2002. Aspergillus on tree nuts: incidence and associations. Mycopathologia, vol. 155, no. 3, pp. 161-169. http://dx.doi.org/10.1023/A:1020419226146 PMid:12617503.
» http://dx.doi.org/10.1023/A:1020419226146 - BLAIZE, M., NORMAND, A.C., IMBERT, S., AL-HATMI, A.M.S., CHRYSSANTHOU, E., CASSAING, S., SCHUTTLER, C., HASSEINE, L., MAHINC, C., COSTA, D., BONNAL, C., RANQUE, S., SAUTOUR, M., RUBIO, E., DELHAES, L., RIAT, A., SENDID, B., KRISTENSEN, L., BRANDENBERGER, M., STUBBE, D., BRUN, S., PIARROUX, R. and FEKKAR, A., 2021. Antifungal susceptibility of 182 Fusarium species isolates from 20 European centers: comparison between EUCAST and gradient concentration strip methods. Antimicrobial Agents and Chemotherapy, vol. 65, no. 12, p. e0149521. http://dx.doi.org/10.1128/AAC.01495-21 PMid:34543091.
» http://dx.doi.org/10.1128/AAC.01495-21 - BLIGH, E.G. and DYER, W.J., 1959. A rapid method of total lipid extraction and purification. Canadian Journal of Biochemistry and Physiology, vol. 37, no. 8, pp. 911-917. http://dx.doi.org/10.1139/o59-099 PMid:13671378.
» http://dx.doi.org/10.1139/o59-099 - BOTONDI, R., BARONE, M. and GRASSO, C., 2021. A review into the effectiveness of ozone technology for improving the safety and preserving the quality of fresh-cut fruits and vegetables. Foods, vol. 10, no. 4, p. 748. http://dx.doi.org/10.3390/foods10040748 PMid:33915979.
» http://dx.doi.org/10.3390/foods10040748 - DAS, K., TIWARI, R., SHRIVASTAVA, D. and BILASPUR, B.C., 2010. Techniques for evaluation of medicinal plant products as antimicrobial agent: current methods and future trends. Journal of Medicinal Plants Research, vol. 4, pp. 104-111.
- DAVIES, C.R., WOHLGEMUTH, F., YOUNG, T., VIOLET, J., DICKINSON, M., SANDERS, J.W., VALLIERES, C. and AVERY, S.V., 2021. Evolving challenges and strategies for fungal control in the food supply chain. Fungal Biology Reviews, vol. 36, pp. 15-26. http://dx.doi.org/10.1016/j.fbr.2021.01.003 PMid:34084209.
» http://dx.doi.org/10.1016/j.fbr.2021.01.003 - DILLON, D., COMBES, R., MCCONVILLE, M. and ZEIGER, E., 1992. Ozone is mutagenic in Salmonella. Environmental and Molecular Mutagenesis, vol. 19, no. 4, pp. 331-337. http://dx.doi.org/10.1002/em.2850190412 PMid:1600961.
» http://dx.doi.org/10.1002/em.2850190412 - DUBOIS, M., GILLES, K., HAMILTON, J.K., REBERS, P.A. and SMITH, F., 1951. A colorimetric method for the determination of sugars. Nature, vol. 168, no. 4265, p. 167. http://dx.doi.org/10.1038/168167a0 PMid:14875032.
» http://dx.doi.org/10.1038/168167a0 - DUBOIS, M., GILLES, K.A., HAMILTON, J.K., REBERS, P.A. and SMITH, F., 1956. Colorimetric method for determination of sugars and related substances. Analytical Chemistry, vol. 28, no. 3, pp. 350-356. http://dx.doi.org/10.1021/ac60111a017
» http://dx.doi.org/10.1021/ac60111a017 - EL-DESOUKY, T.A., SHAROBA, A.M.A., EL-DESOUKY, A.I., EL-MANSY, H.A. and NAGUIB, K., 2012. Effect of ozone gas on degradation of aflatoxin B1 and Aspergillus Flavus fungal. Journal of Environmental & Analytical Toxicology, vol. 2, no. 2, p. 1000128. http://dx.doi.org/10.4172/2161-0525.1000128
» http://dx.doi.org/10.4172/2161-0525.1000128 - GUZEL-SEYDIM, Z.B., GREENE, A.K. and SEYDIM, A.C., 2004. Use of ozone in the food industry. Lebensmittel-Wissenschaft + Technologie, vol. 37, no. 4, pp. 453-460. http://dx.doi.org/10.1016/j.lwt.2003.10.014
» http://dx.doi.org/10.1016/j.lwt.2003.10.014 - HEATH, R.L., 2008. Modification of the biochemical pathways of plants induced by ozone: what are the varied routes to change? Environmental Pollution, vol. 155, no. 3, pp. 453-463. http://dx.doi.org/10.1016/j.envpol.2008.03.010 PMid:18456378.
» http://dx.doi.org/10.1016/j.envpol.2008.03.010 - HOIGNÉ, J. and BADER, H., 1983. Rate constants of reactions of ozone with organic and inorganic compounds in water—I: non-dissociating organic compounds. Water Research, vol. 17, no. 2, pp. 173-183. http://dx.doi.org/10.1016/0043-1354(83)90098-2
» http://dx.doi.org/10.1016/0043-1354(83)90098-2 - ISHIZAKI, K., SHINRIKI, N., IKEHATA, A. and UEDA, T., 1981. Degradation of nucleic acids with ozone. I. Degradation of nucleobases, ribonucleosides and ribonucleoside-5′-monophosphates. Chemical & Pharmaceutical Bulletin, vol. 29, no. 3, pp. 868-872. http://dx.doi.org/10.1248/cpb.29.868 PMid:7249159.
» http://dx.doi.org/10.1248/cpb.29.868 - JABLONSKI, J.E., FU, T.J., JACKSON, L.S. and GENDEL, S.M., 2010. Determination of protein levels in soy and peanut oils by colorimetric assay and ELISA. Journal of AOAC International, vol. 93, no. 1, pp. 213-220. PMid:20334183.
- JOINT FAO/WHO EXPERT COMMITTEE ON FOOD ADDITIVES, 2006. Evaluation of certain food contaminants Geneva: World Health Organization. Sixty-fourth report of the Joint FAO/WHO Expert Committee on Food Additives. WHO technical report series, 930.
- JØRGENSEN, K., 2005. Occurrence of ochratoxin A in commodities and processed food--a review of EU occurrence data. Food Additives and Contaminants, vol. 22, suppl. 1, pp. 26-30. http://dx.doi.org/10.1080/02652030500344811 PMid:16332618.
» http://dx.doi.org/10.1080/02652030500344811 - KELLS, S.A., MASON, L.J., MAIER, D.E. and WOLOSHUK, C.P., 2001. Efficacy and fumigation characteristics of ozone in stored maize. Journal of Stored Products Research, vol. 37, no. 4, pp. 371-382. http://dx.doi.org/10.1016/S0022-474X(00)00040-0 PMid:11463399.
» http://dx.doi.org/10.1016/S0022-474X(00)00040-0 - KHADRE, M.A., YOUSEF, A.E. and KIM, J.-G., 2001. Microbiological aspects of ozone applications in food: a review. Journal of Food Science, vol. 66, no. 9, pp. 1242-1252. http://dx.doi.org/10.1111/j.1365-2621.2001.tb15196.x
» http://dx.doi.org/10.1111/j.1365-2621.2001.tb15196.x - KHALIL, N.M., EL-GHANY, M.N. and RODRÍGUEZ-COUTO, S., 2019. Antifungal and anti-mycotoxin efficacy of biogenic silver nanoparticles produced by Fusarium chlamydosporum and Penicillium chrysogenum at non-cytotoxic doses. Chemosphere, vol. 218, pp. 477-486. http://dx.doi.org/10.1016/j.chemosphere.2018.11.129 PMid:30497030.
» http://dx.doi.org/10.1016/j.chemosphere.2018.11.129 - KLUCZKOVSKI, A.M., 2019. Fungal and mycotoxin problems in the nut industry. Current Opinion in Food Science, vol. 29, pp. 56-63. http://dx.doi.org/10.1016/j.cofs.2019.07.009
» http://dx.doi.org/10.1016/j.cofs.2019.07.009 - KO, Y.T. and LIN, Y.L., 2004. 1,3-beta-glucan quantification by a fluorescence microassay and analysis of its distribution in foods. Journal of Agricultural and Food Chemistry, vol. 52, no. 11, pp. 3313-3318. http://dx.doi.org/10.1021/jf0354085 PMid:15161189.
» http://dx.doi.org/10.1021/jf0354085 - LIEN, K.W., WANG, X., PAN, M.H. and LING, M.P., 2019. Assessing aflatoxin exposure risk from peanuts and peanut products imported to Taiwan. Toxins, vol. 11, no. 2, p. 80. http://dx.doi.org/10.3390/toxins11020080 PMid:30717134.
» http://dx.doi.org/10.3390/toxins11020080 - MANOUSARIDIS, G., NERANTZAKI, A., PALEOLOGOS, E., TSIOTSIAS, A., SAVVAIDIS, I.N. and KONTOMINAS, M., 2005. Effect of ozone on microbial, chemical and sensory attributes of shucked mussels. Food Microbiology, vol. 22, no. 1, pp. 1-9. http://dx.doi.org/10.1016/j.fm.2004.06.003
» http://dx.doi.org/10.1016/j.fm.2004.06.003 - MCKENZIE, K.S., KUBENA, L.F., DENVIR, A.J., ROGERS, T.D., HITCHENS, G.D., BAILEY, R.H., HARVEY, R.B., BUCKLEY, S.A. and PHILLIPS, T.D., 1998. Aflatoxicosis in turkey poults is prevented by treatment of naturally contaminated corn with ozone generated by electrolysis. Poultry Science, vol. 77, no. 8, pp. 1094-1102. http://dx.doi.org/10.1093/ps/77.8.1094 PMid:9706072.
» http://dx.doi.org/10.1093/ps/77.8.1094 - MENDONÇA, M.A., ARAÚJO, W.M.C., BORGO, L.A. and ALENCAR, E.R., 2017. Lipid profile of different infant formulas for infants. PLoS One, vol. 12, no. 6, p. e0177812. http://dx.doi.org/10.1371/journal.pone.0177812 PMid:28570611.
» http://dx.doi.org/10.1371/journal.pone.0177812 - MIR, S.A., SHAH, M.A., MIR, M.M., SIDIQ, T., SUNOOJ, K.V., SIDDIQUI, M.W., MARSZAŁEK, K. and KHANEGHAH, A.M., 2022. Recent developments for controlling microbial contamination of nuts. Critical Reviews in Food Science and Nutrition In press. http://dx.doi.org/10.1080/10408398.2022.2038077 PMid:35170397.
» http://dx.doi.org/10.1080/10408398.2022.2038077 - NORLIA, M., JINAP, S., NOR-KHAIZURA, M.A.R., RADU, S., CHIN, C.K., SAMSUDIN, N.I.P. and FARAWAHIDA, A.H., 2019. Molecular characterisation of aflatoxigenic and non-aflatoxigenic strains of Aspergillus section Flavi isolated from imported peanuts along the supply chain in Malaysia. Toxins, vol. 11, no. 9, p. 501. http://dx.doi.org/10.3390/toxins11090501 PMid:31470527.
» http://dx.doi.org/10.3390/toxins11090501 - OBADI, M., ZHU, K.-X., PENG, W., NOMAN, A., MOHAMMED, K. and ZHOU, H.-M., 2018. Characterization of oil extracted from whole grain flour treated with ozone gas. Journal of Cereal Science, vol. 79, pp. 527-533. http://dx.doi.org/10.1016/j.jcs.2017.12.007
» http://dx.doi.org/10.1016/j.jcs.2017.12.007 - OLIVEIRA, J.M., ALENCAR, E.R., BLUM, L.E.B., FERREIRA, W.F.S., BOTELHO, S.C.C., RACANICCI, A.M.C., LEANDRO, E.S., MENDONÇA, M.A., MOSCON, E.S., BIZERRA, L.V.A.S. and SILVA, C.R., 2020. Ozonation of Brazil nuts: decomposition kinetics, control of Aspergillus flavus and the effect on color and on raw oil quality. LWT, vol. 123, p. 109106. http://dx.doi.org/10.1016/j.lwt.2020.109106
» http://dx.doi.org/10.1016/j.lwt.2020.109106 - ONO, E., HIROOKA, E., ROSSI, C. and ONO, M., 2011. Mycotoxins in seeds and nuts. In: V.R. PREEDY, R.R. WATSON and V.B. PATEL, eds. Nuts and seeds in health and disease prevention Amsterdam: Elsevier, pp. 121-127.
- OUF, S.A. and ALI, E.M., 2021. Does the treatment of dried herbs with ozone as a fungal decontaminating agent affect the active constituents? Environmental Pollution, vol. 277, p. 116715. http://dx.doi.org/10.1016/j.envpol.2021.116715 PMid:33652183.
» http://dx.doi.org/10.1016/j.envpol.2021.116715 - OUF, S.A., MOUSSA, T.A., ABD-ELMEGEED, A.M. and ELTAHLAWY, S.R., 2016. Anti-fungal potential of ozone against some dermatophytes. Brazilian Journal of Microbiology, vol. 47, no. 3, pp. 697-702. http://dx.doi.org/10.1016/j.bjm.2016.04.014 PMid:27287337.
» http://dx.doi.org/10.1016/j.bjm.2016.04.014 - OUYANG, Q., DUAN, X., LI, L. and TAO, N., 2019. Cinnamaldehyde exerts its antifungal activity by disrupting the cell wall integrity of Geotrichum citri-aurantii. Frontiers in Microbiology, vol. 10, no. 55, pp. 1-9. http://dx.doi.org/10.3389/fmicb.2019.00055 PMid:30761105.
» http://dx.doi.org/10.3389/fmicb.2019.00055 - PAES, J., FARONI, L., MARTINS, M., CECON, P. and HELENO, F., 2017. Reaction kinetics of ozone gas in wheat flour. Engenharia Agrícola, vol. 37, no. 3, pp. 520-528. http://dx.doi.org/10.1590/1809-4430-eng.agric.v37n3p520-528/2017
» http://dx.doi.org/10.1590/1809-4430-eng.agric.v37n3p520-528/2017 - PAGÉS, M., KLEIBER, D. and VIOLLEAU, F., 2020. Ozonation of three different fungal conidia associated with apple disease: importance of spore surface and membrane phospholipid oxidation. Food Science & Nutrition, vol. 8, no. 10, pp. 5292-5297. http://dx.doi.org/10.1002/fsn3.1618 PMid:33133532.
» http://dx.doi.org/10.1002/fsn3.1618 - PICKOVA, D., OSTRY, V. and MALIR, F., 2021. A recent overview of producers and important dietary sources of aflatoxins. Toxins, vol. 13, no. 3, p. 186. http://dx.doi.org/10.3390/toxins13030186 PMid:33802572.
» http://dx.doi.org/10.3390/toxins13030186 - PROCTOR, A.D., AHMEDNA, M., KUMAR, J.V. and GOKTEPE, I., 2004. Degradation of aflatoxins in peanut kernels/flour by gaseous ozonation and mild heat treatment. Food Additives and Contaminants, vol. 21, no. 8, pp. 786-793. http://dx.doi.org/10.1080/02652030410001713898 PMid:15370830.
» http://dx.doi.org/10.1080/02652030410001713898 - PRUDENTE JUNIOR, A.D. and KING, J.M., 2002. Efficacy and safety evaluation of ozonation to degrade aflatoxin in corn. Journal of Food Science, vol. 67, no. 8, pp. 2866-2872. http://dx.doi.org/10.1111/j.1365-2621.2002.tb08830.x
» http://dx.doi.org/10.1111/j.1365-2621.2002.tb08830.x - RAHIMI, A., SASANI, E., REZAIE, S., DALLAL, M.M.S., MAHMOUDI, S., AHMADI, A., GHAFFARI, M., AALA, F. and KHODAVAISY, S., 2021. Molecular identification of aflatoxigenic Aspergillus species in dried nuts and grains collected from Tehran, Iran. Journal of Environmental Health Science & Engineering, vol. 19, no. 2, pp. 1795-1799. http://dx.doi.org/10.1007/s40201-021-00734-6 PMid:34900308.
» http://dx.doi.org/10.1007/s40201-021-00734-6 - RAHMATI, E., KHOSHTAGHAZA, M.H., BANAKAR, A. and EBADI, M.T., 2022. Decontamination technologies for medicinal and aromatic plants: a review. Food Science & Nutrition, vol. 10, no. 3, pp. 784-799. http://dx.doi.org/10.1002/fsn3.2707 PMid:35311169.
» http://dx.doi.org/10.1002/fsn3.2707 - RODRIGUES, P., VENÂNCIO, A. and LIMA, N., 2012. Aflatoxigenic fungi and aflatoxins in Portuguese almonds. The Scientific World Journal, vol. 2012, p. 471926. http://dx.doi.org/10.1100/2012/471926 PMid:22666128.
» http://dx.doi.org/10.1100/2012/471926 - SANDHU, H.P., MANTHEY, F.A. and SIMSEK, S., 2011. Quality of bread made from ozonated wheat (Triticum aestivum L.) flour. Journal of the Science of Food and Agriculture, vol. 91, no. 9, pp. 1576-1584. http://dx.doi.org/10.1002/jsfa.4350 PMid:21445841.
» http://dx.doi.org/10.1002/jsfa.4350 - SAVI, G., PIACENTINI, K. and SCUSSEL, V., 2015. Ozone treatment efficiency in aspergillus and penicillium growth inhibition and mycotoxin degradation of stored wheat grains (Triticum aestivum L.). Journal of Food Processing and Preservation, vol. 39, no. 6, pp. 940-948. http://dx.doi.org/10.1111/jfpp.12307
» http://dx.doi.org/10.1111/jfpp.12307 - SULEIMAN, M.N., EMUA, S.A. and TAIGA, A., 2008. Effect of aques leaf extracts on a spot fungus (Fusarium sp) isolated from Compea. American-Eurasian Journal of Sustainable Agriculture, vol. 2, pp. 261-263.
- TOURNAS, V.H., NIAZI, N.S. and KOHN, J.S., 2015. Fungal presence in selected tree nuts and dried fruits. Microbiology Insights, vol. 8, pp. 1-6. http://dx.doi.org/10.4137/MBI.S24308 PMid:26056470.
» http://dx.doi.org/10.4137/MBI.S24308 - TROMBETE, F., FREITAS-SILVA, O., SALDANHA, T., VENÂNCIO, A. and FRAGA, M., 2016. Ozone against mycotoxins and pesticide residues in food: current applications and perspectives. International Food Research Journal, vol. 23, pp. 2545-2556.
- VALLONE, L. and STELLA, S., 2014. Evaluation of antifungal effect of gaseous ozone in a meat processing plant. Italian Journal of Food Safety, vol. 3, no. 2, p. 1680. http://dx.doi.org/10.4081/ijfs.2014.1680 PMid:27800339.
» http://dx.doi.org/10.4081/ijfs.2014.1680 - VENKATACHALAM, M. and SATHE, S.K., 2006. Chemical composition of selected edible nut seeds. Journal of Agricultural and Food Chemistry, vol. 54, no. 13, pp. 4705-4714. http://dx.doi.org/10.1021/jf0606959 PMid:16787018.
» http://dx.doi.org/10.1021/jf0606959 - VETTRAINO, A.M., BIANCHINI, L., CARADONNA, V., FORNITI, R., GOFFI, V., ZAMBELLI, M., TESTA, A., VINCIGUERRA, V. and BOTONDI, R., 2019. Ozone gas as a storage treatment to control Gnomoniopsis castanea, preserving chestnut quality. Journal of the Science of Food and Agriculture, vol. 99, no. 13, pp. 6060-6065. http://dx.doi.org/10.1002/jsfa.9883 PMid:31226223.
» http://dx.doi.org/10.1002/jsfa.9883 - VISAGIE, C.M., HOUBRAKEN, J., FRISVAD, J.C., HONG, S.-B., KLAASSEN, C.H.W., PERRONE, G., SEIFERT, K.A., VARGA, J., YAGUCHI, T. and SAMSON, R.A., 2014. Identification and nomenclature of the genus Penicillium. Studies in Mycology, vol. 78, no. 1, pp. 343-371. http://dx.doi.org/10.1016/j.simyco.2014.09.001 PMid:25505353.
» http://dx.doi.org/10.1016/j.simyco.2014.09.001 - WANG, L., SHAO, H., LUO, X., WANG, R., LI, Y., LI, Y., LUO, Y. and CHEN, Z., 2016. Effect of ozone treatment on deoxynivalenol and wheat quality. PLoS One, vol. 11, no. 1, p. e0147613. http://dx.doi.org/10.1371/journal.pone.0147613 PMid:26812055.
» http://dx.doi.org/10.1371/journal.pone.0147613 - WU, Q., XIE, L. and XU, H., 2018. Determination of toxigenic fungi and aflatoxins in nuts and dried fruits using imaging and spectroscopic techniques. Food Chemistry, vol. 252, pp. 228-242. http://dx.doi.org/10.1016/j.foodchem.2018.01.076 PMid:29478536.
» http://dx.doi.org/10.1016/j.foodchem.2018.01.076 - ZHU, F., 2018. Effect of ozone treatment on the quality of grain products. Food Chemistry, vol. 264, pp. 358-366. http://dx.doi.org/10.1016/j.foodchem.2018.05.047 PMid:29853388.
» http://dx.doi.org/10.1016/j.foodchem.2018.05.047 - ZORLUGENÇ, B., ZORLUGENÇ, F.K., OZTEKIN, S. and EVLIYA, I.B., 2008. The influence of gaseous ozone and ozonated water on microbial flora and degradation of aflatoxin B(1) in dried figs. Food and Chemical Toxicology, vol. 46, no. 12, pp. 3593-3597. http://dx.doi.org/10.1016/j.fct.2008.09.003 PMid:18824207.
» http://dx.doi.org/10.1016/j.fct.2008.09.003
Publication Dates
-
Publication in this collection
22 June 2022 -
Date of issue
2024
History
-
Received
08 May 2022 -
Accepted
31 May 2022