Abstract
This study aimed to develop multicomponent oleogels to achieve desirable technological properties, affordability, and enhanced sensory acceptance for future food applications. Two Central Composite Rotatable Designs (CCRD) were performed with three independent variables, monoacylglycerols (MG), candelilla wax (CLX), and hardfat (HF). One design used soybean oil oleogels (SB) and the other used high oleic sunflower oil oleogels (SF). The variable responses evaluated were hardness (N), oil loss (%), thermal stability, and visual strength. For SB oleogels, CLX was related to the variable that was superior concerning all technological properties as >2 N in hardness, no oil loss, and higher stability to thermal treatment. For SF oleogels, the MG and the interactions of MG, CLX, and HF showed significant technological properties, indicating that the three oleogelators can co-crystallize better together in this oil, thus obtaining the same results as CLX alone in higher amounts.
Keywords:
Oleogels; Multicomponent system; Physical properties; Structuration; Low sat; Zero trans
Resumo
O objetivo deste estudo foi desenvolver oleogéis multicomponentes com propriedades tecnológicas desejáveis, baixo preço e boa aceitação sensorial, visando a futuras aplicações em alimentos. Para isso, dois delineamentos compostos centrais rotacionais (DCCR) com três variáveis independentes foram utilizados. As variáveis independentes foram monoacilgliceróis (MG), cera de candelilla (CLX) e hardfat (HF). Um primeiro DCCR foi feito para o óleo de soja (SB) e um segundo para o óleo de girassol alto oleico (SF). As variáveis resposta avaliadas foram dureza (N), perda de óleo (%), estabilidade térmica e estabilidade visual. Em oleogéis de SB, a CLX foi a única variável significativa em todas as propriedades tecnológicas: a CLX foi responsável por alcançar dureza > 2 N, ausência de perda de óleo e alta estabilidade térmica. Os oleogéis de SF, além de CLX, MG e das interações MG, CLX e HF, também apresentaram efeito significativo nas propriedades tecnológicas. Isso mostra que, especialmente no SF e na presença da combinação ternária MG:CLX:HF, os três estruturantes podem cocristalizar e oleogéis, com propriedades tecnológicas similares às dos oleogéis com CLX, em altas concentrações, podem ser obtidos.
Palavras-chave:
Oleogéis; Sistemas multicomponentes; Propriedades físicas; Estruturação; Baixo saturados; Zero trans
HIGHLIGHTS
• Highlights Monoglycerides (MG), candelilla wax (CLW), and crambe hardfat (HF) oleogels were evaluated as a fat replacer.
• Two different oils- soybean (SB) and high oleic sunflower (SF)- were evaluated as lipid matrix.
• CLW was the oleogelator that primarily influenced hardness and stability for SB.
• In SF oleogels, the MG and HF were also significant about physical properties.
1 Introduction
In response to new regulations (U.S. Food and Drug Administration, 2013U.S. Food and Drug Administration – FDA. (2013). Tentative determination regarding partially hydrogenated oils (Vol. 78, No. 217). Maryland, EUA: FDA., 2015 U.S. Food and Drug Administration – FDA. (2015). Final determination regarding partially hydrogenated oils. Maryland, EUA: FDA.), recommendations (Food and Agriculture Organization of the United Nations, 2010Food and Agriculture Organization of the United Nations – FAO. (2010). Fats and fatty acids in human nutrition. Report of an expert consultation (FAO Food and Nutrition Paper, 91). Rome: FAO.; U.S. Department of Health, 2015U.S. Department of Health. Human Services. U.S. Department of Agriculture. (2015). 2015-2020 Dietary Guidelines for Americans (8th ed.). Rockville: U.S. Department of Health.), and changes in consumer preferences regarding fat consumption, the search for a healthier fat alternative to reduce saturated and trans-fat content has drawn the attention of food scientists and food producers in recent years. However, if unsaturated fatty acids directly replace Saturated Fatty Acids (SFA), the final structure of the product will not match the desired technological characteristics and consumer expectations (functional and sensorial properties). Consequently, a new strategy called oleogelation to structure liquid edible oil has been advocated to formulate healthier alternatives (oleogels) for saturated and trans-fats (Vieira et al., 2015Vieira, S. A., McClements, D. J., & Decker, E. A. (2015). Challenges of utilizing healthy fats in foods. Advances in Nutrition, 6(3), 309S-317S. PMid:25979504. http://dx.doi.org/10.3945/an.114.006965
http://dx.doi.org/10.3945/an.114.006965...
).
Oleogels are liquid lipid materials (oils) that are structured by a three-dimensional network formed of solid lipid or non-lipid materials (oleogelators) added in small concentrations (<10%) (Dassanayake et al., 2011Dassanayake, L. S. K., Kodali, D. R., & Ueno, S. (2011). Formation of oleogels based on edible lipid materials. Current Opinion in Colloid & Interface Science, 16(5), 432-439. http://dx.doi.org/10.1016/j.cocis.2011.05.005
http://dx.doi.org/10.1016/j.cocis.2011.0...
).
Oleogels might have a critical role in the food industry as healthier fat sources; however, like most novel-designed food systems, some issues must be addressed or minimized in order to achieve acceptable technological properties that make them available for industrial food applications (Patel et al., 2020Patel, A. R., Nicholson, R. A., & Marangoni, A. G. (2020). Applications of fat mimetics for the replacement of saturated and hydrogenated fat in food products. Current Opinion in Food Science, 33, 61-68. http://dx.doi.org/10.1016/j.cofs.2019.12.008
http://dx.doi.org/10.1016/j.cofs.2019.12...
). Nevertheless, the primary challenge with oleogels relates to their ability to mimic the mouthfeel and texture of commonly used fats. In this regard, the synergistic interaction between two different oleogelators for example, can alter the microstructural network and enhance the strength of the solid-like systems (Doan et al., 2018aDoan, C. D., Tavernier, I., Danthine, S., Rimaux, T., & Dewettinck, K. (2018a). Physical compatibility between wax esters and triglycerides in hybrid shortenings and margarines prepared in rice bran oil. Journal of the Science of Food and Agriculture, 98(3), 1042-1051. PMid:28718922. http://dx.doi.org/10.1002/jsfa.8553
http://dx.doi.org/10.1002/jsfa.8553...
, 2018bDoan, C. D., Tavernier, I., Okuro, P. K., & Dewettinck, K. (2018b). Internal and external factors affecting the crystallization, gelation and applicability of wax-based oleogels in food industry. Innovative Food Science & Emerging Technologies, 45, 42-52. http://dx.doi.org/10.1016/j.ifset.2017.09.023
http://dx.doi.org/10.1016/j.ifset.2017.0...
). Some multicomponent systems with food-grade combinations have been studied, such as waxes combinations (Doan et al., 2017aDoan, C. D., Tavernier, I., Sintang, M. D. B., Danthine, S., Van de Walle, D., Rimaux, T., & Dewettinck, K. (2017a). Crystallization and gelation behavior of low- and high melting waxes in rice bran oil: a case-study on berry wax and sunflower wax. Food Biophysics, 12(1), 97-108. http://dx.doi.org/10.1007/s11483-016-9467-y
http://dx.doi.org/10.1007/s11483-016-946...
; Jana & Martini, 2016Jana, S., & Martini, S. (2016). Phase behavior of binary blends of four different waxes. Journal of the American Oil Chemists’ Society, 93(4), 543-554. http://dx.doi.org/10.1007/s11746-016-2789-6
http://dx.doi.org/10.1007/s11746-016-278...
); candelilla wax (CLX) + high melting point triacylglycerol’s (TAG) (Ramírez-Gómez et al., 2016Ramírez-Gómez, N. O., Acevedo, N. C., Toro-Vázquez, J. F., Ornelas-Paz, J. J., Dibildox-Alvarado, E., & Pérez-Martínez, J. D. (2016). Phase behavior, structure and rheology of candelilla wax/fully hydrogenated soybean oil mixtures with and without vegetable oil. Food Research International, 89(Pt 1), 828-837. PMid:28460985. http://dx.doi.org/10.1016/j.foodres.2016.10.025
http://dx.doi.org/10.1016/j.foodres.2016...
); waxes + monoacylglycerol (MG) (Silva et al., 2018bSilva, T. L. T., Chaves, K. F., Fernandes, G. D., Rodrigues, J. B., Bolini, H. M. A., & Arellano, D. B. (2018b). Sensory and technological evaluation of margarines with reduced saturated fatty acid contents using oleogel technology. Journal of the American Oil Chemists’ Society, 95(6), 673-685. http://dx.doi.org/10.1002/aocs.12074
http://dx.doi.org/10.1002/aocs.12074...
; Ögutcu & Yilmaz, 2014Ögutcu, M., & Yilmaz, E. (2014). Oleogels of virgin olive oil with carnauba wax and monoglyceride as spreadable products. Grasas y Aceites, 65(3), 1-11. http://dx.doi.org/10.3989/gya.0349141
http://dx.doi.org/10.3989/gya.0349141...
; Pérez-martínez et al., 2019Pérez-martínez, J. D., Sánchez-becerril, M., Marangoni, A. G., Toro-vazquez, J. F., Ornelas-paz, J. J., & Ibarra-junquera, V. (2019). Structuration, elastic properties scaling, and mechanical reversibility of candelilla wax oleogels with and without emulsifiers. Food Research International, 122, 471-478. https://doi.org/10.1016/j.foodres.2019.05.020.
https://doi.org/10.1016/j.foodres.2019.0...
); MG + phytosterols (Bin Sintang et al., 2017Bin Sintang, M. D., Rimaux, T., Van de Walle, D., Dewettinck, K., & Patel, A. R. (2017). Oil structuring properties of monoglycerides and phytosterols mixtures. European Journal of Lipid Science and Technology, 119(3), 1-14. http://dx.doi.org/10.1002/ejlt.201500517
http://dx.doi.org/10.1002/ejlt.201500517...
); MG + HF (Silva & Danthine, 2021Silva, T. L. T., & Danthine, S. (2021). Effect of high-intensity ultrasound on the oleogelation and physical properties of high melting point monoglycerides and triglycerides oleogels. Journal of Food Science, 86(2), 343-356. PMid:33448022. http://dx.doi.org/10.1111/1750-3841.15589
http://dx.doi.org/10.1111/1750-3841.1558...
); to name a few. These studies evaluated the benefit of reducing the total amount of oleogelators needed by synergic binary systems. However, in some cases, a binary mixture was not satisfactory. As an alternative, some ternary systems has recently been studied as hardfat +sorbitan monostearate (SMS) + CLX (Godoi et al., 2019Godoi, K. R. R., Basso, R. C., Ming, C. C., da Silva, V. M., da Cunha, R. L., Barrera-Arellano, D., Ribeiro, A. P. B., & Ribeiro, B. (2019). Physicochemical and rheological properties of soybean organogels : interactions between different structuring agents. Food Research International, 124, 108475. PMid:31466657. http://dx.doi.org/10.1016/j.foodres.2019.05.023
http://dx.doi.org/10.1016/j.foodres.2019...
, 2020Godoi, K. R. R., Basso, R. C., Ming, C. C., da Silva, A. Á., Cardoso, L. P., & Ribeiro, A. P. B. (2020). Crystallization, microstructure and polymorphic properties of soybean oil organogels in a hybrid structuring system. Food Research International, 137, 109460. PMid:33233134. http://dx.doi.org/10.1016/j.foodres.2020.109460
http://dx.doi.org/10.1016/j.foodres.2020...
), MG + beeswax + propolis wax (Fayaz et al., 2017Fayaz, G., Goli, S. A. H., Kadivar, M., Valoppi, F., Barba, L., Calligaris, S., & Nicoli, M. C. (2017). Potential application of pomegranate seed oil oleogels based on monoglycerides, beeswax and propolis wax as partial substitutes of palm oil in functional chocolate spread. Lebensmittel-Wissenschaft + Technologie, 86, 523-529. http://dx.doi.org/10.1016/j.lwt.2017.08.036
http://dx.doi.org/10.1016/j.lwt.2017.08....
), CLX + MG + hardfat (Silva et al., 2018aSilva, T. L. T., Arellano, D. B., & Martini, S. (2018a). Physical properties of Candelilla Wax, monoacylglycerols, and fully hydrogenated oil oleogels. Journal of the American Oil Chemists’ Society, 95(7), 797-811. http://dx.doi.org/10.1002/aocs.12096
http://dx.doi.org/10.1002/aocs.12096...
), phytosterol + γ-oryzanol + lecithin (Okuro et al., 2018Okuro, P. K., Malfatti-Gasperini, A. A., Vicente, A. A., & Cunha, R. L. (2018). Lecithin and phytosterols-based mixtures as hybrid structuring agents in different organic phases. Food Research International, 111, 168-177. PMid:30007673. http://dx.doi.org/10.1016/j.foodres.2018.05.022
http://dx.doi.org/10.1016/j.foodres.2018...
). It is important to note that the limits and effects of each oleogelator as well as binary/ternary combinations of the physical properties of fats warrant additional exploration. One of the tools with which to evaluate these effects is the use of statistical designs (Giacomozzi et al., 2018Giacomozzi, A. S., Carrín, M. E., & Palla, C. A. (2018). Muffins elaborated with optimized monoglycerides oleogels : from solid fat replacer obtention to product quality evaluation. Journal of Food Science, 83(6), 1505-1515. PMid:29786854. http://dx.doi.org/10.1111/1750-3841.14174
http://dx.doi.org/10.1111/1750-3841.1417...
; Godoi et al., 2019Godoi, K. R. R., Basso, R. C., Ming, C. C., da Silva, V. M., da Cunha, R. L., Barrera-Arellano, D., Ribeiro, A. P. B., & Ribeiro, B. (2019). Physicochemical and rheological properties of soybean organogels : interactions between different structuring agents. Food Research International, 124, 108475. PMid:31466657. http://dx.doi.org/10.1016/j.foodres.2019.05.023
http://dx.doi.org/10.1016/j.foodres.2019...
; Paglarini et al., 2018Paglarini, C. S., Furtado, G. F., Biachi, J. P., Vidal, V. A. S., Martini, S., Forte, M. B. S., Cunha, R. L., & Pollonio, M. A. R. (2018). Functional emulsion gels with potential application in meat products. Journal of Food Engineering, 222, 29-37. http://dx.doi.org/10.1016/j.jfoodeng.2017.10.026
http://dx.doi.org/10.1016/j.jfoodeng.201...
).
Based on these previous studies and the need to continue to explore the use of multicomponent oleogels, the objective of this study was to produce an oleogel for future application in foods by using different combinations of a ternary oleogelator system with MG, HF and CLX. To evaluate whether or not the oleogelators interact among themselves and how they influence the technological properties, a Central Composite Rotatable Design (CCRD) was used to screen the concentrations in two liquid oils: soybean and high oleic sunflower oils.
2 Material and methods
2.1 Materials
Previous research has shown that the gelling properties of waxes depend not only on the type of wax but also on the oil used (Martini et al., 2015Martini, S., Tan, C. Y., & Jana, S. (2015). Physical Characterization of Wax/Oil Crystalline Networks. Journal of Food Science, 80(5), C989-C997. PMid:25850679. http://dx.doi.org/10.1111/1750-3841.12853
http://dx.doi.org/10.1111/1750-3841.1285...
). Therefore, two different oils with specific compositions were used in this study. Soybean oil (SB) used in the study was purchased from the local market (Campinas, SP, Brazil), and high oleic sunflower oil (SF) was donated by ChemyUnion S.A. (Sorocaba, SP, Brazil). CLX was obtained from Koster Keunen Holland BV (Raamburg, NL). MG GRINDSTED® CRYSTALLIZER was obtained from DuPont Nutrition & Health (Barueri, SP, Brazil), and the fully HF was kindly donated by ChemyUnion S.A. (Sorocaba, SP, Brazil).
2.2 Characterization of the lipid material
2.2.1 Fatty acid composition
The fatty acid composition of the oils, HF and MG, were determined using an Agilent 6850 Series Gas Chromatography (GC) system (Agilent Technologies, Santa Clara, CA, USA) equipped with a capillary column, following esterification according to a method described by Hartman & Lago (1973)Hartman, L., & Lago, R. C. A. (1973). Rapid preparation of fatty acid methyl esters from lipids. Laboratory Practice, 22, 475-476, 494. PMid:4727126.. Fatty acid methyl esters were separated using AOCS method Ce 2-66 (American Oil Chemists’ Society, 2009American Oil Chemists’ Society – AOCS. (2009). Official Methods and Recommended Practices of the American Oil Chemists’ Society (5th ed.). USA: AOCS.), employing a 60-m DB-23 capillary column (50% cyanopropyl methylpolysiloxane; Agilent Technologies) with an internal diameter of 0.25 mm, coated with a 0.25-µm film. Chromatography was performed under the following conditions: heating at 110 °C for 5 min in an oven; followed by heating to 215 °C at a rate of 5 °C/min and holding at 215 °C for 24 min. The detector temperature was 280 °C, the injector temperature was 250 °C, helium was used as the carrier gas, the split ratio was 1:50, and the injection volume was 1.0 µL. The qualitative composition was determined via area normalization and was expressed as the mass percentage. The analysis was performed in triplicate.
2.2.2 Melting point (MP)
The MP of the pure raw materials was measured using differential scanning calorimetry (DSC, TA Instruments, New Castle, DE). Between 10 and 15 mg of sample was weighted and hermetically sealed in aluminum pans and placed in the DSC. Samples were kept at 25 °C for 1 min for stabilization in the DSC, and then cooled to -20 °C at 5 °C/min. In order to allow for complete crystallization, the samples were held at -20 °C for 90 min. Finally, the samples were heated to 100 °C at 5 °C/min, and the MP temperature was measured based on the peak temperature of the higher melting peak (Kerr et al., 2011Kerr, R. M., Tombokan, X., Ghosh, S., & Martini, S. (2011). Crystallization behavior of anhydrous milk fat - sunflower oil wax blends. Journal of Agricultural and Food Chemistry, 59(6), 2689-2695. PMid:21344886. http://dx.doi.org/10.1021/jf1046046
http://dx.doi.org/10.1021/jf1046046...
).
2.3 Samples preparation
Samples (500 mL) were prepared in duplicate by heating the oils to 80 °C under stirring using a heated stirring plate (350 rpm). Once the temperature was reached, the structuring agents were slowly added at the concentrations predefined in Table 1 and mixed until complete dissolution. In addition, the mixture was kept under agitation for 3 min. Hot samples were placed in 50 mL beakers and aluminum cylinders (20 mL) and stored at 5 °C for 24 h plus 25 °C for 24 h in order to form the gel. The physical properties of the gels formed as a result of this process were measured as described in detail below. All analyses described below were performed at 25 °C.
Experimental design matrix for optimization of oleogels according to the central composite rotatable design (experimental real values are in parenthesis)
2.4 Physical analysis
2.4.1 Hardness
The oleogel hardness was evaluated after conditioning 40 mL of the sample in a 50 mL beaker for 24 h at 5 °C and 24 h at 25 °C (48 h stabilization) using a texture stable MicroSystems model TA-XT2i (Godalming — UK) with a cylindrical probe (25 mm of diameter and 35 mm of high) and compression speed 1.0 mm/s. Four replicates of each sample were evaluated (Rocha et al., 2013Rocha, J. C. B., Lopes, J. D., Mascarenhas, M. C. N., Arellano, D. B., Guerreiro, L. M. R., & da Cunha, R. L. (2013). Thermal and rheological properties of organogels formed by sugarcane or candelilla wax in soybean oil. Food Research International, 50(1), 318-323. http://dx.doi.org/10.1016/j.foodres.2012.10.043
http://dx.doi.org/10.1016/j.foodres.2012...
).
2.4.2 Thermal stability and visual evaluation
Conditioned samples in a 50 mL beaker after 48 h of stabilization were evaluated at 25 °C and under temperature cycling (120 h at 40 °C) (Silva et al., 2019aSilva, T. L. T., Arellano, D. B., & Martini, S. (2019a). Interactions between candelilla wax and saturated triacylglycerols in oleogels. Food Research International, 121, 900-909. PMid:31108823. http://dx.doi.org/10.1016/j.foodres.2019.01.018
http://dx.doi.org/10.1016/j.foodres.2019...
). Samples were evaluated in terms of flowing behavior and superficial oil exudation. For this determination, a scale was developed to quantify the stability of the sample based on its behavior, where samples that were visually hard and did not flow or presented superficial oil exudation received a grade of 5. A sample that did not flow but showed slight superficial oil exudation was graded with a 4. Samples that started to flow slowly but exhibited a visual strong and acceptable structure were graded with a 3, and samples graded with a 2 were those that flowed easily. Samples graded with a 1 were those that only showed an increase in viscosity and turbidity compared to oil (Godoi et al., 2019Godoi, K. R. R., Basso, R. C., Ming, C. C., da Silva, V. M., da Cunha, R. L., Barrera-Arellano, D., Ribeiro, A. P. B., & Ribeiro, B. (2019). Physicochemical and rheological properties of soybean organogels : interactions between different structuring agents. Food Research International, 124, 108475. PMid:31466657. http://dx.doi.org/10.1016/j.foodres.2019.05.023
http://dx.doi.org/10.1016/j.foodres.2019...
).
2.4.3 Oil Loss
The oil loss method used was based on previous research (Lopez-Martínez et al., 2015Lopez-martínez, A., Charó-alonso, M. A., Marangoni, A. G., & Toro-vazquez, J. F. (2015). Monoglyceride organogels developed in vegetable oil with and without ethylcellulose. Food Research International, 72, 37-46. http://dx.doi.org/10.1016/j.foodres.2015.03.019
http://dx.doi.org/10.1016/j.foodres.2015...
). Approximately 20 mL of oleogels were placed in small aluminum cylinders (3 cm diameter and 2 cm high). The cylinders were poured onto a weighted Petri plate with a filter paper (15 cm diameter) after 48 h stabilization. The Petri plate + filter paper was weighed after 24 h. All oleogel excess was removed from the top of the system (plate + filter) and kept in the cylinders during the weighing to ensure that only the oil loss in the filter paper was measured. Four replicates of each sample were measured. Oil loss was calculated using Equation 1.
where Mf is related to the final mass (g) of the paper filter, Mi is the initial mass of the paper filter, and Moleogel is the total mass of the oleogel used in each replicate.
2.4.4 Microstructure
The crystal microstructure was evaluated using polarized light microscopy (PLM). An aliquot of crystallized sample was taken and placed between a slide and cover slide. The crystal microstructure was evaluated using a 20× magnification objective in a PLM (Olympus BX 41 Tokyo, Japan) equipped with an Infinity 2 digital camera (Lumenera Scientific, Infinity 2, Ottawa, ON, Canada). The size and number of crystals were measured using Image Pro-Plus software version 7.0 for Windows (Media Cybernetics, Rockville, MD, USA).
2.5 Experiment design
To study the way in which the concentration of MG, CLX, and HF affects the formation of an oleogel for application in food systems, a CCRD for three independent variables was performed. The independent variables (factors) were the concentration of the oleogelators MG, CLX, and HF. The dependent variables (responses) were hardness, oil loss, visual stability at 25 °C, and thermal stability. Each factor in the design was studied at five different levels with two fractional points (−1 and +1), one central point (0), and two axial points encoded as −α (-1.68) and +α (+1.68), as shown in Table 1. Three replicates at the central point were performed to evaluate the pure error. The ranges of the variables were determined based on our previous experiments (Silva et al., 2018bSilva, T. L. T., Chaves, K. F., Fernandes, G. D., Rodrigues, J. B., Bolini, H. M. A., & Arellano, D. B. (2018b). Sensory and technological evaluation of margarines with reduced saturated fatty acid contents using oleogel technology. Journal of the American Oil Chemists’ Society, 95(6), 673-685. http://dx.doi.org/10.1002/aocs.12074
http://dx.doi.org/10.1002/aocs.12074...
, 2019aSilva, T. L. T., Arellano, D. B., & Martini, S. (2019a). Interactions between candelilla wax and saturated triacylglycerols in oleogels. Food Research International, 121, 900-909. PMid:31108823. http://dx.doi.org/10.1016/j.foodres.2019.01.018
http://dx.doi.org/10.1016/j.foodres.2019...
). They were chosen to obtain oleogels with a maximum concentration of CLX of 3% and total oleogelators of 10% for future use in food applications without compromising the sensory properties and SFA reduction. The entire experiment plan resulted in 17 runs the levels of which are listed in Table 1. For modeling the responses, a second-order polynomial function was fitted to the experiment results. The three-dimensional (3D) surface graphs were built to visualize the primary and interactive effects of the independent variables on the dependent ones. From the analysis of the response surfaces and equations generated, it was possible to determine the better concentration of each oleogelator and their effect on the different physical properties measured. Validation of the equations was performed by comparing the experiment and equation results.
2.6 Statistical analysis
Statistic 7.0.0 (Stat-Ease Inc., Minneapolis, MN, USA) software was used to design the experiment and process the results by Analysis of Variance (ANOVA). The quality of fit of the models was expressed by the coefficients of determination (R2 and adjusted R2), and the F-test tested the statistical significance.
3 Results and discussion
3.1 Lipid material characterization
The raw material used in this study was characterized according to its chemical composition and MP (Table 2). The SB oil was primarily composed of C18:2 and C18:1. Because of the higher amounts of fatty acids with double bonds in the oil, SB oil presented the lowest MP. The SF was composed mainly of C18:1 and showed a higher MP than that of SB. The oleogelators studied showed similar composition between HF and MG, with a higher amount of C18 and C22. However, even though the oleogelators showed similar compositions, both the molecular structure and MP were different.
Fatty acid composition and melting point (°C) of the soybean oil (SB), high oleic sunflower oil (SF), hardfat (HF), and monoacylglycerol (MG).
It should be noted that CLX was not chemically characterized in this study. Consequently, for discussions on CLX, the composition obtained by Doan et al. (2017b) Doan, C. D., To, C. M., De Vrieze, M., Lynen, F., Danthine, S., Brown, A., Dewettinck, K., & Patel, A. R. (2017b). Chemical profiling of the major components in natural waxes to elucidate their role in liquid oil structuring. Food Chemistry, 214, 717-725. PMid:27507530. http://dx.doi.org/10.1016/j.foodchem.2016.07.123
http://dx.doi.org/10.1016/j.foodchem.201...
was used as a basis. The authors stated that CLX comprises approximately 72% of hydrocarbons, 15% of wax esters, 9% of free fatty acids, and 2% of free fatty alcohols. The hydrocarbon fraction and the wax esters are mainly composed of n-alkanes with saturated chains of 29–33 carbons, where C31 makes up 82% of this fraction.
3.2 Effect of oleogelators on oleogel formation and physical properties
The CCRD matrix from the experiment design with the independent variables and responses is presented in Table 1. Table 3 shows the regression coefficients for these responses.
Regression coefficient values of different responses for oleogels using Response Surface Methodology (RSM)
Hardness: Observing the SB oleogels, the hardness is mainly influenced by the CLX amount where samples with at least 2.39% (+1) could form an oleogel with at least 1 N (samples 2, 6, and 12). The highest hardness was observed when MG and HF were in the central point or lower (samples 6 and 12). As observed in Table 1, the only positive factor significant for hardness was CLX linear (p = 0.003) and quadratic (p = 0.017). The interaction CLX:MG was also significant but resulted in a negative coefficient (p = 0.028, C=-0.49) (Table 3). Compared to other waxes, CLX presented a greater degree of hardness. It was assumed that the gel formation of CLX is a result of a significant reorganization of the crystalline phase at lower temperatures because CLX crystallizes into very fine linear particles that are further organized into an open aggregate-like structure (Patel et al., 2015Patel, A. R., Babaahmadi, M., Lesaffer, A., & Dewettinck, K. (2015). Rheological Profiling of Organogels Prepared at Critical Gelling Concentrations of Natural Waxes in a Triacylglycerol Solvent. Journal of Agricultural and Food Chemistry, 63(19), 4862-4869. PMid:25932656. http://dx.doi.org/10.1021/acs.jafc.5b01548
http://dx.doi.org/10.1021/acs.jafc.5b015...
). The formation of such crystalline microplatelets by CLX is attributed to a high proportion of linear hydrocarbons (n-alkanes) (Toro-Vazquez et al., 2010Toro-Vazquez, J. F., Morales-Rueda, J., Mallia, V. A., & Weiss, R. G. (2010). Relationship between molecular structure and thermo-mechanical properties of candelilla wax and amides derived from (R)-12-hydroxystearic acid as gelators of safflower oil. Food Biophysics, 5(3), 193-202. http://dx.doi.org/10.1007/s11483-010-9159-y
http://dx.doi.org/10.1007/s11483-010-915...
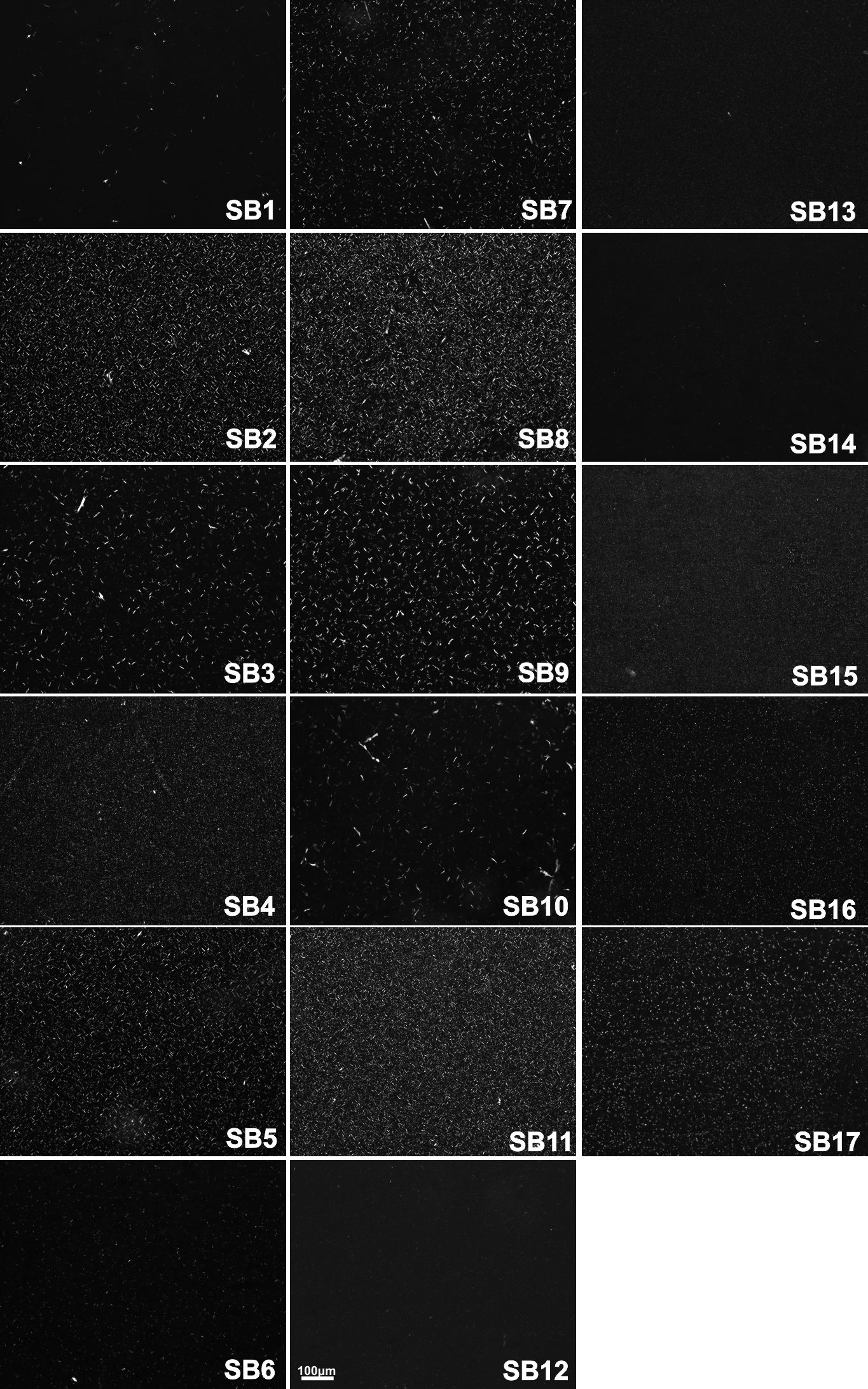
). It is difficult to observe these very small microplatelets (~8µm), but they are present as shown in Figure 1, samples SB6 and SB12.
Polarized light microscopy pictures of crystals obtained for oleogels with soybean oil at 25 °C (20x). For samples concentration 1-17, see Table 1.
The increase in MG and HF seems to generate a softer gel even with CLX kept at a higher concentration, as observed for samples 4 and 8. This is why the interaction factor MG and CLX in Table 3 presented a significant negative coefficient. In samples 8 and 4, it was possible to see more crystals in a needle shape than in samples 6 and 12. The microstructures in samples 8 and 4 showed needle-like crystals characteristic of MG and HF (Kesselman & Shimoni, 2007Kesselman, E., & Shimoni, E. (2007). Imaging of oil/monoglyceride networks by polarizing near-field scanning optical microscopy. Food Biophysics, 2(2–3), 117-123. http://dx.doi.org/10.1007/s11483-007-9038-3
http://dx.doi.org/10.1007/s11483-007-903...
), suggesting that MG and HF in SB oil did not interact with the CLX very well.
The addition of MG also modified the network morphology of BEW-based oleogels, adding a combination of spherulitic crystals with tiny needle-like crystals in the background (Barroso et al., 2020Barroso, N. G., Okuro, P. K., Ribeiro, A. P. B., & Cunha, R. L. (2020). Tailoring properties of mixed-component oleogels: wax and monoglyceride interactions towards flaxseed oil structuring. Gels, 6(1), 5. PMid:32023926. http://dx.doi.org/10.3390/gels6010005
http://dx.doi.org/10.3390/gels6010005...
). Similar behavior in which crystal sized CLX-oleogels increase in higher concentrations of HF followed by a softer gel was observed for different HF (palm, soybean, crambe, and palm kernel sources) (Silva et al., 2019aSilva, T. L. T., Arellano, D. B., & Martini, S. (2019a). Interactions between candelilla wax and saturated triacylglycerols in oleogels. Food Research International, 121, 900-909. PMid:31108823. http://dx.doi.org/10.1016/j.foodres.2019.01.018
http://dx.doi.org/10.1016/j.foodres.2019...
). However, the negative effect was observed only for higher concentrations of HF in the system (50% or more). When used in lower amounts (0.25HF/0.75CLX), the combination resulted in improved physical properties and a synergic interaction (Silva et al., 2019aSilva, T. L. T., Arellano, D. B., & Martini, S. (2019a). Interactions between candelilla wax and saturated triacylglycerols in oleogels. Food Research International, 121, 900-909. PMid:31108823. http://dx.doi.org/10.1016/j.foodres.2019.01.018
http://dx.doi.org/10.1016/j.foodres.2019...
; Ramírez-Gómez et al., 2016Ramírez-Gómez, N. O., Acevedo, N. C., Toro-Vázquez, J. F., Ornelas-Paz, J. J., Dibildox-Alvarado, E., & Pérez-Martínez, J. D. (2016). Phase behavior, structure and rheology of candelilla wax/fully hydrogenated soybean oil mixtures with and without vegetable oil. Food Research International, 89(Pt 1), 828-837. PMid:28460985. http://dx.doi.org/10.1016/j.foodres.2016.10.025
http://dx.doi.org/10.1016/j.foodres.2016...
)
For SF oleogels, CLX was not the most and only positive significant factor affecting hardness. Conversely here, the interactions with MG (MG and CLX; MG and HF) also showed a positive effect on hardness (p < 0.05; Table 3). Table 1 shows the confirmation of these results by runs 3, 8, 10, and 11 which also showed hardness higher than 1N in addition to runs 2, 6, and 12 (higher in CLX). This means that the increase in the levels of MG and HF in samples with higher amounts of CLX did not negatively affect hardness in SF, as observed for SB samples. These results are very interesting as using only waxes to structure oleogels results in acceptable hardness. However, the complete replacement of saturated fats with waxes-oloegels also reduces the solid content, plasticity, and spreadability in the final products. Additionally, this might result in a waxy mouthfeel that would negatively affect the sensorial properties. Therefore, a combination of waxes with fat hardstocks (MG and HF) could both limit the high consumption of saturated and/or trans-fats and achieve the desired spreadability and sensory appeal (Doan et al., 2018aDoan, C. D., Tavernier, I., Danthine, S., Rimaux, T., & Dewettinck, K. (2018a). Physical compatibility between wax esters and triglycerides in hybrid shortenings and margarines prepared in rice bran oil. Journal of the Science of Food and Agriculture, 98(3), 1042-1051. PMid:28718922. http://dx.doi.org/10.1002/jsfa.8553
http://dx.doi.org/10.1002/jsfa.8553...
).
This difference in hardness due to the different oils used for samples 8, 10, 11, and 13 can be observed on the crystal microstructure (Figures 1 and 2). For example, the lower amounts of CLX formed runs 3 and 11 which showed a lower amount of crystals in the SB oil (Figure 1) than in the SF oil (Figure 2). On SF oleogels, these runs presented an increase in the number of crystals, indicating that more MG and HF could crystallize (needles-like), forming a harder network in the SF oil. High amounts of 3 oleogelators form runs 10 and 8. In this case, the MG and HF might have co-crystallized with the microplatelets of the CLX in the SF because we could not see the MG/HF needle-like crystals in these samples as we observed for the SB.
Polarized light microscopy pictures of crystals obtained for oleogels with high oleic sunflower oil at 25 °C (20x). For samples concentration 1-17, see Table 1.
The positive influence of high amounts of MG and HF in SF and not in SB might be due to the difference in chemical composition between the oils. The high content of oleic in SF and consequently higher MP (Table 2) can influence oleogel stabilization because the first 24 h of oleogel formation occurs at 5 °C, slightly higher than SF MP. Some higher MP components from the SF might crystallize at this temperature, enabling interactions between the oil, MG, and HF, further influencing the CLX network. Even after oleogels were placed at 25 °C, the resultant stable crystals remained. A similar benefit using only MG was found when a high oleic oil was used and stabilized at a lower temperature (Palla et al., 2017Palla, C., Giacomozzi, A., Genovese, D. B., & Carrín, M. E. (2017). Multi – objective optimization of high oleic sunflower oil and monoglycerides oleogels: searching for rheological and textural properties similar to margarine. Food Structure, 12, 1-14. http://dx.doi.org/10.1016/j.foostr.2017.02.005
http://dx.doi.org/10.1016/j.foostr.2017....
). On the other hand, the higher level of unsaturation (18:2 versus 18:1 in SB versus SF) could also influence and promote a lack of structuration as observed in the MG-flaxseed oil oleogel (Barroso et al., 2020Barroso, N. G., Okuro, P. K., Ribeiro, A. P. B., & Cunha, R. L. (2020). Tailoring properties of mixed-component oleogels: wax and monoglyceride interactions towards flaxseed oil structuring. Gels, 6(1), 5. PMid:32023926. http://dx.doi.org/10.3390/gels6010005
http://dx.doi.org/10.3390/gels6010005...
). According to the authors, this would be due to the fact that the presence of a greater degree of unsaturation represents a solvent with a larger molar volume (higher conformational freedom caused by the bended chains and hydrophobicity), which may reflect oleogelator–solvent interactions.
Oil loss: In SB samples, oil loss was zero (Table 1) only for the samples that showed hardness higher than 1 N, such as runs 2, 6, and 12. However, none of the factors was significant (p > 0.05; Table 3), even though oil migration was previously influenced by crystal size, shape and distribution, the degree of branching, elasticity, and the mechanical strength of the material (Blake & Marangoni, 2015Blake, A. I., & Marangoni, A. G. (2015). Plant wax crystals display platelet-like morphology. Food Structure, 3, 30-34. http://dx.doi.org/10.1016/j.foostr.2015.01.001
http://dx.doi.org/10.1016/j.foostr.2015....
).
SF sample runs 4, 6, 8, 10, and 12 did not present any oil loss (Table 1). However, MG (L) and CLX (L and Q) were significant factors for oil loss in SF oleogels (Table 3). Previous oleogels optimized with MG showed good oil loss only with 10% of MG (Palla et al., 2017Palla, C., Giacomozzi, A., Genovese, D. B., & Carrín, M. E. (2017). Multi – objective optimization of high oleic sunflower oil and monoglycerides oleogels: searching for rheological and textural properties similar to margarine. Food Structure, 12, 1-14. http://dx.doi.org/10.1016/j.foostr.2017.02.005
http://dx.doi.org/10.1016/j.foostr.2017....
), whereas the maximum used in our study was 4%, reinforcing the importance of multicomponents to reduce the amount of oil loss using less amount of oleogelators (Silva et al., 2019aSilva, T. L. T., Arellano, D. B., & Martini, S. (2019a). Interactions between candelilla wax and saturated triacylglycerols in oleogels. Food Research International, 121, 900-909. PMid:31108823. http://dx.doi.org/10.1016/j.foodres.2019.01.018
http://dx.doi.org/10.1016/j.foodres.2019...
). This influence of MG and CLX on oil loss can be examined in Table 1, where sample 6, for example, showed no oil migration and a total oleogelator of 4.41%. The MG concentration, in this case, was only 0.81%. The oil loss results confirmed our theory regarding the interaction of MG, HF, and CLX with high oleic oils when oleogelation was induced at 5 °C.
Thermal stability and visual stability: Observing the thermal stability and visual stability of the samples, runs 2, 4, 6, 8, 10, 12, and 14, showed samples visually hard and graded as 5 for both oils before thermal treatment (Table 1). This was because the samples did not flow when the beaker was turned, and no superficial exudation was observed, indicating that the ternary oleogelator mixture had formed a self-sustainable crystal network (Godoi et al., 2019Godoi, K. R. R., Basso, R. C., Ming, C. C., da Silva, V. M., da Cunha, R. L., Barrera-Arellano, D., Ribeiro, A. P. B., & Ribeiro, B. (2019). Physicochemical and rheological properties of soybean organogels : interactions between different structuring agents. Food Research International, 124, 108475. PMid:31466657. http://dx.doi.org/10.1016/j.foodres.2019.05.023
http://dx.doi.org/10.1016/j.foodres.2019...
; Wijarnprecha et al., 2019Wijarnprecha, K., de Vries, A., Santiwattana, P., Sonwai, S., & Rousseau, D. (2019). Microstructure and rheology of oleogel-stabilized water-in-oil emulsions containing crystal-stabilized droplets as active fi llers. Lebensmittel-Wissenschaft + Technologie, 115, 108058. http://dx.doi.org/10.1016/j.lwt.2019.04.059
http://dx.doi.org/10.1016/j.lwt.2019.04....
). The only difference in visual stability between SB and SF samples scored as 5 was noted in run 3. As discussed above, the MG and HF in high amounts could compensate for the lack of CLX in SF and form a firmer gel. This improvement could also be observed visually.
However, when submitted to thermal treatment, few samples kept the visual structure as 5 (Figure 3 and Figure 4). For example, runs 6 and 12 were the only samples that kept their structure for both oils, which were also the hardest ones, even though all oleogelators showed a MP (Table 2) much higher than the cyclization temperature used (40 °C). Similar results were found for a ternary oleogel with CLX:HF:SMS, where only oleogels with CLX as the major structuring agent were able to return their primary structure after thermal treatment, regardless of the oleogelator MP (Godoi et al., 2020Godoi, K. R. R., Basso, R. C., Ming, C. C., da Silva, A. Á., Cardoso, L. P., & Ribeiro, A. P. B. (2020). Crystallization, microstructure and polymorphic properties of soybean oil organogels in a hybrid structuring system. Food Research International, 137, 109460. PMid:33233134. http://dx.doi.org/10.1016/j.foodres.2020.109460
http://dx.doi.org/10.1016/j.foodres.2020...
). We can thus assume that the microstructure formed on samples with lower amounts of CLX was not strong enough to survive thermal cycles. Sample SF13 was the only score of 0 due to the fact that this sample showed phase separation after storage, as shown in Figure 4. There are three criteria for oleogel stability: (1) temperature of cooling; (2) the oleogelator concentration necessary to immobilize a liquid component; and (3) the length of time that a gel persists in a sealed tube at room temperature without macroscopic phase separation or flowing when inverted. Clearly, the longer the n-alkane, as in CLX, or the carbon chain, for MG and HF, the more efficiently the carbon chain acts as a low molecular oleogelator (Abdallah & Weiss, 2000Abdallah, D. J., & Weiss, R. G. (2000). n-Alkanes gel n-alkanes (and many other organic liquids). Langmuir, 16(2), 352-355. http://dx.doi.org/10.1021/la990795r
http://dx.doi.org/10.1021/la990795r...
).
The soybean oleogels (Sb) visual appearance at 25 °C after thermal stability, for samples concentration (1-17), please see Table 1.
The visual appearance of the high sunflower oleogels (SF) at 25 °C after thermal stability, for samples concentration (1-17), please see Table 1.
In fact, the p-values lower than 0.05 (Table 3) were considered for ANOVA (Table 4) hardness, oil loss, and thermal and visual stability. The coefficient of determination (R2) of the model could be used to check the experiment data variability. R2 values indicated that the models were able to explain in percentage 66.81 (SB)/83.07 (SF); 64.41(SF); 56.89 (SB)/63.26 (SF); 53.91 (SB)/ 68.22(SF) of experimental data variability, for hardness, oil loss, thermal stability, and visual stability, respectively. The computed F-values for regressions were much more significant than the tabulated F-values, reflecting the statistical significance of the models which are described in the footnote in Table 4.
Equations that represent the models with the significant factors for the experimental data are presented in Table 5, and the effects of the independent variables (MG, CLX, and HF) are illustrated in the response surfaces in Figure 5. A desired oleogel for shortening replacement would present a hardness value higher than 2 N, no oil loss, and visual and thermal stability of 5 scores within the total concentration of oleogelator no higher than 10%. The model was validated using the multicomponent system of 2:3:3 of MG:CLX:HF. The surface responses show that for hardness and oil loss, there is a good match between values obtained through the model and experimental (difference <0.3 N and 0%), with low relative deviations (<12%). For thermal and visual stability, acceptable results are also achieved but with a higher relative deviation (>18%), except for the visual attribute for SB samples (0.8%). This means that the model accurately could predict the effect of the variation on MG, HF, and CLX on oleogel formation and technological properties.
Equations and validation (experimental values and predicting values) obtained from the models.
Response surfaces for high oleic sunflower oleogels, hardness (a), (b) and (c), oil loss (d), thermal stability (e), and visual stability (f), (g), and (h) as a function of the MG: monoacylglycerol, CLX: candelilla wax and HF: hardfat.
An earlier study compared the CLX oleogelator power to its primary chemical pure compound, the n-alkane 32C. It was observed that the minor compounds present in CLX changed the microstructure and physical properties, positively increasing solubility and structuring power (Ramírez-Gómez et al., 2016Ramírez-Gómez, N. O., Acevedo, N. C., Toro-Vázquez, J. F., Ornelas-Paz, J. J., Dibildox-Alvarado, E., & Pérez-Martínez, J. D. (2016). Phase behavior, structure and rheology of candelilla wax/fully hydrogenated soybean oil mixtures with and without vegetable oil. Food Research International, 89(Pt 1), 828-837. PMid:28460985. http://dx.doi.org/10.1016/j.foodres.2016.10.025
http://dx.doi.org/10.1016/j.foodres.2016...
). These connections might be what make binary, ternary, or multicomponent systems sometimes very fascinating. The oleogelators in some cases complement each other’s and form satisfactory oleogels, sometimes even with lower amounts of oleogelators which is very interesting for the food industry since, as observed in Table 1, these new ternary systems formed have no more than 23.76% (w/w) of SFA. The lower amounts of SFA in the oleogels (Table 1), compared with shortenings for fillings available on Brazilian market (~50%) (Silva et al., 2019bSilva, T. L. T., Martini, S., & Arellano, D. B. (2019b). Chemical composition and nutritional information of fats used in fillings of sandwich cookies. Journal of the American Oil Chemists’ Society, 96(10), 1173-1179. http://dx.doi.org/10.1002/aocs.12256
http://dx.doi.org/10.1002/aocs.12256...
), would result in at least 50% of replacement in the SFA and moreover, 100% of trans-fatty acids replacement for food application.
In order to show the potential of the mixture between MG, HF and CLX, this system was further applied in fillings for cookies (Silva et al., 2021Silva, T. L. T., Fernandes, G. D., & Arellano, D. B. (2021). Development of reduced saturated fat cookie fillings using multicomponent oleogels. Journal of the American Oil Chemists’ Society, 48(11), 1069-1082. http://dx.doi.org/10.1002/aocs.12527
http://dx.doi.org/10.1002/aocs.12527...
) and margarine (Silva et al., 2018bSilva, T. L. T., Chaves, K. F., Fernandes, G. D., Rodrigues, J. B., Bolini, H. M. A., & Arellano, D. B. (2018b). Sensory and technological evaluation of margarines with reduced saturated fatty acid contents using oleogel technology. Journal of the American Oil Chemists’ Society, 95(6), 673-685. http://dx.doi.org/10.1002/aocs.12074
http://dx.doi.org/10.1002/aocs.12074...
). When the appropriate concentration of each oleogelator was used per individual food product, an acceptable product with good technological properties such as oil binding capacity, consistency, adhesiveness, and melting properties can be produced (Silva et al., 2021Silva, T. L. T., Fernandes, G. D., & Arellano, D. B. (2021). Development of reduced saturated fat cookie fillings using multicomponent oleogels. Journal of the American Oil Chemists’ Society, 48(11), 1069-1082. http://dx.doi.org/10.1002/aocs.12527
http://dx.doi.org/10.1002/aocs.12527...
) along with highly important sensory acceptance like that of a commercial product and the same shelf-life (Silva et al., 2018bSilva, T. L. T., Chaves, K. F., Fernandes, G. D., Rodrigues, J. B., Bolini, H. M. A., & Arellano, D. B. (2018b). Sensory and technological evaluation of margarines with reduced saturated fatty acid contents using oleogel technology. Journal of the American Oil Chemists’ Society, 95(6), 673-685. http://dx.doi.org/10.1002/aocs.12074
http://dx.doi.org/10.1002/aocs.12074...
).
4 Conclusion
Oleogels can have a uniquely critical future role in the food industry by enhancing the health attributes of many food products. In this experiment, we observed that the CCRD represented an excellent statistical tool for predicting the oleogelation process for multicomponent systems oleogelators by predicting the technological properties of the oleogels as a result of oleogelator concentrations and interactions.
The results obtained for the multicomponent oleogel formed by CLX:MG:HF in SB and SF oils were dependent on the type of oleogelators and the lipid matrix used. In SB oil, CLX was the oleogelator that contributed mainly to hardness, oil loss, and thermal resistance. In SF, besides CLX alone the interactions of CLX with MG and HF were also significant and positively affected the physical properties of the oleogels as hardness, oil loss, and thermal and visual stability.
The significant interaction among CLX, MG, and HF emphasizes the benefit of using a multicomponent system. The synergic combinations can reduce the amount of each oleogelator necessary to achieve the required physical properties that will impact directly the price, waxy-mouth feel, and sensory acceptance.
Acknowledgements
The authors would like to thank Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (National Council for Scientific and Technological Development) for their financial support of this work and scholarship (process number 140477/2015-2).
-
Cite as: Silva, T. L. T., Fernandes, G. D., & Arellano, D. B. (2022). The combination of monoglycerides, wax and hardfat on oleogels structuration. Brazilian Journal of Food Technology, 25, e2021137. https://doi.org/10.1590/1981-6723.13721
-
Funding: CNPq - National Council for Scientific and Technological Development (process number 140477/2015-2).
References
- Abdallah, D. J., & Weiss, R. G. (2000). n-Alkanes gel n-alkanes (and many other organic liquids). Langmuir, 16(2), 352-355. http://dx.doi.org/10.1021/la990795r
» http://dx.doi.org/10.1021/la990795r - American Oil Chemists’ Society – AOCS. (2009). Official Methods and Recommended Practices of the American Oil Chemists’ Society (5th ed.). USA: AOCS.
- Barroso, N. G., Okuro, P. K., Ribeiro, A. P. B., & Cunha, R. L. (2020). Tailoring properties of mixed-component oleogels: wax and monoglyceride interactions towards flaxseed oil structuring. Gels, 6(1), 5. PMid:32023926. http://dx.doi.org/10.3390/gels6010005
» http://dx.doi.org/10.3390/gels6010005 - Bin Sintang, M. D., Rimaux, T., Van de Walle, D., Dewettinck, K., & Patel, A. R. (2017). Oil structuring properties of monoglycerides and phytosterols mixtures. European Journal of Lipid Science and Technology, 119(3), 1-14. http://dx.doi.org/10.1002/ejlt.201500517
» http://dx.doi.org/10.1002/ejlt.201500517 - Blake, A. I., & Marangoni, A. G. (2015). Plant wax crystals display platelet-like morphology. Food Structure, 3, 30-34. http://dx.doi.org/10.1016/j.foostr.2015.01.001
» http://dx.doi.org/10.1016/j.foostr.2015.01.001 - Dassanayake, L. S. K., Kodali, D. R., & Ueno, S. (2011). Formation of oleogels based on edible lipid materials. Current Opinion in Colloid & Interface Science, 16(5), 432-439. http://dx.doi.org/10.1016/j.cocis.2011.05.005
» http://dx.doi.org/10.1016/j.cocis.2011.05.005 - Doan, C. D., Tavernier, I., Danthine, S., Rimaux, T., & Dewettinck, K. (2018a). Physical compatibility between wax esters and triglycerides in hybrid shortenings and margarines prepared in rice bran oil. Journal of the Science of Food and Agriculture, 98(3), 1042-1051. PMid:28718922. http://dx.doi.org/10.1002/jsfa.8553
» http://dx.doi.org/10.1002/jsfa.8553 - Doan, C. D., Tavernier, I., Okuro, P. K., & Dewettinck, K. (2018b). Internal and external factors affecting the crystallization, gelation and applicability of wax-based oleogels in food industry. Innovative Food Science & Emerging Technologies, 45, 42-52. http://dx.doi.org/10.1016/j.ifset.2017.09.023
» http://dx.doi.org/10.1016/j.ifset.2017.09.023 - Doan, C. D., Tavernier, I., Sintang, M. D. B., Danthine, S., Van de Walle, D., Rimaux, T., & Dewettinck, K. (2017a). Crystallization and gelation behavior of low- and high melting waxes in rice bran oil: a case-study on berry wax and sunflower wax. Food Biophysics, 12(1), 97-108. http://dx.doi.org/10.1007/s11483-016-9467-y
» http://dx.doi.org/10.1007/s11483-016-9467-y - Doan, C. D., To, C. M., De Vrieze, M., Lynen, F., Danthine, S., Brown, A., Dewettinck, K., & Patel, A. R. (2017b). Chemical profiling of the major components in natural waxes to elucidate their role in liquid oil structuring. Food Chemistry, 214, 717-725. PMid:27507530. http://dx.doi.org/10.1016/j.foodchem.2016.07.123
» http://dx.doi.org/10.1016/j.foodchem.2016.07.123 - Fayaz, G., Goli, S. A. H., Kadivar, M., Valoppi, F., Barba, L., Calligaris, S., & Nicoli, M. C. (2017). Potential application of pomegranate seed oil oleogels based on monoglycerides, beeswax and propolis wax as partial substitutes of palm oil in functional chocolate spread. Lebensmittel-Wissenschaft + Technologie, 86, 523-529. http://dx.doi.org/10.1016/j.lwt.2017.08.036
» http://dx.doi.org/10.1016/j.lwt.2017.08.036 - Food and Agriculture Organization of the United Nations – FAO. (2010). Fats and fatty acids in human nutrition. Report of an expert consultation (FAO Food and Nutrition Paper, 91). Rome: FAO.
- Giacomozzi, A. S., Carrín, M. E., & Palla, C. A. (2018). Muffins elaborated with optimized monoglycerides oleogels : from solid fat replacer obtention to product quality evaluation. Journal of Food Science, 83(6), 1505-1515. PMid:29786854. http://dx.doi.org/10.1111/1750-3841.14174
» http://dx.doi.org/10.1111/1750-3841.14174 - Godoi, K. R. R., Basso, R. C., Ming, C. C., da Silva, V. M., da Cunha, R. L., Barrera-Arellano, D., Ribeiro, A. P. B., & Ribeiro, B. (2019). Physicochemical and rheological properties of soybean organogels : interactions between different structuring agents. Food Research International, 124, 108475. PMid:31466657. http://dx.doi.org/10.1016/j.foodres.2019.05.023
» http://dx.doi.org/10.1016/j.foodres.2019.05.023 - Godoi, K. R. R., Basso, R. C., Ming, C. C., da Silva, A. Á., Cardoso, L. P., & Ribeiro, A. P. B. (2020). Crystallization, microstructure and polymorphic properties of soybean oil organogels in a hybrid structuring system. Food Research International, 137, 109460. PMid:33233134. http://dx.doi.org/10.1016/j.foodres.2020.109460
» http://dx.doi.org/10.1016/j.foodres.2020.109460 - Hartman, L., & Lago, R. C. A. (1973). Rapid preparation of fatty acid methyl esters from lipids. Laboratory Practice, 22, 475-476, 494. PMid:4727126.
- Jana, S., & Martini, S. (2016). Phase behavior of binary blends of four different waxes. Journal of the American Oil Chemists’ Society, 93(4), 543-554. http://dx.doi.org/10.1007/s11746-016-2789-6
» http://dx.doi.org/10.1007/s11746-016-2789-6 - Kerr, R. M., Tombokan, X., Ghosh, S., & Martini, S. (2011). Crystallization behavior of anhydrous milk fat - sunflower oil wax blends. Journal of Agricultural and Food Chemistry, 59(6), 2689-2695. PMid:21344886. http://dx.doi.org/10.1021/jf1046046
» http://dx.doi.org/10.1021/jf1046046 - Kesselman, E., & Shimoni, E. (2007). Imaging of oil/monoglyceride networks by polarizing near-field scanning optical microscopy. Food Biophysics, 2(2–3), 117-123. http://dx.doi.org/10.1007/s11483-007-9038-3
» http://dx.doi.org/10.1007/s11483-007-9038-3 - Lopez-martínez, A., Charó-alonso, M. A., Marangoni, A. G., & Toro-vazquez, J. F. (2015). Monoglyceride organogels developed in vegetable oil with and without ethylcellulose. Food Research International, 72, 37-46. http://dx.doi.org/10.1016/j.foodres.2015.03.019
» http://dx.doi.org/10.1016/j.foodres.2015.03.019 - Martini, S., Tan, C. Y., & Jana, S. (2015). Physical Characterization of Wax/Oil Crystalline Networks. Journal of Food Science, 80(5), C989-C997. PMid:25850679. http://dx.doi.org/10.1111/1750-3841.12853
» http://dx.doi.org/10.1111/1750-3841.12853 - Ögutcu, M., & Yilmaz, E. (2014). Oleogels of virgin olive oil with carnauba wax and monoglyceride as spreadable products. Grasas y Aceites, 65(3), 1-11. http://dx.doi.org/10.3989/gya.0349141
» http://dx.doi.org/10.3989/gya.0349141 - Okuro, P. K., Malfatti-Gasperini, A. A., Vicente, A. A., & Cunha, R. L. (2018). Lecithin and phytosterols-based mixtures as hybrid structuring agents in different organic phases. Food Research International, 111, 168-177. PMid:30007673. http://dx.doi.org/10.1016/j.foodres.2018.05.022
» http://dx.doi.org/10.1016/j.foodres.2018.05.022 - Paglarini, C. S., Furtado, G. F., Biachi, J. P., Vidal, V. A. S., Martini, S., Forte, M. B. S., Cunha, R. L., & Pollonio, M. A. R. (2018). Functional emulsion gels with potential application in meat products. Journal of Food Engineering, 222, 29-37. http://dx.doi.org/10.1016/j.jfoodeng.2017.10.026
» http://dx.doi.org/10.1016/j.jfoodeng.2017.10.026 - Palla, C., Giacomozzi, A., Genovese, D. B., & Carrín, M. E. (2017). Multi – objective optimization of high oleic sunflower oil and monoglycerides oleogels: searching for rheological and textural properties similar to margarine. Food Structure, 12, 1-14. http://dx.doi.org/10.1016/j.foostr.2017.02.005
» http://dx.doi.org/10.1016/j.foostr.2017.02.005 - Patel, A. R., Babaahmadi, M., Lesaffer, A., & Dewettinck, K. (2015). Rheological Profiling of Organogels Prepared at Critical Gelling Concentrations of Natural Waxes in a Triacylglycerol Solvent. Journal of Agricultural and Food Chemistry, 63(19), 4862-4869. PMid:25932656. http://dx.doi.org/10.1021/acs.jafc.5b01548
» http://dx.doi.org/10.1021/acs.jafc.5b01548 - Patel, A. R., Nicholson, R. A., & Marangoni, A. G. (2020). Applications of fat mimetics for the replacement of saturated and hydrogenated fat in food products. Current Opinion in Food Science, 33, 61-68. http://dx.doi.org/10.1016/j.cofs.2019.12.008
» http://dx.doi.org/10.1016/j.cofs.2019.12.008 - Pérez-martínez, J. D., Sánchez-becerril, M., Marangoni, A. G., Toro-vazquez, J. F., Ornelas-paz, J. J., & Ibarra-junquera, V. (2019). Structuration, elastic properties scaling, and mechanical reversibility of candelilla wax oleogels with and without emulsifiers. Food Research International, 122, 471-478. https://doi.org/10.1016/j.foodres.2019.05.020
» https://doi.org/10.1016/j.foodres.2019.05.020 - Ramírez-Gómez, N. O., Acevedo, N. C., Toro-Vázquez, J. F., Ornelas-Paz, J. J., Dibildox-Alvarado, E., & Pérez-Martínez, J. D. (2016). Phase behavior, structure and rheology of candelilla wax/fully hydrogenated soybean oil mixtures with and without vegetable oil. Food Research International, 89(Pt 1), 828-837. PMid:28460985. http://dx.doi.org/10.1016/j.foodres.2016.10.025
» http://dx.doi.org/10.1016/j.foodres.2016.10.025 - Rocha, J. C. B., Lopes, J. D., Mascarenhas, M. C. N., Arellano, D. B., Guerreiro, L. M. R., & da Cunha, R. L. (2013). Thermal and rheological properties of organogels formed by sugarcane or candelilla wax in soybean oil. Food Research International, 50(1), 318-323. http://dx.doi.org/10.1016/j.foodres.2012.10.043
» http://dx.doi.org/10.1016/j.foodres.2012.10.043 - Silva, T. L. T., Arellano, D. B., & Martini, S. (2018a). Physical properties of Candelilla Wax, monoacylglycerols, and fully hydrogenated oil oleogels. Journal of the American Oil Chemists’ Society, 95(7), 797-811. http://dx.doi.org/10.1002/aocs.12096
» http://dx.doi.org/10.1002/aocs.12096 - Silva, T. L. T., Chaves, K. F., Fernandes, G. D., Rodrigues, J. B., Bolini, H. M. A., & Arellano, D. B. (2018b). Sensory and technological evaluation of margarines with reduced saturated fatty acid contents using oleogel technology. Journal of the American Oil Chemists’ Society, 95(6), 673-685. http://dx.doi.org/10.1002/aocs.12074
» http://dx.doi.org/10.1002/aocs.12074 - Silva, T. L. T., Arellano, D. B., & Martini, S. (2019a). Interactions between candelilla wax and saturated triacylglycerols in oleogels. Food Research International, 121, 900-909. PMid:31108823. http://dx.doi.org/10.1016/j.foodres.2019.01.018
» http://dx.doi.org/10.1016/j.foodres.2019.01.018 - Silva, T. L. T., Martini, S., & Arellano, D. B. (2019b). Chemical composition and nutritional information of fats used in fillings of sandwich cookies. Journal of the American Oil Chemists’ Society, 96(10), 1173-1179. http://dx.doi.org/10.1002/aocs.12256
» http://dx.doi.org/10.1002/aocs.12256 - Silva, T. L. T., & Danthine, S. (2021). Effect of high-intensity ultrasound on the oleogelation and physical properties of high melting point monoglycerides and triglycerides oleogels. Journal of Food Science, 86(2), 343-356. PMid:33448022. http://dx.doi.org/10.1111/1750-3841.15589
» http://dx.doi.org/10.1111/1750-3841.15589 - Silva, T. L. T., Fernandes, G. D., & Arellano, D. B. (2021). Development of reduced saturated fat cookie fillings using multicomponent oleogels. Journal of the American Oil Chemists’ Society, 48(11), 1069-1082. http://dx.doi.org/10.1002/aocs.12527
» http://dx.doi.org/10.1002/aocs.12527 - Toro-Vazquez, J. F., Morales-Rueda, J., Mallia, V. A., & Weiss, R. G. (2010). Relationship between molecular structure and thermo-mechanical properties of candelilla wax and amides derived from (R)-12-hydroxystearic acid as gelators of safflower oil. Food Biophysics, 5(3), 193-202. http://dx.doi.org/10.1007/s11483-010-9159-y
» http://dx.doi.org/10.1007/s11483-010-9159-y - U.S. Department of Health. Human Services. U.S. Department of Agriculture. (2015). 2015-2020 Dietary Guidelines for Americans (8th ed.). Rockville: U.S. Department of Health.
- U.S. Food and Drug Administration – FDA. (2013). Tentative determination regarding partially hydrogenated oils (Vol. 78, No. 217). Maryland, EUA: FDA.
- U.S. Food and Drug Administration – FDA. (2015). Final determination regarding partially hydrogenated oils Maryland, EUA: FDA.
- Vieira, S. A., McClements, D. J., & Decker, E. A. (2015). Challenges of utilizing healthy fats in foods. Advances in Nutrition, 6(3), 309S-317S. PMid:25979504. http://dx.doi.org/10.3945/an.114.006965
» http://dx.doi.org/10.3945/an.114.006965 - Wijarnprecha, K., de Vries, A., Santiwattana, P., Sonwai, S., & Rousseau, D. (2019). Microstructure and rheology of oleogel-stabilized water-in-oil emulsions containing crystal-stabilized droplets as active fi llers. Lebensmittel-Wissenschaft + Technologie, 115, 108058. http://dx.doi.org/10.1016/j.lwt.2019.04.059
» http://dx.doi.org/10.1016/j.lwt.2019.04.059
Edited by
Publication Dates
-
Publication in this collection
01 June 2022 -
Date of issue
2022
History
-
Received
06 Aug 2021 -
Accepted
31 Jan 2022