Abstract
The aim of the present study was to evaluate the activity of macrophages, and the production of TNF-α and antibodies against experimental infection by Leptospira serovar Pomona in mice genetically selected for High (H) or Low (L) humoral immune response. To evaluate macrophagic activity, peritoneal and splenic lavages were performed for determination of oxygen (H2O2) and nitrogen (NO) intermediates. The production of the tumor necrosis factor (TNF-α) was investigated through bioassays in serum and homogenates of splenic and hepatic cells of control and infected animals, as was as specific antibodies production. The immune response against serovar Pomona in those lines, was characterized by high antibody production, especially in later periods of the infectious process, whereas values of bacterial recovery in culture medium were lower. The production of reactives oxygen and nitrogen intermediate, also helped to eliminate Leptospira Pomona in both lines; H2O2 production an important factor in H IV-A, as well as NO production in L IV-A, especially in later post-inoculation periods. The same was detected for TNF-α. Results suggest that such lines could be an important model to investigate the pathogenesis and the immune response of animals against the several Leptospira serovars.
Immunological aspects; Biozzi mice; Leptospira; Macrophages; Cytokines
VETERINARY MICROBIOLOGY
Production of reactive oxygen (H2O2) and nitrogen (NO) intermediates and TNF-α in mice genetically selected for high (H) and low (L) antibody response and experimentally infected with Leptospira serovar pomona
Haanwinckel, Maria Cristina SantosI,II,* * Corresponding Author. Mailing address: Avenida Elias Garcia, 163, 1º Esquerdo, Lisboa Portugal, 1050-099.; Email: crishaan@yahoo.com.br ; Oliveira, Silvio Luis deI,II
IDepartamento de Microbiologia-Imunologia, Instituto de Biociências, Universidade Estadual Paulista, Botucatu, SP, Brasil
IIDepartamento de Doenças Tropicais e Diagnóstico por Imagem, Faculdade de Medicina, Universidade Estadual Paulista, Botucatu, SP, Brasil
ABSTRACT
The aim of the present study was to evaluate the activity of macrophages, and the production of TNF-α and antibodies against experimental infection by Leptospira serovar Pomona in mice genetically selected for High (H) or Low (L) humoral immune response. To evaluate macrophagic activity, peritoneal and splenic lavages were performed for determination of oxygen (H2O2) and nitrogen (NO) intermediates. The production of the tumor necrosis factor (TNF-α) was investigated through bioassays in serum and homogenates of splenic and hepatic cells of control and infected animals, as was as specific antibodies production. The immune response against serovar Pomona in those lines, was characterized by high antibody production, especially in later periods of the infectious process, whereas values of bacterial recovery in culture medium were lower. The production of reactives oxygen and nitrogen intermediate, also helped to eliminate Leptospira Pomona in both lines; H2O2 production an important factor in HIV-A, as well as NO production in LIV-A, especially in later post-inoculation periods. The same was detected for TNF-α. Results suggest that such lines could be an important model to investigate the pathogenesis and the immune response of animals against the several Leptospira serovars.
Keywords: Immunological aspects; Biozzi mice; Leptospira; Macrophages; Cytokines.
INTRODUCTION
Leptospirosis is a widespread zoonotic infection of results ranging from subclinical infection, to a fatal presentation named Weil's disease. Despite the overall impact of this disease, the mechanisms of pathogenicity, host defense and protective immunity of Leptospira still remain unclear, especially for those related to the production of mediated immune response for the infection control, which is not similar in all hosts and depends on the infective serovar (8).
Protection against the several Leptospira serovars involves different factors such as specific antibodies and the complement system. However, the phagocytic activity of macrophages and granulocytes also seems to be efficient against leptospirosis. Thus, phagocytic cells actively participate in the inflammatory process, even in the absence of specific antibodies (12, 16, 28).
Activated macrophages have microbicidal properties through the action of lysosomal enzymes, release of cytokines such as TNF-α and IFN-γ, and reactive products of nitrogen and oxygen (2).
In guinea pigs infected with Leptospira serovar Icterohaemorrhagiae, there was a high production of the TNF- α relative to controls (20). In hamsters infected with Leptospira serovar Pyrogenes, TNF-α expression was identified in renal tissues through real-time PCR from the third day post-infection (18).
Initially from albino mice colonies outbred, Biozzi et al (5) other lines were developed and they were genetic selected for the study of the characterization of the mechanisms of regulation that are involved in the immune response. After this study, other studies have been done that generated the development of five selections (I to V).
This model has been obtain based on the bidirectional genetic selection of mice that had high response (H) and the ones that had a low response (L) in terms of production of antibodies against complex natural antigens for a period of 15 to 20 generations, until they have reached the highest divergence in the immune humoral response. The selection has modified the response not only of the selecting antigen but also of others antigens not really related with this process (10). The high and the low capacity of response are result of additional effect of alleles localized in different independent loci (polygenic control). Which have been accumulated progressively in the groups H and L during the selection process. These homozygotic lines represent extreme phenotypes that can be found in natural heterogeneous populations.
In the present study, genetically selected mice lines (selection IV-A) were used for the quantitative production of antibodies (6, 15). as well as changes in the metabolic activity of macrophages. This two lines of mice, high line (HIV-A) and low line (LIV-A) were genetically selected for their respective high and low antibody formation after immunization with sheep erythrocytes. The extensive quantitative difference between them involves not only all classes of immunoglobulin but also antibody synthesis in response to a wide range of unrelated antigens, cytodynamics of their B cells response and in the macrophage functions, namely those concerning the metabolism and presentation of the antigen to lymphocytes (6).
Mice were experimentally infected with Leptospira serovar Pomona in order to evaluate antibody production levels and peritoneal and spleenic macrophages activity through quantification of oxygen (H2O2) and nitrogen (NO) reactive intermediates and TNF-α production in serum and homogenates of splenic and hepatic cells of control and infected animals.
MATERIAL AND METHODS
Mice and Inoculum
In this study have been used thirty male mice of the IV-A Selection for high humoral response (HIV-A) and other 30 mice of the IV-A selection for low humoral response (LIV-A) with 4-6 weeks of age developed at the Immunology Lab of São Paulo Biological Institute and they were maintain in the Department of Microbiology and Immunology, Biosciences Institute, São Paulo State University (UNESP).
Leptospira strains were obtained from the Bacterial Zoonoses Lab, University of São Paulo (USP). Virulence of Leptospira interrogans serovar Pomona strain Fromm was maintained by iterative passages in Golden Syrian hamsters.
The mice were inoculated intraperitoneally with 1 mL inoculum containing 2x107 leptospires identified through agglutinin-absorption tests (27) and quantified according to the method described by Faine, (11). Twenty-four negative controls were also used. Before sacrifice, mice were anesthetized by light ether inhalation and partially bled by orbital plexus puncture to obtain serum for the in microscopic agglutination test. The animals were sacrificed at days 4, 7, 14, 21, 28 and 35 post-inoculation under the supervision of the Ethics Committee of Botucatu Medical College, São Paulo State University, Brazil, process 383/2004.
Recovery of leptospires
To assess the degree of infection by leptospires, fragments of kidneys and liver from the infected animals were cultured and isolated through the by serial dilution technique, according to Santa Rosa (27) and Passos et al (23).
Antibody titration
Anti-Leptospira interrogans serovar Pomona antibodies were detected in sera through microscopic agglutination test on slides according to the method described by Faine (11). Pomona and 24 other serovars were used to exclude mice previously infected or containing a different serovar.
Macrophage activity
After killing the animals, they were maintained in an aseptic laminar flow hood and they were fixed in a lying back position for dissection. The abdominal wall was exposed taking the skin out from that region. After that a cold solution of 10ml of PBS (Buffer solution phosphate pH 7,2) was inject in central superior abdominal region. Subsequently, the abdominal cavity was massaged and liquid was taken with needle from the peritoneal cavity. This procedure was done three times. Then, samples from spleen were taken and homogenised with PBS to obtain suspensions. The abdominal suspension and spleen suspension were kept in plastic tubes, maintain on ice and centrifuge after being collect for 10 minutes at 1500 rpm.
After centrifugation, the cellular suspension was maintained in complete medium for cell culture (MCCC). From each peritoneal suspension 50 μl aliquots were made and were supplemented with a solution that contained 0,45ml of neutral red at 0,02% and incubate at 37ºC for 10 minutes for a subsequent counting. The macrophages were identified by the incorporation of the neutral red and counted Neubauer chamber and the cellular concentration was adjusted to 2X106 macrophages/ml of MCCC. Small volumes of 0,1ml of suspension were distributed in micro plates (Corning plates) for cell culture. After an incubation period of 2 hours at 37ºC with 5% of CO2, the non-adherents cells were taken using a wash with RPMI 1640 medium.
Hydrogen peroxide production
After obtaining macrophages by peritoneal lavages and splenic tissue suspensions, of hydrogen peroxide (H2O2) production was quantified through the method described by Pick & Keisari (24) and adapted by Pick & Mizel (25). H2O2 production was investigated without stimuli or with the addition of interferon gamma (IFN-γ) and Phorbol-Myristate Acetate (PMA). Results are express as in nanomoles of H2O2 per 2x105 cells, by comparing OD with standard curve of known H2O2 concentration.
Nitrite production
NO2- production by the macrophages culture was assessed through colorimetric method based on Griess Reaction (13). NO production was analyzed by the addition or not of IFN-γ. Results are express as in micromoles (umoles) of NO per 2x105 cells, by comparing OD with standard curve of known NO2_ concentration.
Tumour necrosis factor (TNF- α ) bioassay
The murine tumor-line fibroblasts named L929, which is sensitive to TNF-α was used in the bioassay to detect this cytokine in serum and homogenates of splenic and hepatic cells from both lines through the procedure adapted from Di Giovine et al (9) and Pourshafie et al (26). TNF-α levels in the macrophages culture supernatants were calculated on a standard curve plotted with recombinant TNF-α murine (R & D Systems, Minneapolis, MN, USA) used at concentrations from 40 to 4000 U / mL.
Statistical Analysis
To compare the serological titers obtained from mice HIV-A and LIV-A mice, parametric analysis of variance - ANOVA was used for independent samples, besides non-parametric Kruskal-Wallis test. Means of H2O2, NO and cytokine productions were compared by using repeated measures ANOVA and Student-Newman-Keuls t test for múltiple comparisons (29). Significance level was 5% for all tests.
RESULTS
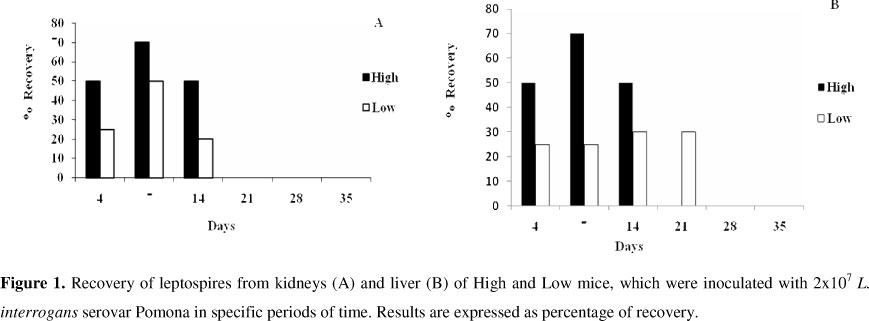
The results expressed in Figures 1A and 1B indicate that the bacterial recovery from liver and kidneys occurred from the 4th day post-inoculation in both lines, peaking at the 7th day post-inoculation in HIV-A and at the 7th, 14th? and 21st days of infection in LIV-A mice. After the 21st day, the values decreased. In LIV-A line, the agent could be isolated for a longer period compared to HIV-A.
In the present study, antibody production in the lines was associated with the leptospire recovery rates in renal and hepatic tissues. The antibody titers produced by mice are presented in Figure 2. In the negative control animals there was no detection of any titration values.
The analysis of such results indicates that there was no significant statistical difference (p< 0.05) between the genetically selected lines HIV-A and LIV-A, although antibody levels were higher in HIV-A than in LIV-A, especially between the 7th and 21st days post-inoculation.
The susceptibility and/or resistance were measured according the rate recovery of leptospires in culture, the presence of antibodies in a time graphical representation and by the characteristics of the lesions in kidney tissues through histopatological analysis (data not shown). Meanwhile, although the lines have different levels of production of antibodies and different rates of recovery of leptospires, mainly in the initial period of post-inoculation, they maintained the characteristics of resistance of the infectious agent has in the murine model.
As regards H2O2 production by peritoneal macrophages without stimuli, significant values were only observed at the 14th day in HIV-A compared to LIV-A lines (Figure 3A). When stimulated with IFN-γ, macrophages of control HIV-A produced higher H2O2 concentration than those of control LIV-A (Figure 3C). However, this apparent increase was not significant, probably due to the great variability of individual data.
The treatment of peritoneal macrophages with PMA significantly increased H2O2 production (Figure 3B). Comparing the production of H2O2 between lines, significant differences were observed at the 14th (H > L) and 21st (L > H) days of infection. The simultaneous stimulus with IFN-γ and PMA significantly increased H2O2 production at the 14th (H > L) and 21st (L > H) days of infection (Figure 3D).
H2O2 production by splenic macrophages without stimuli was higher at the 14th and 35th days in both HIV-A and LIV-A lines (Figure 4A). However, the treatment with IFN-γ resulted in differences between lines: H > L at the 21st day and L > H at the 35th day (Figure 4C). In PMA-stimulated macrophages, great alterations were observed at the 14th and 35th days between the infected lines and their respective controls, as well as between lines (Figure 4B). With the additional stimulus of IFN-γ and PMA, there was a difference between lines: L>H at the 14th day (Figure 4D).
As regards NO production by peritoneal macrophages, differences between lines were only detected at the 21st day, when LIV-A produced more NO than HIV-A mice (Figure 5A). The treatment with IFN-γ increased NO production in both lines over the experiment (Figure 5B). NO production by splenic macrophages stimulated or not with IFN-γ was not significantly different between infected mice and their respective controls, as well as between HIV-A and LIV-A lines.
TNF-α production in serum and splenic cells of control HIV-A and LIVA mice was not significantly different. However, differences between lines were observed in the serum of LIV-A line, which produced more TNF-α than HIV-A at the 21th day post-inoculation (Figure 6A). In splenic cells, significant differences were observed at the 4th and 35th days of infection, when HIV-A line produced more TNF-α than LIV-A (Figure 6B). TNF-α production by hepatic cells was higher than that observed in other analyzed tissues and fluids. In the liver, HIV-A mice had a significant inhibition relative to controls at the 7th and 14th days, recovering at the subsequent periods with a high production at the 21st, 28th and 35th days (Figure 6C).
DISCUSSION
Mouse lines selected for the differentiation in the humoral response may present varied degrees of antigenic response due to genetic variations in immunoglobulin production and macrophagic activity.
In the present study, the infectious agent was recovered until the 21th day from the liver of LIV-A mice, whereas HIV-A mice completely controlled the infectious process in the same period. Such results may be related to the higher metabolism of macrophages from low-responder relative to high-responder animals, making difficult for macrophages from LIV-A mice to present antigenic determinants to specific antibodies.
However, the formation of circulating antibodies probably reduced drastically the agent possibility of diffusion in tissues, contributing to the agent elimination after the 21th day in both lines, which made difficult the isolation of leptospires in later post-inoculation periods; this was also observed by Adler & Faine (3, 4). The decreasing values of recovery from kidneys and liver coincide with periods of increased detectable immunoglobulin production in the microscopic agglutination test, demonstrating a positive correlation between these parameters, which corroborates other studies (22).
HIV-A mice are more resistant than LIV-A mice when the humoral immune response represents the main mechanism of protection against microorganisms. However, there was no significant difference in the production of antibodies against L. Pomona between HIV-A and LIV-A lines, although HIV-A animals produced more antibodies between 7 and 21 days. These results partially disagree with those obtained by Marinho et al (22), who analyzed the production of antibodies against L. Icterohaemorrhagiae. HIV-A line produced a statistically significant response relative to LIV-A, maintaining the multispecific effect normally observed in such lines.
The endogenous production of hydrogen peroxide (H2O2) in peritoneal and splenic cells from HIV-A line may have helped to control the infectious process, since a higher production of such metabolite was observed at statistically significant concentrations at the 14th day, which agrees with the results obtained in other studies (21). Although LIV-A line presented a basal H2O2 production in most of the evaluated periods, this production was higher relative to controls at the 14th and 35th days in the spleen and at the 28th and 35th days in the peritoneum, when this line again completely controlled the infectious process.
As regards NO production, there were differences between lines considering infected animals without stimulus: LIV-A produced more NO than HIV-A in the peritoneum at the 14th, 21st and 28th days; in HIV-A line this difference was only observed at the 35th day (HIV-A > LIV-A).
Comparing such results with those obtained for bacterial recovery, NO production contributed to control the infectious process at later infection periods (from the 14th day), which was similar to the results obtained by Marangoni et al (19).
Marinho (21) analyzed NO production in IV-A selection lines against the infection triggered by Leptospira serovar Icterohaemorrhagiae and observed that IFN-γ-treated cells had high NO levels. This effect was also observed in the present study. The treatment with IFN-γ and/or PMA on H2O2 production and with IFN-γ on NO production indicated that in some evaluated compartments and periods L. Pomona was a "messenger" for this cell to produce such metabolite.
Darrah et al (7) studied knockout mice to evaluate H2O2 and NO production and observed that the combination of both radicals resulted in a synergistic effect, with rapid and efficient pathogen killing by activated macrophages, especially under IFN-γ stimulus. In the present study, a positive interaction between both metabolites seems to have occurred, since such intermediaries were higher, especially in the infectious process control periods.
NO production requires the presence of IFN-γ, which plays an important role as a macrophage activating factor and a primary signal for the transcription of the enzyme Inducible Nitric Oxide Synthase (INOS). In innate immunity, signals for macrophage activation may originate from the pathogen and natural killer cells (NK cells), which are a source of IFN-γ (1).
In addition, Marinho et al (22) analyzed TNF-α production by HIV-A and LIV-A lines against Leptospira serovar Icterohaemorrhagiae and observed a high production in LIV-A at the beginning of the infection and this level remained stable until the 14th day. Following this period, there was an inhibition of TNF-α production, exactly at the moment leptospires were not recovered anymore.
Production of tumor necrosis factor (TNF-α) as a parameter for macrophagic activity was also evaluated in the present experiment. TNF-α levels in the serum from infected LIV-A line were higher at the 7th, 21st and 35th days. However, significant differences between lines were only observed at the 21st day. HIV-A splenic cells produced more TNF-α than those of LIV-A at the 4th and 35th days post-inoculation. In hepatic cells, TNF-α production was different between lines only at the 35th day (HIV-A > LIV-A).
These results indicate that TNF-α was an adjuvant in the infectious process control in both lines, and the analysis of the production of this cytokine in HIV-A hepatic cells evidenced its importance in the infection control, which agrees with the data obtained by Marangoni et al (20), who analyzed TNF-α production by hepatic macrophages, correlating it to the presence of lipopolysaccharides (LPS) from Leptospira serovar Icterohaemorrhagiae.
The analyses of other cytokines can be determinant to evaluate the profile of a broaden immune response of the different serovars of leptospires in the lines genetically selected. By Marinho et al. (22) the line HIV had the profile Th2 with more production of antibodies, IL-4 and worst tissues lesions, while the line LIV-A had the profile Th1 with higher production of IFN-γ, higher macrophage activity and lesser damaged tissues.
The differences in the recovery rates and in the cellular and humoral immune response of Leptospira serovar Pomona relative to other serovars investigated in previous studies may be related to antibody production control mechanisms and differentiated cellular response, but especially to the infectious agent adaptability factors, since serovars had different virulence in some hosts, i.e. they are species-adapted (14, 17).
In general, the study of the infection by Leptospira serovar Pomona in lines indicated that macrophagic activity and TNF-α production play an important role in the infectious process control, which varied according to the evaluated compartments and periods. Compared to studies with other serovars, the present results indicate partial differences in an immune trait genetically selected by an external nonspecific agent.
ACKNOWLEDGMENTS
We thank the National Council for Scientific and Technological Development (CNPq) for the financial support; Prof. Dr. Silvio Arruda Vasconcellos and the biologist Zenaíde Maria de Moraes from the Bacterial Zoonoses Lab, Department of Veterinary Hygiene and Animal Health, College of Veterinary Medicine and Animal Science, University of São Paulo (USP), São Paulo City, São Paulo State, Brazil; Prof. Dr. Jane Megid, Department of Veterinary Hygiene and Public Health, College of Veterinary Medicine and Animal Science, São Paulo State University (UNESP), Botucatu Municipality, São Paulo State, Brazil.
Submitted: April 12, 2010; Returned to authors for corrections: May 24, 2010; Approved: January 13, 2011.
- 1. Abbas, A.K.; Lichtman, A.H.; Pober, J.S. (2002). Citocinas. In: Abbas, A.K., Lichtman, A.H., Pober, J.S. (eds). Imunologia celular e molecular 4ª ed. Revinter, Rio de Janeiro, Brasil, p.235-269
- 2. Adams, H.R. (1996). Physiologic, pathophysiologic, and therapeutic implications for endogenous nitric oxide. J. Am. Vet. Med. Assoc. 209, 1297-1302.
- 3. Adler, B.; Faine, S. (1976). Susceptibility of mice treat with cyclophosphamide to lethal infection with Leptospira interrogans serovar pomona. Infect. Immun. 14, 703-708.
- 4. Adler, B.; Faine, S. (1978). Serological and protective-antibody response of rabbits to leptospiral antigens. J. Med. Microbiol. 11, 401-409.
- 5. Biozzi, G.; Stiffel, C.; Mouton, D.; Bouthillier, Y.; Decreusefond, C. (1972). Cytodynamics of the immune response in the two lines of mice genetically selected for "high" and "low" antibody synthesis. J. Exp. Med 135, 1071-1094.
- 6. Cabrera, W.H.K.; Ibañez, O.M.; Oliveira, S.L.; De Sant'anna, O.A.; Siqueira, M.; Mouton, D.; Biozzi, G. (1982). Evidence for distinct polygenic regulation of antibody responses to some unrelated antigens in lines of mice selected for high or low antibody responses to somatic antigens of Salmonella. Immunogenetics. 16, 583-592.
- 7. Darrah, P.A.; Hondalus, M.K.; Chen, Q.; Ischiropoulos, H.; Mosser, D.M. (2000). Cooperation between reactive oxygen and nitrogen intermediates in killing of Rodococcus equi by activated macrophages. Infect. Immun. 68, 3587-3593.
- 8. Davis, J.M.; Haake, D.A.; Ramakrishnan, L. (2009) Leptospira interrogans stably infects zebrafish embryos, altering phagocyte behavior and homing to specific tissues. PLoS Negl Trop Dis http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2693671/?tool=pubmed
- 9. Di Giovine, F.S.; Nuki, G.; Duff, G.W. (1988). Tumour necrosis factor in synovial exudates. Ann. Rheum. Dis. 47, 768-772.
- 10. 10 Dockrell, H.M.; Taverne, J.; Lelchuk, R.; Depledge, P.; Brown, I.N.; Playfair, J.H. (1985). Macrophage functions in Biozzi mice. Immunol 55, 501-519.
- 11. Faine, S. (1982). Guidelines for the control of leptospirosis World Health Organization, Geneva.
- 12. Faine, S. (ed) (1994). Leptospira and Leptospirosis, CRC-Press, Boca-Raton, Florida.
- 13. Green, L.C.; Tannenbaun, S.R. (1981). Nitrate synthesis in the germfree and conventional rat. Science. 212, 56-58.
- 14. Hathaway, S.C.; Blackmore, D.K.; Marshall, R.B. (1983). Leptospirosis and the maintenance host: a laboratory mouse model. Res. Vet. Sci. 34, 82-89.
- 15. Ibanez, O.M.; Mouton, D.; Oliveira, S.L.; Ribeiro Filho, O.G.; Piatti, R.M.; Sant´Anna, O.A.; Massa, S.; Biozzi, G.; Siqueira, M. (1988). Polygenic control of quantitative antibody responsiveness: restrictions of the multispecific effect related to the selection antigen. Immunogenetics. 28, 6-12.
- 16. Isogai, E.; Isogai, H.; Fujii, N.; Oguma, K. (1990). Macrophage activation by leptospiral lipopolysaccharide. Zentralbl. Bakteriol. 273, 200-208.
- 17. Klimpel, G.R.; Mathias, M.A.; Vinetz, J.M. (2003). Leptospira interrogans activation of human peripheral blood mononuclear cells: Preferential expansion of TCR yδ+ T cells vs TCRαβ+ T cells. J. Immunol. 171, 1447-1455.
- 18. Lowanitchapat, A.; Payungporn, S.; Sereemaspun, A.; Ekpo, P.; Phulsuksombati, D.; Poovorawan, Y.; Chirathaworn, C. (2009). Expression of TNF-α, TGF-β, IP-10 and IL-10 mRNA in kidneys of hamsters infected with pathogenic Leptospira. Comp Immunol Microbiol Infect Dis. http://www.citeulike.org/article/5541032
- 19. Marangoni, A.; Accardo, S.; Aldini, R.; Guardigli, M.; Cavrini, F.; Sambri, V.; Montagnani, M.; Roda, A.; Cavenini, R. (2006). Production of reactive oxygen species and expression of inducible nitric oxide synthase in rat isolated Kupffer cells stimulated by Leptospira interrogans and Borrelia burgdorferi World J. Gastroenterol. 12, 3077-3081.
- 20. Marangoni, A.; Aldini, R.; Sambri, V.; Giacani, L.; Di Leo, K.; Cevenini, R. (2004). Production de tumor factorby Treponema pallidum, Borrelia burgdorferi, s.l, and Leptospira interrogans is isolated rat Kupffer cells. FEMS Immunol. Med. Micriobiol. 40, 187-191.
- 21. Marinho, M. (2000). Resposta imune à infecção por Leptospira interrogans sorovar icterohaemorrhagiae em linhagens de camundongos geneticamente selecionados Botucatu, São Paulo, Brasil, 102p. (D.Sc. Thesis. Faculdade de Medicina, UNESP).
- 22. Marinho, M.; Langoni, H.; Oliveira, S.L.; Carreira, R.; Perri, S.H.V.; Luvizoto, M.C. (2003). Resposta humoral, recuperação bacteriana e lesões histológicas em camundongos em geneticamente selecionados para bons e maus respondedores de anticorpos e balb/c, frente à infecção por Leptospira interrogans sorovar icterohaemorrhagiae. Pesq. Vet. Bras. 23, 5-12.
- 23. Passos, E.C.; Vasconcellos, A.S.; Ito, F.O.; Yasuda, P.H.; Nürmberger Jr, R. (1988). Isolamento de Leptospiras a partir do tecido renal de hamsters experimentalmente infectados com L. interrogans sorotipo pomona: Emprego das técnicas de Pipeta Pasteur e das diluições seriadas em meio de cultura de Fletcher tratado com 5-fluor-uracil ou sulfato de neomicina. Rev. Fac. Med. Vet. Zoot. Univ. São Paulo. 25, 221-235.
- 24. Pick, E.; Keisari, Y. (1980). A simple colorimetric method for the measurement of hydrogen peroxide produced by cells in culture. J. Immunol. Methods. 38, 161-170.
- 25. Pick, E.; Mizel, D. (1981). Rapid microassays for the measurement of superoxide and hydrogen peroxide production by macrophages in culture using an automatic enzyme immunoassay reader. J. Immunol. Methods. 46, 211-226.
- 26. Pourshafie, M.; Ayub, Q.; Barrow, W.W.; (1993). Comparative effects of Mycobacterium avium glycopeptidolipid and lipopeptide fragment on the function and ultrastructure of mononuclear cells. Clin. Exp. Immunol. 93, 72-79.
- 27. Santa Rosa, C.A. (1970). Diagnóstico laboratorial das leptospiroses. Rev. Microbiol. 1, 97-109.
- 28. Werts, C.; Tapping, R.I.; Mathison, J.C.; Chuang, T.H.; Kravchenko, V.; Saint Girons, I.; Haake, D.A.; Godowski, P.J.; Hayashi, F.; Ozinsky, A.; Underhill, D.M.; Kirschning, C.J.; Wagner, H.; Aderem, A.; Tobias, P.S.; Ulevitch, R.J. (2001). Leptospiral lipopolysaccharide activates cells through a TLR2- dependent mechanism. Nat. Immunol. 2, 346-352.
- 29. Zar, J.H. (1996). Bioestatistical analysis 3ª ed. Prentice Hall, Upper Saddle River.
Publication Dates
-
Publication in this collection
06 June 2011 -
Date of issue
June 2011
History
-
Received
12 Apr 2010 -
Accepted
13 Jan 2011 -
Reviewed
24 May 2010