Abstract
Exclusion of the transcription factor Max from the nucleus of retinal ganglion cells is an early, caspase-independent event of programmed cell death following damage to the optic axons. To test whether the loss of nuclear Max leads to a reduction in neuroprotection, we developed a procedure to overexpress Max protein in rat retinal tissue in vivo. A recombinant adeno-associated viral vector (rAAV) containing the max gene was constructed, and its efficiency was confirmed by transduction of HEK-293 cells. Retinal ganglion cells were accessed in vivo through intravitreal injections of the vector in rats. Overexpression of Max in ganglion cells was detected by immunohistochemistry at 2 weeks following rAAV injection. In retinal explants, the preparation of which causes damage to the optic axons, Max immunoreactivity was increased after 30 h in vitro, and correlated with the preservation of a healthy morphology in ganglion cells. The data show that the rAAV vector efficiently expresses Max in mammalian retinal ganglion cells, and support the hypothesis that the Max protein plays a protective role for retinal neurons.
Retina; Max expression; Transcription factor; Viral vector; Gene therapy; Neuroprotection
Braz J Med Biol Res, March 2005, Volume 38(3) 375-379 (Short Communication)
Modulation of the expression of the transcription factor Max in rat retinal ganglion cells by a recombinant adeno-associated viral vector
H. Petrs-Silva1, V. Chiodo2, L.B. Chiarini1, W.W. Hauswirth2 and R. Linden1
1Instituto de Biofísica, Universidade Federal do Rio de Janeiro, Rio de Janeiro, RJ, Brasil
2Department of Ophthalmology, University of Florida College of Medicine, Gainesville, FL, USA
 Text
Text
 References
References
 Correspondence and Footnotes
Correspondence and Footnotes
Correspondence and Footnotes
Correspondence and Footnotes
Correspondence and Footnotes
Correspondence and Footnotes
Abstract
Exclusion of the transcription factor Max from the nucleus of retinal ganglion cells is an early, caspase-independent event of programmed cell death following damage to the optic axons. To test whether the loss of nuclear Max leads to a reduction in neuroprotection, we developed a procedure to overexpress Max protein in rat retinal tissue in vivo. A recombinant adeno-associated viral vector (rAAV) containing the max gene was constructed, and its efficiency was confirmed by transduction of HEK-293 cells. Retinal ganglion cells were accessed in vivo through intravitreal injections of the vector in rats. Overexpression of Max in ganglion cells was detected by immunohistochemistry at 2 weeks following rAAV injection. In retinal explants, the preparation of which causes damage to the optic axons, Max immunoreactivity was increased after 30 h in vitro, and correlated with the preservation of a healthy morphology in ganglion cells. The data show that the rAAV vector efficiently expresses Max in mammalian retinal ganglion cells, and support the hypothesis that the Max protein plays a protective role for retinal neurons.
Key words: Retina, Max expression, Transcription factor, Viral vector, Gene therapy, Neuroprotection
The transcription factor Max is a basic helix-loop-helix/leucine zipper protein, which forms heterodimers with members of the Myc protein family (1). Myc/Max heterodimers exhibit sequence-specific DNA binding and function as transcription factors. Max may also form homodimers which recognize the same DNA target sequence as Myc/Max, but which are unable to function as transcriptional activators (2). The functional data support a dual physiological role for Max, either as an obligatory DNA-binding partner for Myc, or as a negative regulator of Myc activity, depending on the relative concentration of both partners. The transcription factor c-Myc regulates both cell proliferation and apoptosis (3). Deregulated expression of c-Myc is sufficient to drive programmed cell death in response to growth inhibitory signals, but the precise mechanisms are still unclear (4).
Recently,we showed that the transcription factor Max, which is found mostly within the nucleus of normal cells, undergoes exclusion from the nucleus early upon induction of programmed cell death in retinal ganglion cells, followed by complete loss of Max immunoreactivity as apoptotic cell death proceeds (5). The early nuclear exclusion of Max probably leads to the loss of its transcriptional regulatory activity, which may be an early and necessary step for the execution of cell death (5). Max is ubiquitously expressed and its absence leads to early embryonic lethality (6). It has been shown that the selective over-expression of Max in endothelial cells blocks apoptosis promoted by serum withdrawal (7), suggesting a cell protective function for this protein. If, indeed, the early loss of nuclear Max reduces neuroprotection, then its overexpression might counteract the retrograde degeneration of retinal ganglion cells following axon damage. Modulation of gene expression is, however, notoriously difficult in postmitotic, differentiated neurons. This article describes a technique successfully used to modulate the expression of Max in retinal ganglion cells, together with preliminary results indicating a decrease in the sensitivity of transduced neurons to retrograde degeneration.
Adeno-associated viruses (AAVs) belong to the Parvoviridae family, small animal viruses with a linear single-stranded DNA genome, that replicate in the presence of a helper virus such as adenovirus (8). Gene transfer vectors based on AAV combine a number of advantages: AAV is naturally defective for replication and is considered nonpathogenic (9). It can transduce both proliferating and post-mitotic cells, transfer a gene to a number of cell types efficiently, and mediate long-term gene expression in the central nervous system, including the retina, as well as in the lungs, liver, or muscle cells, with minimal toxicity and cellular immune responses (10). A disadvantage of AAV is the limited packaging size of the virus, which cannot exceed 5,000 nucleotides. This, however, is not relevant for the delivery of Max, which is encoded by a relatively small gene.
Recombinant AAV (rAAV) vectors have been proved suitable for long-term transgene expression in the eye (11), and have been used to transfect a variety of retinal cell types including photoreceptors (12), retinal pigment epithelial cells (13), Müller cells (12), and retinal ganglion cells (14). The efficiency of transfection of particular cell types in the eye is determined by a number of variables including the site of injection, the AAV serotype and titer, and the specific gene promoter and enhancing elements used. The experiments described below employed an AAV containing the max gene, and capable of efficient transduction of retinal ganglion cells in vivo leading to overexpression of the Max protein.
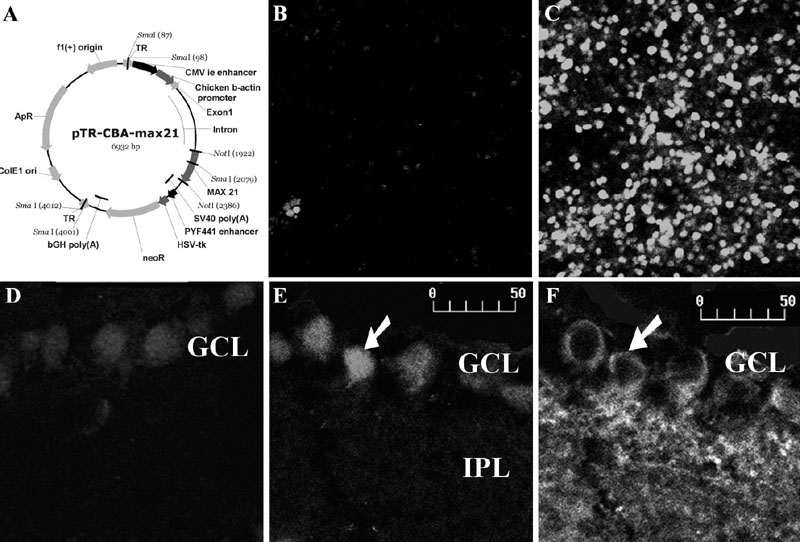
rAAV stocks were produced by co-transfection of both an rAAV vector plasmid and a helper plasmid containing only minimally required AAV and adenovirus sequences, into HEK-293 cells. The vector plasmid has the pTR-UF backbone (15) containing the max gene under the control of a hybrid CMV/CBA promoter, all flanked by AAV2 inverted terminal repeats, the only AAV sequence necessary in cis, and functions as the origin of DNA replication and the packaging signal (16) (Figure 1A). After 72 h the cells were lysed and centrifuged and the vector-containing supernatant was centrifuged in an Iodixanol density gradient to separate the vectors from cell debris. The vector fraction was further purified and concentrated by column chromatography. Vector titers were determined by quantitative PCR relative to a known standard and vector purity was assayed by protein gel analysis (17). All the steps cited above were carried out to assure that the high titer vector stocks were separated from impurities.
To determine the efficiency of transduction of the AAV-max vector, HEK-293 cells (2 x 106) were infected with 2 x 1013 particles/ml. Four days after infection, the cells were analyzed by confocal microscopy following immunocytochemistry for Max protein. To obtain photomicrographs representative of the relative intensity of immunolabeling in the various experimental groups, the confocal microscope was set for the most intensely stained image, and all photomicrographs were obtained with the same settings. HEK-293 cells infected with rAAV-max displayed a much stronger immunofluorescence signal than uninfected 293 cells (Figure 1B,C). In fields containing similar numbers of cells, the average intensity of immunofluorescence in cells transfected with rAAV was approximately seven times higher than in uninfected cells (35.3 vs 5.3 arbitrary units, respectively, measured with the NIH-ImageJ program on exposure-matched photomicrographs).
To assess the ability of rAAV-max to transduce nervous tissue in vivo, retinal ganglion cells were targeted in newborn rats via intravitreal injection. Each eye received 1 µl of the rAAV-max stock, which represents 2 x 1010 vector particles. Because there is a delay in rAAV-mediated transgene expression due to the time required for conversion of the recombinant viral DNA to a transcriptionally active double-stranded form (18), retinas were examined 2 weeks after the injection. At postnatal day 15, no cell-mediated immune response was detected in the eye or in the retina of the experimental rats. In sections of the infected postnatal day 15 retina, there was increased immunoreactivity for Max relative to uninfected retinas (Figure 1D,E). Ganglion cells, which were distinguished from other cells within the ganglion cell layer by staining with an antibody to ßIII-tubulin, were highly immunoreactive for Max (Figure 1D-F), showing efficient transduction of the rAAV-max vector in these cells.
Dissection of retinal tissue for preparation of explants necessarily damages optic nerve axons that originate in the ganglion cells. To test the effect of rAAV-max transduction in such axotomized retinal ganglion cells, infected retinal explants were maintained in vitro for 30 h after dissection. The retinas exhibited increased Max immunostaining after 30 h in vitro compared with uninfected retinas (Figure 2A,B). Double labeling of both DNA fragmentation with the TUNEL procedure, and Max by immunohistochemistry (Figure 2C,D), showed that all TUNEL-positive cells were devoid of Max staining, as previously shown (5). However, cells with intense immunoreactivity for Max still showed an apparently healthy morphology even at 30 h after axon damage (Figure 2C), suggesting that overexpression of Max may have a protective effect upon the axotomized ganglion cells.
A surprisingly deleterious effect on retinal survival was described for the proto-oncogene Bcl-2. Retina infected with AAV containing Bcl-2 was more susceptible to ganglion cell loss than control retina following either axon injury or intravitreal NMDA (19). This result was unexpected, because Bcl-2 had been shown to have a protective effect both in transgenic models and in vitro. The paradoxical effect of Bcl-2 upon overexpression highlights the importance of carefully examining the results of modulation of gene expression in vivo, particularly in complex tissues such as the retina and other parts of the central nervous system.
Viral vectors are efficient gene transfer vehicles in various tissues both in vitro and in vivo. Four main groups of viral vectors are currently used in gene transfer protocols: retroviral, adenoviral, adeno-associated viral, and lentiviral vectors. We choose rAAV-mediated gene transfer both because it results in persistent transgene expression and because rAAV is able to infect a variety of cell types, including postmitotic neurons such as the retinal ganglion cells. The purification of rAAV vectors is important to produce highly concentrated stocks, without either cell remnants or serum proteins that might trigger immune responses. We were able to produce stocks of 1013 particles/ml that, after intravitreal injection into rats, showed no signs of stimulating an immune response.
The experiments reported here demonstrate that rAAV vectors containing the max cDNA were a) infective and drove the expression of the Max protein in both the HEK-293 cell line and in retinal ganglion cells in vivo, and b) showed a potential
protective function for retinal ganglion cells. The results support the hypothesis that the early nuclear exclusion of Max from axon-damaged ganglion cells may be a necessary step for the execution of retrograde degeneration (5), and raise the possibility that modulation of the expression of this transcription factor may be a novel target for the development of gene-based therapy for degenerative retinopathies such as glaucoma, that targets retinal ganglion cells
(20).
A, Map of the recombinant adeno-associated viral (rAAV)-max vector. f1(+) = origin; f1 = bacteriophage origin of replication; TR = terminal repeats; CMV ie enhancer = cytomegalovirus immediate early enhancer; max21 = coding sequence of the human max gene; SV40 poly(A) = transgene polyadenylation site from Simian virus 40; neoR = coding sequence of the neomycin resistance gene; PYF441 enhancer = enhancer from polyoma virus; bGH poly(A) = bovine growth hormone polyadenylation signal; HSV-tk = thymidine kinase promoter of herpesvirus; ColE1 ori = E. coli origin of replication; ApR, ampicillin resistance sequence. B,C, Test of transfection efficiency. Samples of HEK-293 cells, either uninfected (B), or at 96 h after infection with 2 x 1013 particles/ml (C), were immunostained with anti-Max antibody (Santa Cruz). Notice the overexpression of Max protein after rAAV-max infection. D-F, Immunohistochemistry for Max (D,E) or for ßIII-tubulin (F) in retinal sections 2 weeks after intravitreal injection of either PBS (D) or rAAV-max (E,F). Notice the overexpression of Max in ßIII-tubulin-immunoreactive retinal ganglion cells (arrows) in the matching E and F photomicrographs. Retinal layers: GCL = ganglion cell layer; IPL = inner plexiform layer. Scale = 50 µm.
A,B, Immunostaining for Max in explants at 30 h in vitro, from retinae intravitreally injected either with PBS (A) or with recombinant adeno-associated viral (rAAV)-max (B). Notice the increased immunoreactivity in the retina injected with rAAV-max, even after 30 h in vitro. C,D, Matching confocal photomicrographs of the ganglion cell layer (GCL) of retinal explants after 30 h in vitro stained either for Max (C) or TUNEL (D). Notice that condensed TUNEL-positive nuclei belong to cells devoid of Max (arrowheads), while Max overexpressing cells show a healthy morphology (arrows). Scale = 50 µm.
Acknowledgments
We thank José Nilson dos Santos, Tom Doyle and Min Ding for excellent technical assistance, Dr. Robert N. Eisenman (Fred Hutchinson Cancer Research Center, Seattle, WA, USA) for kindly providing max cDNA, and Tim Vough for help with the confocal microscope.
Address for correspondence: H. Petrs-Silva, Instituto de Biofísica, UFRJ, CCS, Bloco G, 21949-900, Rio de Janeiro, RJ, Brasil. Fax: +55-21-2280-8193. E-mail: petrs@biof.ufrj.br
Presented at the XI Congresso Brasileiro de Biologia Celular, Campinas, SP, Brazil, July 15-18, 2004. Research supported by CNPq, CAPES, FAPERJ, PRONEX, The John Simon Guggenheim Foundation (to R. Linden), and by the National Institutes of Health (grants EY 11123 and EY 13053), the Foundation Fighting Blindness, Research to Prevent Blindness, the Macular Vision Research Foundation, and the Steinbach Foundation (a grant to W.W. Hauswirth). Received August 27, 2004. Accepted December 1, 2004.
- 1. Blackwood EM & Eisenman RN (1991). Max: a helix-loop-helix zipper protein that forms a sequence-specific DNA binding complex with Myc. Science, 251: 1211-1217.
- 2. Amati B, Dalton S, Brooks MW, Littlewood TD, Evan GI & Land H (1992). Transcriptional activation by the human c-Myc oncoprotein in yeast requires interaction with Max. Nature, 359: 423-426.
- 3. Evan GI, Wyllie AH, Gilbert CS, Littlewood TD & Land H (1992). Induction of apoptosis in fibroblasts by c-myc protein. Cell, 69: 119-128.
- 4. Pelengaris S, Khan M & Evan G (2002). c-MYC: more than just a matter of life and death. Nature Reviews. Cancer, 10: 764-776.
- 5. Petrs-Silva H, de Freitas FG, Linden R & Chiarini LB (2004). Early nuclear exclusion of the transcription factor Max is associated with retinal ganglion cell death independent of caspase activity. Journal of Cellular Physiology, 198: 179-187.
- 6. Shen-Li H, O'Hagan RC, Hou Jr H, Horner 2nd JW, Lee HW & DePinho RA (2000). Essential role for Max in early embryonic growth and development. Genes and Development, 14: 17-22.
- 7. Shichiri M, Kato H, Doi M, Marumo F & Hirata Y (1999). Induction of Max by adrenomedullin and calcitonin gene-related peptide antagonizes endothelial apoptosis. Molecular Endocrinology, 13: 1353-1363.
- 8. Muzyczka N & Berns KI (2001). Parvoviridae: the viruses and their replication. In: Knipe DM & Howley PM (Editors), Fields Virology Vol. 2. Lippincott, Williams and Wilkins, Philadelphia, PA, USA, 2327-2359.
- 9. Muzyczka N (1992). Use of adeno-associated virus as a general transduction vector for mammalian cells. Current Topics in Microbiology and Immunology, 158: 97-129.
- 10. Samulski RJ, Sally M & Muzyczka N (1999). Adeno-associated viral vectors. In: Friedmann T (Editor), Development of Human Gene Therapy Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, USA, 131-172.
- 11. Chaum E & Hatton MP (2002). Gene therapy for genetic and acquired retinal diseases. Survey of Ophthalmology, 47: 449-469.
- 12. Liang FQ, Aleman TS, Dejneka NS, Dudus S, Fisher KJ, Maguire AM, Jacobson SG & Bennett J (2001). Long-term protection of retinal structure but not function using rAAV. CNTF in animal models of retinitis pigmentosa. Molecular Therapy, 4: 461-472.
- 13. Hansen KA, Sugino IK, Yagi F, Wang H, Tsukahara I, Gullapalli V, Bennett J & Zarbin MA (2003). Adeno-associated virus encoding green fluorescent protein as a label for retinal pigment epithelium. Investigative Ophthalmology and Visual Science, 44: 772-780.
- 14. Guy J, Qi X, Muzyczka N & Hauswirth WW (1999). Reporter expression persists 1 year after adeno-associated virus-mediated gene transfer to the optic nerve. Archives of Ophthalmology, 117: 929-937.
- 15. Zolotukhin S, Hauswirth WW & Muzyczka N (1996). A `humanized' green fluorescent protein cDNA adapted for high-level expression in mammalian cells. Journal of Virology, 70: 4646-4654.
- 16. McCarty DM, Christensen M & Muzyczka N (1991). Sequences required for coordinate induction of adeno-associated virus p19 and p40 promoters by Rep protein. Journal of Virology, 65: 2936-2945.
- 17. Zolotukhin S, Potter M, Zolotukhin I et al. (2002). Production and purification of serotype 1, 2, and 5 recombinant adeno-associated viral vectors. Methods, 28: 158-167.
- 18. Janson CG, McPhee SW, Leone P, Freese A & During MJ (2001). Viral-based gene transfer to the mammalian CNS for functional genomic studies. Trends in Neurosciences, 24: 706-712.
- 19. Simon PD, Vorwerk CK, Mansukani SS, Chen SJ, Wilson JM, Zurakowski D, Bennett J & Dreyer EB (1999). Bcl-2 gene therapy exacerbates excitotoxicity. Human Gene Therapy, 10: 1715-1720.
- 20. Nickells RW (1999). Apoptosis of retinal ganglion cells in glaucoma: an update of the molecular pathways involved in cell death. Survey of Ophthalmology, 43 (Suppl 1): S151-S161.
Correspondence and Footnotes
Publication Dates
-
Publication in this collection
08 Mar 2005 -
Date of issue
Mar 2005
History
-
Received
27 Aug 2004 -
Accepted
01 Dec 2004