Abstract
The N-acylhydrazone (NAH) analogues N-methyl 2-thienylidene 3,4-benzoylhydrazine (LASSBio-785) and N-benzyl 2-thienylidene 3,4-benzoylhydrazine (LASSBio-786) were prepared from 2-thienylidene 3,4-methylenedioxybenzoylhydrazine (LASSBio-294). The ability of LASSBio-785 and LASSBio-786 to decrease central nervous system activity was investigated in male Swiss mice. LASSBio-785 or LASSBio-786 (30 mg/kg, ip) reduced locomotor activity from 209 ± 26 (control) to 140 ± 18 (P < 0.05) or 146 ± 15 crossings/min (P < 0.05), respectively. LASSBio-785 (15 or 30 mg/kg, iv) also reduced locomotor activity from 200 ± 15 to 116 ± 29 (P < 0.05) or 60 ± 16 crossings/min (P < 0.01), respectively. Likewise, LASSBio-786 (15 or 30 mg/kg, iv) reduced locomotor activity from 200 ± 15 to 127 ± 10 (P < 0.01) or 96 ± 14 crossings/min (P < 0.01), respectively. Pretreatment with flumazenil (20 mg/kg,ip) prevented the locomotor impairment induced by NAH analogues (15 mg/kg, iv), providing evidence that the benzodiazepine (BDZ) receptor is involved. This finding was supported by the structural similarity of NAH analogues to midazolam. However, LASSBio-785 showed weak binding to the BDZ receptor. LASSBio-785 or LASSBio-786 (30 mg/kg,ip, n = 10) increased pentobarbital-induced sleeping time from 42 ± 5 (DMSO) to 66 ± 6 (P < 0.05) or 75 ± 4 min (P < 0.05), respectively. The dose required to achieve 50% hypnosis (HD50) following iv injection of LASSBio-785 or LASSBio-786 was 15.8 or 9.5 mg/kg, respectively. These data suggest that both NAH analogues might be useful for the development of new neuroactive drugs for the treatment of insomnia or for use in conjunction with general anesthesia.
N-acylhydrazone; Locomotor activity; Sedation; Hypnosis; Benzodiazepine receptor; Flumazenil
Introduction
During an ongoing research program aimed at developing novel treatments for cardiovascular disorders, novel N-acylhydrazone (NAH) compounds based on 2-thienylidene 3,4-methylenedioxybenzoylhydrazine (LASSBio-294) were synthesized. LASSBio-294 was synthesized from safrole 11. Barreiro EJ, Fraga CAM, Miranda ALP, Rodrigues CR. Strategy of molecular simplification in rational drug design: the discovery of a new cardioactive agent. Quim Nova 2002; 25: 129-148, doi: 10.1590/S0100-40422002000100022.
https://doi.org/10.1590/S0100-4042200200...
,22. Lima PC, Lima LM, da Silva KC, Leda PH, de Miranda AL, Fraga CA, et al. Synthesis and analgesic activity of novel N-acylarylhydrazones and isosters, derived from natural safrole. Eur J Med Chem 2000; 35: 187-203, doi: 10.1016/S0223-5234(00)00120-3.
https://doi.org/10.1016/S0223-5234(00)00...
, an abundant Brazilian natural product obtained from Ocotea pretiosa
33. Barreiro EJ, Fraga CAM. The utilization of the safrole, principal chemical constituent of sassafras oil, in the synthesis of compounds actives in the arachidonic acid cascade: antiinflammatory, analgesic and antithrombotic. Quim Nova 1999; 22: 744-759, doi: 10.1590/S0100-40421999000500019.
https://doi.org/10.1590/S0100-4042199900...
. LASSBio-294 was prepared as a bioactive 6-aryl-4,5-heterocyclic-fused pyridazinone compound, part of a family of compounds known as potent and selective phosphodiesterase inhibitors 44. Dal Piaz V, Giovannoni MP, Castellana C, Palacios JM, Beleta J, Domenech T, et al. Novel heterocyclic-fused pyridazinones as potent and selective phosphodiesterase IV inhibitors. J Med Chem 1997; 40: 1417-1421, doi: 10.1021/jm970105l.
https://doi.org/10.1021/jm970105l...
, and its cardiac inotropic properties were evaluated. LASSBio-294 exhibited significant positive cardiac inotropic activity due to increased calcium accumulation in the sarcoplasmic reticulum 55. Sudo RT, Zapata-Sudo G, Barreiro EJ. The new compound, LASSBio 294, increases the contractility of intact and saponin-skinned cardiac muscle from Wistar rats. Br J Pharmacol 2001; 134: 603-613, doi: 10.1038/sj.bjp.0704291.
https://doi.org/10.1038/sj.bjp.0704291...
. Additionally, LASSBio-294 induced the relaxation of aortic rings, an effect mediated by the guanylate cyclase/cyclic guanylate monophosphate pathway 66. Silva CL, Noel F, Barreiro EJ. Cyclic GMP-dependent vasodilatory properties of LASSBio 294 in rat aorta. Br J Pharmacol 2002; 135: 293-298, doi: 10.1038/sj.bjp.0704473.
https://doi.org/10.1038/sj.bjp.0704473...
.
In order to identify new drug candidates with enhanced vasodilatory properties and decreased side effects, two novel analogues of LASSBio-294 were synthesized by the introduction of a methyl and a benzyl group in the amide nitrogen of the NAH moiety, to yield LASSBio-785 (N-methyl 2-thienylidene 3,4-benzoylhydrazine) and LASSBio-786 (N-benzyl 2-thienylidene 3,4-benzoylhydrazine), respectively. LASSBio-785 exhibited improved vasodilator properties (IC50: 10.2 ± 0.5 µM) and was seven times more potent than LASSBio-294 (74 µM) in aortic rings pre-contracted with phenylephrine 77. Silva AG, Zapata-Sudo G, Kummerle AE, Fraga CA, Barreiro EJ, Sudo RT. Synthesis and vasodilatory activity of new N-acylhydrazone derivatives, designed as LASSBio-294 analogues. Bioorg Med Chem 2005; 13: 3431-3437, doi: 10.1016/j.bmc.2005.03.003.
https://doi.org/10.1016/j.bmc.2005.03.00...
, and both derivatives were recently shown to inhibit platelet aggregation 88. Brito FC, Kummerle AE, Lugnier C, Fraga CA, Barreiro EJ, Miranda AL. Novel thienylacylhydrazone derivatives inhibit platelet aggregation through cyclic nucleotides modulation and thromboxane A2 synthesis inhibition. Eur J Pharmacol 2010; 638: 5-12, doi: 10.1016/j.ejphar.2010.04.003.
https://doi.org/10.1016/j.ejphar.2010.04...
. While investigating the hemodynamic effect of LASSBio-785 in rats, we noted that the animals fell asleep, an effect not observed with the LASSBio-294 prototype compound. Therefore, the current study was designed to investigate the sedative-hypnotic pharmacological properties of LASSBio-785 and the more lipid-soluble LASSBio-786. We also investigated the involvement of the GABAergic system in the sedative-hypnotic activities of both compounds.
Material and Methods
Animals
Male Swiss mice (20-25 g) were housed in a temperature- (24 ± 1°C) and humidity-controlled (60%) room with a 12-h light/dark cycle. The mice were fed a standard diet with water ad libitum. Housing, handling and experimental procedures were in compliance with the recommendations of the Animal Care and Use Committee of Universidade Federal do Rio de Janeiro (protocol #DFBCICB013). The animals were randomly assigned to a single-treatment group and were used only once.
Drugs
Midazolam, sodium pentobarbital and flumazenil were generously donated by Cristália Produtos Químicos e Farmac#xEA;uticos (Brazil). LASSBio-785 and LASSBio-786 were generously donated by Laboratório de Avaliação e Síntese de Substâncias Bioativas (LASSBio, Universidade Federal do Rio de Janeiro, Brazil). All compounds were dissolved in dimethyl sulfoxide (DMSO), except for midazolam, which was dissolved in distilled water, and pentobarbital sodium, which was dissolved in propylene glycol and distilled water.
Methods
Assessment of locomotor activity in mice
Spontaneous locomotor activity was measured in an open field (45 × 45 cm, LE 8811, Panlab, USA) in which 16 infrared photocells were positioned every 2.5 cm. The mice were placed individually in the center of the chamber. The sedative properties of midazolam, LASSBio-785 and LASSBio-786 were compared using locomotor activity as an index of sedation 99. Menegatti R, Silva GM, Zapata-Sudo G, Raimundo JM, Sudo RT, Barreiro EJ, et al. Design, synthesis, and pharmacological evaluation of new neuroactive pyrazolo[3,4-b]pyrrolo[3,4-d]pyridine derivatives within vivo hypnotic and analgesic profile. Bioorg Med Chem 2006; 14: 632-640, doi: 10.1016/j.bmc.2005.08.042.
https://doi.org/10.1016/j.bmc.2005.08.04...
. Locomotor activity was quantified as the number of beam interruptions (crossings) registered by a computer, and data are reported as the number of crossings per minute (crossings/min). After ip oriv injection of vehicle (DMSO), midazolam (2 mg/kg), LASSBio-785 (15 or 30 mg/kg), or LASSBio-786 (15 or 30 mg/kg), locomotor activity was recorded cumulatively over a 40-min session, divided into 8 5-min periods. The doses of derivatives were selected on the basis of preliminary tests with LASSBio-785 (data not shown). Each treatment group consisted of 10 mice. The same positive (midazolam) and negative (vehicle) control groups were used to analyze the effects of LASSBio-785 and LASSBio-786 on locomotor activity.
In another set of experiments, in order to investigate the mechanism involved in the sedative activity of LASSBio-785 and LASSBio-786, the mice were pretreated with a specific antagonist of the benzodiazepine receptor on the GABAA receptor complex (flumazenil, 20 mg/kg,ip) 15 min before the administration of LASSBio-785 or LASSBio-786 (15 mg/kg, iv) 1010. Savic MM, Obradovic DI, Ugresic ND, Cook JM, Yin W, Bokonjic DR. Bidirectional effects of benzodiazepine binding site ligands in the elevated plus-maze: differential antagonism by flumazenil and beta-CCt. Pharmacol Biochem Behav 2004; 79: 279-290, doi: 10.1016/j.pbb.2004.07.013.
https://doi.org/10.1016/j.pbb.2004.07.01...
.
Assessment of pentobarbital-induced sleeping time in mice
Mice were gently positioned in a restraining tube, and sodium pentobarbital (30 mg/kg) was injected intravenously into the tail vein. The time from the loss of the up-righting reflex to its recovery was recorded as the sleeping time. Three consecutive trials up to recovery of this reflex were used to determine the hypnosis endpoint 1111. Mora S, Diaz-Veliz G, Lungenstrass H, Garcia-Gonzalez M, Coto-Morales T, Poletti C, et al. Central nervous system activity of the hydroalcoholic extract ofCasimiroa edulis in rats and mice. J Ethnopharmacol 2005; 97: 191-197, doi: 10.1016/j.jep.2004.10.028.
https://doi.org/10.1016/j.jep.2004.10.02...
. To investigate the hypnotic activity, LASSBio-785 (15 or 30 mg/kg, ip) or LASSBio-786 (15 or 30 mg/kg, ip) was injected 15 min before sodium pentobarbital and the sleeping time was determined as before. Injection of midazolam (2 mg/kg, ip) 15 min before sodium pentobarbital (30 mg/kg, iv) was used as a positive control. Another control group received DMSO (ip) 15 min before sodium pentobarbital administration.
Assessment of LASSBio-785 and LASSBio-786 hypnotic activity in mice
Mice were injected intravenously with LASSBio-785 (10, 15, 20, and 25 mg/kg) or LASSBio-786 (5, 10, 15, and 20 mg/kg) in order to determine the HD50for the hypnotic activity of the compounds based on loss of the righting reflex. Groups of 10 animals were used for each dose. Solutions were prepared immediately before the test. Logarithmic dose-response curves for LASSBio-785 and LASSBio-786 were fitted to calculate the HD50 using a linear regression analysis.
Root mean square (RMS) analysis
The structural similarities of LASSBio-785 and LASSBio-786 to the benzodiazepine (BDZ) drug midazolam were first estimated using the BioMedCAChe 6.0 software. The most stable synperiplanar structural conformers (12) were then superimposed on the corresponding BDZ scaffolds, thus aligning the central NAH framework. The RMS of midazolam was then calculated to enable evaluation of the structure-activity relationships between BDZ and the novel analogues, LASSBio-785 and LASSBio-786. The closer to zero the RMS values are, the higher the structural correlation between them.
Binding assay
A binding assay was performed between LASSBio-785 and the BDZ receptor site (13) or the GABA-gated Cl-channel of the GABAA receptor complex (14) of the rat cerebral cortex using a single concentration (50 µM). [3H]-flunitrazepam (0.4 nM) and [35S]-TBPS (3 nM) were used as the BDZ agonist radioligand and the Cl-channel GABA-gated antagonist radioligand, respectively. Data are reported as percent inhibition of specific binding afforded by LASSBio-785 relative to control, using the equation: % inhibition = 100 - [(measured specific binding / control specific binding) x 100].
Statistical analysis
Data are reported as means ± SE. The Student t-test was used to compare the differences between the positive control and vehicle groups at the same time. Comparisons between vehicle (control) and treatment groups were analyzed by one-way analysis of variance (ANOVA), followed by the Dunnett multiple comparison test. Differences with P < 0.05 were considered to be statistically significant. Statistical analyses were performed using the GraphPad Prism® 4.0 software (USA).
Results
Effects of LASSBio-785 and LASSBio-786 on the locomotor activity of mice
The potential sedative activity of LASSBio-785 and LASSBio-786 at doses of 15 and 30 mg/kg (ip and iv) was evaluated based on measurements of spontaneous locomotor activity of mice in an open field. Figure 1A shows that ipadministration of LASSBio-785 at 15 mg/kg did not significantly reduce the locomotor activity relative to DMSO-treated control mice. However, at a higher dose of LASSBio-785 (30 mg/kg), locomotor activity was reduced 30%. Midazolam (2 mg/kg, ip), which was used as a BDZ-like positive control, significantly reduced locomotor activity 45%. Intraperitoneal administration of LASSBio-786 at 15 and 30 mg/kg also reduced locomotor activity 29 and 30%, respectively (Figure 1B).
Effect of midazolam and two new N-acylhydrazone compounds on locomotor activity of mice. LASSBio-785 (A) and LASSBio-786 (B) were injected (15 and 30 mg/kg, ip), and locomotor activity was determined in an open field test during a 40-min period. Data are reported as means ± SE (n = 10). *P < 0.05 compared to DMSO (Dunnett multiple comparison test).
When injected intravenously at 15 and 30 mg/kg, LASSBio-785 decreased locomotor activity from 200 ± 15 (DMSO, n = 10) to 116 ± 29 (P < 0.05, n = 10) and 60 ± 16 crossings/min (P < 0.01, n = 10), respectively. Midazolam treatment (2 mg/kg, iv) reduced locomotor activity to 120 ± 19 crossings/min (P < 0.01, n = 10). The same effect was observed afteriv administration of 15 and 30 mg/kg LASSBio-786, when locomotor activity decreased to 127 ± 10 (P < 0.01, n = 10) and 96 ± 14 crossings/min (P < 0.01, n = 10), respectively.
The involvement of the BDZ pathway was investigated by examining the influence of pretreatment with flumazenil (20 mg/kg, ip) on the sedative properties of LASSBio-785 (15 mg/kg, iv) and LASSBio-786 (15 mg/kg, iv). Flumazenil did not significantly reduce locomotor activity when compared to the vehicle-pretreated group (170 ± 19 and 209 ± 26 crossings/min, respectively, n = 10). Locomotor activity following LASSBio-785 administration to vehicle- or flumazenil-pretreated mice was 116 ± 29 and 186 ± 5 crossings/min (P < 0.05, n = 10), respectively. Likewise, flumazenil pretreatment also attenuated the depression of locomotor activity induced by LASSBio-786 (from 127 ± 10 to 174 ± 9 crossings/min, P < 0.05, n = 10).
Effects on pentobarbital-induced sleeping time in mice
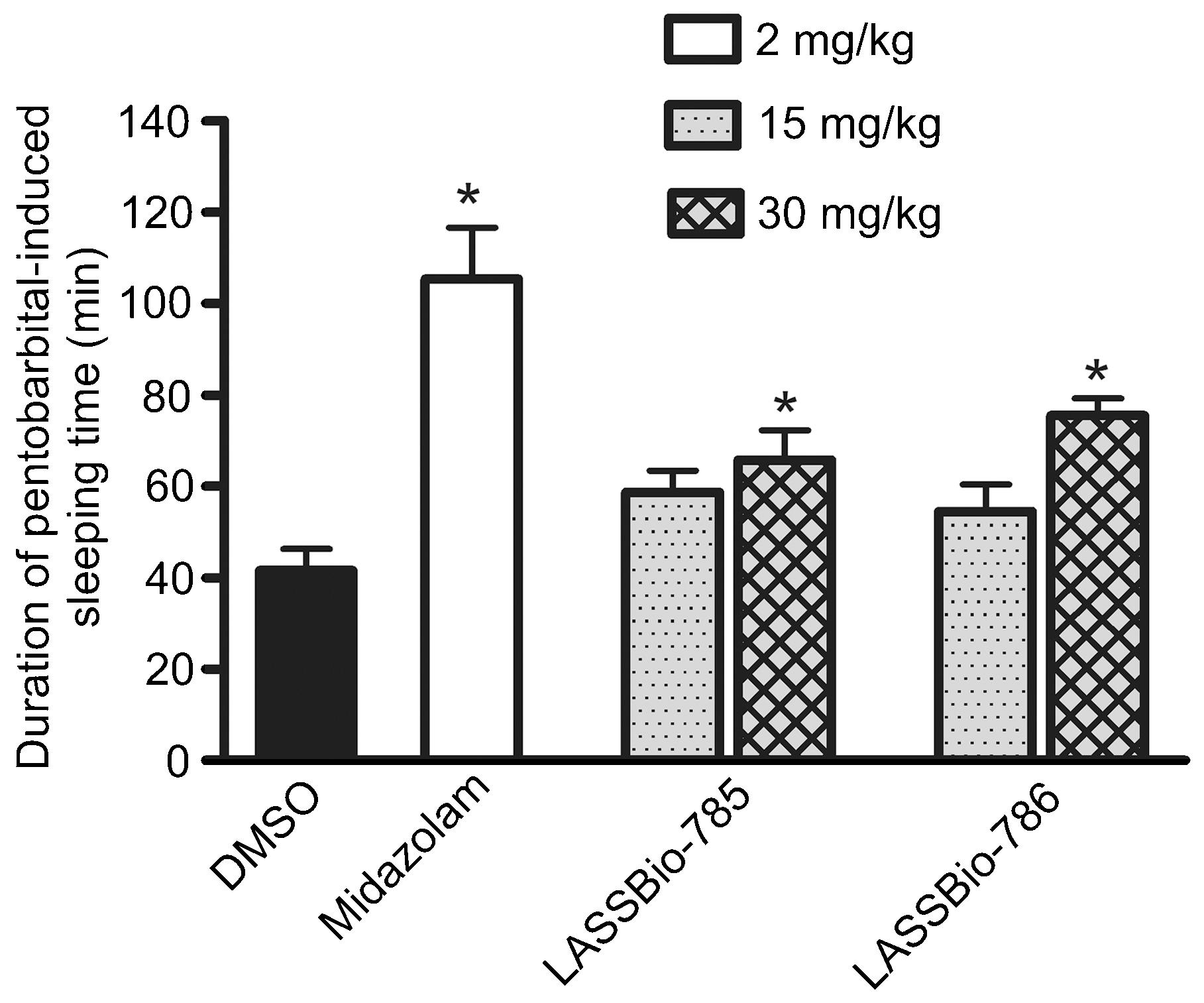
Intravenous injection of sodium pentobarbital (30 mg/kg) to DMSO-treated (ip) control mice induced sleeping time of 42 ± 5 min (Figure 2). Sleeping time was increased in mice pretreated with midazolam (2 mg/kg, ip). Administration of LASSBio-785 and LASSBio-786 at 15 mg/kg (ip) did not alter sodium pentobarbital-induced sleeping time significantly, but at 30 mg/kg these compounds prolonged sleeping time (Figure 2).
Effects of midazolam (2 mg/kg, ip), LASSBio-785 (15 and 30 mg/kg, ip) and LASSBio-786 (15 and 30 mg/kg,ip) on the duration of sodium pentobarbital-induced sleeping time. Data are reported as means ± SE (n = 10). *P < 0.05 compared to DMSO (Dunnett multiple comparison test).
Effects on the hypnotic activity in mice
Intravenous injections of 10, 15, 20, and 25 mg/kg LASSBio-785 induced loss of the righting reflex 20, 40, 70, and 90% of each group, respectively. In mice similarly treated with LASSBio-786 at 5, 10, 15, and 20 mg/kg, the reflex loss was 20, 60, 70, and 80%, respectively. The HD50 values for LASSBio-785 and LASSBio-786, calculated from the log dose-response curves, were 15.8 and 9.5 mg/kg, respectively (Figure 3).
Incidence of hypnosis induced in mice by ivadministration of different doses of (A) LASSBio-785 (10, 15, 20, 25 mg/kg, iv) or (B) LASSBio-786 (5, 10, 15, 20 mg/kg, iv). Simple linear regression (γ = α + βx) of the log dose-response curves was used to calculate the dose causing 50% hypnosis (HD50). Each point represents the percentage of subjects presenting hypnosis in the group (n = 10).
Superimposition of NAH derivatives and BDZ compounds
Figure 4 shows the occurrence of significant structural correlations in pharmacologically relevant regions between the N-alkyl NAH derivatives and the BDZ drug midazolam. The RMS values calculated after superimposing the structures of LASSBio-785 and LASSBio-786 on that of midazolam were 0.358 and 0.378, respectively.
Structural correlation between the N-acylhydrazone (NAH) derivatives LASSBio-785 and LASSBio-786 and the benzodiazepine compound midazolam. The atoms were colored as follows: carbon (gray), hydrogen (white), oxygen (red), and nitrogen (blue). The circles indicate the atoms used for the alignment of the structures.A, Chemical structures of LASSBio-785 (1), LASSBio-786 (2), and midazolam (3). B, Superimposition of compounds 1 vs 3 (yellow structure) and 2vs 3 (yellow structure), aligned by the central NAH framework.
Binding assay
In rat cerebral cortex membranes, LASSBio-785 (50 µM) inhibited binding of the antagonist [3H]-flunitrazepam (0.4 nM) to BDZ receptors by 14.2 and 8% (average 11.1%, n = 2) and of the antagonist [35S]-TBPS (3 nM) to Cl-channel GABAA-gated receptors by 11.2 and 17.6% (average 14.4%, n = 2). LASSBio-786 was not tested in these assays.
Discussion
The main finding of the current study was that LASSBio-785 and LASSBio-786, which respectively have a methyl or benzyl group linked to the amide nitrogen unit of the NAH moiety of LASSBio-294, a cardiac ionotropic 55. Sudo RT, Zapata-Sudo G, Barreiro EJ. The new compound, LASSBio 294, increases the contractility of intact and saponin-skinned cardiac muscle from Wistar rats. Br J Pharmacol 2001; 134: 603-613, doi: 10.1038/sj.bjp.0704291.
https://doi.org/10.1038/sj.bjp.0704291...
and vasodilator compound 66. Silva CL, Noel F, Barreiro EJ. Cyclic GMP-dependent vasodilatory properties of LASSBio 294 in rat aorta. Br J Pharmacol 2002; 135: 293-298, doi: 10.1038/sj.bjp.0704473.
https://doi.org/10.1038/sj.bjp.0704473...
, significantly reduced the locomotor activity of mice measured in an open-field, a protocol that has been used to efficiently monitor the sedative effect of drugs1515. Holland HC, Weldon E. A note on a new technique of recording ambulation in the open field test and its validation. Acta Psychol 1968; 28: 293-300, doi: 10.1016/0001-6918(68)90020-6.
https://doi.org/10.1016/0001-6918(68)900...
. In addition, the effects of both derivatives were sensitive to inhibition by pretreatment with the BDZ receptor antagonist flumazenil and were accompanied by prolongation of the hypnotic effects of pentobarbital-induced sleeping time, but no anxiolytic-like effects were detected with either derivative in the elevated plus-maze test.
Inhibition of neuronal excitability by the neurotransmitter GABA is mainly the result of activation of GABAA receptors, pentameric ligand-gated chloride channels which, via enhanced chloride influx, induce hyperpolarization and reduce firing of action potentials 1616. Gottesmann C. GABA mechanisms and sleep. Neuroscience 2002; 111: 231-239, doi: 10.1016/S0306-4522(02)00034-9.
https://doi.org/10.1016/S0306-4522(02)00...
. These inhibitory effects of GABA are facilitated by BDZ agonists, which act allosterically on the GABAA receptor complex to enhance affinity of the GABA binding site to the neurotransmitter, and it is estimated that 20-30% of the inhibitory neurons in the CNS are GABAergic. There is a wide diversity of GABAAreceptor subtypes, and those most highly expressed in the CNS include the α1βγ2, α2βγ2, α3βγ2, and α5βγ2 isoforms. Previous studies have reported that α1-containing GABAA receptors mediate the sedative, addictive, anterograde amnesic, and part of the anticonvulsant activities of the BDZ diazepam, whereas the α2-containing isoform is the main mediator of its anxiolytic-like effects and also promotes part of its myorelaxant actions, alongside the α3- and α5-containing isoforms 1717. Rudolph U, Knoflach F. Beyond classical benzodiazepines: novel therapeutic potential of GABAA receptor subtypes. Nat Rev Drug Discov 2011; 10: 685-697, doi: 10.1038/nrd3502.
https://doi.org/10.1038/nrd3502...
. The α5-containing GABAA receptors have also been linked to the development of tolerance to the sedative effects of BDZ 1717. Rudolph U, Knoflach F. Beyond classical benzodiazepines: novel therapeutic potential of GABAA receptor subtypes. Nat Rev Drug Discov 2011; 10: 685-697, doi: 10.1038/nrd3502.
https://doi.org/10.1038/nrd3502...
.
LASSBio-785 and LASSBio-786 could facilitate GABAergic inhibition via interactions with the GABAA receptor complex. This hypothesis is supported by the finding that the sedative effects of both compounds were reversed by the BDZ receptor antagonist flumazenil. This view is strengthened by the evidence for structural correlation observed by in silico superimposition of the pharmacophoric points of LASSBio-785 and LASSBio-786 with the BDZ drug midazolam, as detected by the determination of RMS. Despite these findings, preliminary binding analyses using a single concentration of LASSBio-785 have shown only weak interactions between this compound and the BDZ-binding site. Even if a more detailed investigation regarding characterization of the LASSBio-785 and LASSBio-786 binding sites on the GABAA receptor complex is still warranted, it is important to highlight that some currently available sedatives, which are active at α1-containing GABAA receptors, such as zolpidem and zopiclone, do not share structural similarity with BDZs 1818. Crestani F, Martin JR, Mohler H, Rudolph U. Mechanism of action of the hypnotic zolpidemin vivo. Br J Pharmacol 2000; 131: 1251-1254, doi: 10.1038/sj.bjp.0703717.
https://doi.org/10.1038/sj.bjp.0703717...
.
The insertion of methyl and benzyl groups in the NAH subunit of LASSBio-785 and LASSBio-786 is associated with the synperiplanar conformation of both compounds, whereby the amide hydrogen is synperiplanar to the carbonyl oxygen 1212. Kummerle AE, Raimundo JM, Leal CM, da Silva GS, Balliano TL, Pereira MA, et al. Studies towards the identification of putative bioactive conformation of potent vasodilator arylidene N-acylhydrazone derivatives. Eur J Med Chem 2009; 44: 4004-4009, doi: 10.1016/j.ejmech.2009.04.044.
https://doi.org/10.1016/j.ejmech.2009.04...
. The present study shows that this folded structure can play an important role in the central depressor activity of these substances.
In contrast to barbiturates, BDZ-like compounds do not directly open the chloride channel 1717. Rudolph U, Knoflach F. Beyond classical benzodiazepines: novel therapeutic potential of GABAA receptor subtypes. Nat Rev Drug Discov 2011; 10: 685-697, doi: 10.1038/nrd3502.
https://doi.org/10.1038/nrd3502...
. Preliminary binding assays with LASSBio-785 (50 µM) have shown moderate displacement (average 14.4%) of the antagonist [35S]-TBPS from its specific binding sites on the chloride channel of GABAA receptors, suggesting that it may act as an agonist at this target. Thus, the effects of LASSBio-785 (and possibly LASSBio-786) on GABA receptors of the CNS may be associated with both an indirect activation via binding to the BDZ receptor and a direct stimulatory effect on the chloride channel. These mechanisms could explain the increased duration of hypnosis induced by pentobarbital following LASSBio-785 or LASSBio-786 administration.
On the other hand, LASSBio-785 and LASSBio-786 were more potent in reducing locomotor activity when given iv as compared to the iproute. The less intense sedative effects seen following ipadministration could be due to poor absorption into the blood stream or to significant liver metabolism. These aspects, as well as the bioavailability of both derivatives following oral administration, remain to be assessed. Moreover, even if LASSBio-786 was deliberately planned to display greater lipid solubility than LASSBio-785 (lipid solubility ClogP values of 3.85 ± 0.62 and 2.09 ± 0.61, respectively) to favor its transfer across the blood-brain barrier, both compounds were equipotent in promoting sedation following iv injection.
In addition to their sedative effects, classical BDZ-like compounds can also promote anticonvulsant and anxiolytic-like effects. The possibility that LASSBio-785 and LASSBio-786 could display similar properties was also tested, but the results were negative. At 5 mg/kg, iv, neither derivative protected mice from seizures induced by pentylenetetrazole (10 or 15 mg/kg, ip) or promoted anxiolytic-like behaviors in mice submitted to the elevated plus-maze test (data not shown).
Drug-induced impairment of locomotor activity may be affected by several physiological conditions, including hypotension, pain and muscle relaxation. Although blood pressure was not measured in the current study, rats giveniv LASSBio-785 infusion at 7.5 mg.kg-1.min-1 for 10 min displayed decreases of approximately 30% in diastolic (but not systolic or mean) blood pressure and heart rate over the last 3 min, whereas no such changes were detected following similar infusion of LASSBio-786 (19). Such findings suggest that the locomotor impairment induced by LASSBio-785 and LASSBio-786 was not a consequence of hypotension. Furthermore, since LASSBio-785 and LASSBio-786 can each reduce carrageenan-induced hind paw thermal hyperalgesia (data not shown), it appears unlikely that inhibition of locomotor activity is due to nociceptive effects of these derivatives. In addition, we also observed that the sedative effects of LASSBio-785 and LASSBio-786 were not affected by ip pretreatment with either the α-2-adrenoceptor antagonist yohimbine (5 mg/kg) or the opioid receptor antagonist naloxone (1 mg/kg) (data not shown). Finally, as LASSBio-785 administration (30 mg/kg, ip) did not modify the performance of mice in the rotarod test (data not shown), the inhibition of locomotor activity afforded by this dose of the derivative is unrelated to effects on motor coordination per se.
The therapeutic use of currently available hypnotic drugs is limited by the onset of side effects including risk of addiction and hemodynamic and respiratory depression1717. Rudolph U, Knoflach F. Beyond classical benzodiazepines: novel therapeutic potential of GABAA receptor subtypes. Nat Rev Drug Discov 2011; 10: 685-697, doi: 10.1038/nrd3502.
https://doi.org/10.1038/nrd3502...
,2020. Hession PM, Joshi GP. Sedation: not quite that simple. Anesthesiol Clin 2010; 28: 281-294, doi: 10.1016/j.anclin.2010.02.007.
https://doi.org/10.1016/j.anclin.2010.02...
. Even if the available reports on the effects of LASSBio-785 and LASSBio-786 show limited (if any) effects on blood pressure, muscle relaxation or impairment of muscular coordination, more studies are needed to compare the toxicological profiles of these compounds with those of hypnotics currently in use in clinical practice.
It is interesting to note that the HD50 values for the hypnotic effects of LASSBio-785 and LASSBio-786 were similar, yet the former derivative is 10-fold less potent than the latter in relaxing isolated arterial vessel rings 77. Silva AG, Zapata-Sudo G, Kummerle AE, Fraga CA, Barreiro EJ, Sudo RT. Synthesis and vasodilatory activity of new N-acylhydrazone derivatives, designed as LASSBio-294 analogues. Bioorg Med Chem 2005; 13: 3431-3437, doi: 10.1016/j.bmc.2005.03.003.
https://doi.org/10.1016/j.bmc.2005.03.00...
. This difference in the relative potencies of LASSBio-786 and LASSBio-785 in promoting hypnosis and vasodilatation suggests that both effects are the result of actions on distinct targets, and that the CNS effects reported herein are unrelated to modulation of the NO-GMPc system.
Another possible use of LASSBio-785 and LASSBio-786 is as adjuvant drugs in anesthesia. Midazolam and dexmedetomidine have been used successfully to potentiate intravenous and inhalation general anesthetics. The associated increase in pentobarbital-induced sleeping time and the hypnotic effect induced by single-bolus administration indicate that LASSBio-785 and LASSBio-786 may be useful for this purpose.
Two new N-acylhydrazone derivatives of the cardio-inotropic prototype drug LASSBio-294, named LASSBio-785 and LASSBio-786, exhibited sedative and hypnotic properties in mice, which could be potentially relevant towards the development of new neuroactive drugs for the treatment of insomnia and in conjunction with general anesthesia.
The authors thank CNPq, CAPES, FAPERJ, Programa de Apoio aos Núcleos de Excel#xEA;ncia (PRONEX), and Instituto Nacional de Ci#xEA;ncia e Tecnologia de Fármacos e Medicamentos (CNPq/FAPERJ) for financial support and fellowships from CNPq (to G.A.P. Silva, G. Zapata-Sudo, R.T. Sudo, C.A.M. Fraga, E.J.L. Barreiro).
References
-
1Barreiro EJ, Fraga CAM, Miranda ALP, Rodrigues CR. Strategy of molecular simplification in rational drug design: the discovery of a new cardioactive agent. Quim Nova 2002; 25: 129-148, doi: 10.1590/S0100-40422002000100022.
-
2Lima PC, Lima LM, da Silva KC, Leda PH, de Miranda AL, Fraga CA, et al. Synthesis and analgesic activity of novel N-acylarylhydrazones and isosters, derived from natural safrole. Eur J Med Chem 2000; 35: 187-203, doi: 10.1016/S0223-5234(00)00120-3.
-
3Barreiro EJ, Fraga CAM. The utilization of the safrole, principal chemical constituent of sassafras oil, in the synthesis of compounds actives in the arachidonic acid cascade: antiinflammatory, analgesic and antithrombotic. Quim Nova 1999; 22: 744-759, doi: 10.1590/S0100-40421999000500019.
-
4Dal Piaz V, Giovannoni MP, Castellana C, Palacios JM, Beleta J, Domenech T, et al. Novel heterocyclic-fused pyridazinones as potent and selective phosphodiesterase IV inhibitors. J Med Chem 1997; 40: 1417-1421, doi: 10.1021/jm970105l.
-
5Sudo RT, Zapata-Sudo G, Barreiro EJ. The new compound, LASSBio 294, increases the contractility of intact and saponin-skinned cardiac muscle from Wistar rats. Br J Pharmacol 2001; 134: 603-613, doi: 10.1038/sj.bjp.0704291.
-
6Silva CL, Noel F, Barreiro EJ. Cyclic GMP-dependent vasodilatory properties of LASSBio 294 in rat aorta. Br J Pharmacol 2002; 135: 293-298, doi: 10.1038/sj.bjp.0704473.
-
7Silva AG, Zapata-Sudo G, Kummerle AE, Fraga CA, Barreiro EJ, Sudo RT. Synthesis and vasodilatory activity of new N-acylhydrazone derivatives, designed as LASSBio-294 analogues. Bioorg Med Chem 2005; 13: 3431-3437, doi: 10.1016/j.bmc.2005.03.003.
-
8Brito FC, Kummerle AE, Lugnier C, Fraga CA, Barreiro EJ, Miranda AL. Novel thienylacylhydrazone derivatives inhibit platelet aggregation through cyclic nucleotides modulation and thromboxane A2 synthesis inhibition. Eur J Pharmacol 2010; 638: 5-12, doi: 10.1016/j.ejphar.2010.04.003.
-
9Menegatti R, Silva GM, Zapata-Sudo G, Raimundo JM, Sudo RT, Barreiro EJ, et al. Design, synthesis, and pharmacological evaluation of new neuroactive pyrazolo[3,4-b]pyrrolo[3,4-d]pyridine derivatives within vivo hypnotic and analgesic profile. Bioorg Med Chem 2006; 14: 632-640, doi: 10.1016/j.bmc.2005.08.042.
-
10Savic MM, Obradovic DI, Ugresic ND, Cook JM, Yin W, Bokonjic DR. Bidirectional effects of benzodiazepine binding site ligands in the elevated plus-maze: differential antagonism by flumazenil and beta-CCt. Pharmacol Biochem Behav 2004; 79: 279-290, doi: 10.1016/j.pbb.2004.07.013.
-
11Mora S, Diaz-Veliz G, Lungenstrass H, Garcia-Gonzalez M, Coto-Morales T, Poletti C, et al. Central nervous system activity of the hydroalcoholic extract ofCasimiroa edulis in rats and mice. J Ethnopharmacol 2005; 97: 191-197, doi: 10.1016/j.jep.2004.10.028.
-
12Kummerle AE, Raimundo JM, Leal CM, da Silva GS, Balliano TL, Pereira MA, et al. Studies towards the identification of putative bioactive conformation of potent vasodilator arylidene N-acylhydrazone derivatives. Eur J Med Chem 2009; 44: 4004-4009, doi: 10.1016/j.ejmech.2009.04.044.
-
13Speth RC, Wastek GJ, Yamamura HI. Benzodiazepine receptors: temperature dependence of [3H]flunitrazepam binding. Life Sci 1979; 24: 351-357, doi: 10.1016/0024-3205(79)90331-X.
-
14Lewin AH, de Costa BR, Rice KC, Skolnick P. Meta- and para-isothiocyanato-t-butylbicycloorthobenzoate: irreversible ligands of the gamma-aminobutyric acid-regulated chloride ionophore. Mol Pharmacol 1989; 35: 189-194.
-
15Holland HC, Weldon E. A note on a new technique of recording ambulation in the open field test and its validation. Acta Psychol 1968; 28: 293-300, doi: 10.1016/0001-6918(68)90020-6.
-
16Gottesmann C. GABA mechanisms and sleep. Neuroscience 2002; 111: 231-239, doi: 10.1016/S0306-4522(02)00034-9.
-
17Rudolph U, Knoflach F. Beyond classical benzodiazepines: novel therapeutic potential of GABAA receptor subtypes. Nat Rev Drug Discov 2011; 10: 685-697, doi: 10.1038/nrd3502.
-
18Crestani F, Martin JR, Mohler H, Rudolph U. Mechanism of action of the hypnotic zolpidemin vivo Br J Pharmacol 2000; 131: 1251-1254, doi: 10.1038/sj.bjp.0703717.
-
19Silva G. Investigação da atividade depressora de derivados N-acilidrazônicos LASSBio-785 e LASSBio-786 sobre o sistema nervoso central. [Master's thesis]. Rio de Janeiro: Universidade Federal do Rio de Janeiro; 2010.
-
20Hession PM, Joshi GP. Sedation: not quite that simple. Anesthesiol Clin 2010; 28: 281-294, doi: 10.1016/j.anclin.2010.02.007.
Publication Dates
-
Publication in this collection
19 Mar 2013 -
Date of issue
Mar 2013
History
-
Received
28 Oct 2012 -
Accepted
23 Nov 2012