Abstract
The study investigates the potential of Morus alba L. (mulberry) young and ripe fruit extracts against lung cancer cells. Cancer ranking as the second leading cause of global mortality, it is essential to investigate natural compounds like phytochemicals for therapeutic benefits. The research investigates presence of phytochemicals and antioxidant activity of both young (MAF-Y) and ripe (MAF-R) mulberry fruit extracts. Results revealed presence of various secondary metabolites, particularly high phenolic content and antioxidant properties in MAF-R. Both extracts demonstrated significant cytotoxicity against lung adenocarcinoma cells (A549), with IC50 18.4 ± 3.01µg/ml (MAF-R) and 29.41 ±3.6 µg/ml (MAF-Y). Moreover, the extracts effectively inhibited cell migration. Treatment of extracts elevated reactive oxygen species (ROS) production which resulted in disruption of mitochondrial membrane potential, and induced the process of apoptosis in lung carcinoma cells. This was evidenced through various assays including differential staining and DNA fragmentation analysis. These findings underscore the potential of mulberry fruit extracts as promising candidates for cancer prevention and treatment due to their antioxidant properties, cytotoxic effects, and ability to induce apoptosis in lung cancer cells.
Keywords:
Antioxidant activity; Apoptosis; Cytotoxicity; Lung cancer; Morus alba L
INTRODUCTION
Lung cancer is an uncontrolled proliferation of cells in lung tissue, a disease responsible for causing high fatalities (Rom et al., 2000). According to the GLOBCAN 2020 report, 19.3 million instances of all cancer types were recorded, of which 2.21 million cases with a mortality rate of 18% were of lung cancer, which was responsible for 1.79 million deaths globally (Ferlay et al., 2021). On a global basis, it has been speculated that the majority of cancer-related mortality can be prevented. An effective intervention can minimize cancer-related mortality and human impairment (Wilson et al., 2017; Aghajanpour et al., 2017).
Various studies have reported that certain plant foods if incorporated in our diet, reduces risk of occurrence of cancer (Tao et al., 2018; Li et al., 2016). Vegetables, legumes, fruits, spices, tea and mushrooms contains array of phytochemical which helps in prevention of different kind of cancers (Seidel et al., 2017; Xu et al., 2016; Sidhu, Kabir, Huffman, 2007). Nowadays, the concept of “Functional foods” is emerging worldwide. Functional foods are the traditional foods containing high nutritional components, which not only fulfill the basic nutritional requirements, it additionally provides health benefits which helps in strengthening the immunity which aids in prevention of many diseases (Ozen, Pons, Tur, 2012). Plant-based dietary phytochemicals have significant therapeutic potential; their demonstration of anti-mutagenic and anti-carcinogenic properties raises the possibility of employing these bioactive ingredients to prevent cancer (Chen, Kong, 2005; Pan, Ghai, Ho, 2008).
Mulberry plant is placed under category of functional foods as it not only providing necessary nutrition but also contains bioactive compound of therapeutic importance (Kadam, Dhumal, Khyade, 2019). Thus, for the present study we have selected Morus alba L.; various studies reported that extract from different parts of M. alba can be potentially used in treatment of diseases like diabetes (Jiao et al., 2017), asthma (Jung et al., 2014), cough, hypertension, blood circulation disorders, arteriosclerosis (Eo, Lim, 2016; Yuan, Zhao, 2017). Mulberry fruits are well studied for their role in enhancing human immunity (Wang et al., 2018; Chang et al., 2015; Shin et al., 2013). Phytochemicals like phenols, alkaloids, anthocyanins, tannins, flovanoids have been reported from the fruits of M. alba (Seo et al., 2015; Li et al., 2021; Kim et al., 2014; Lee et al., 2018).
Various studies shows that mulberries contain phytochemicals that contribute to a variety of biological activities such as antioxidant (Jiang et al., 2013), antibacterial (Budiman et al., 2017), anticancer (Aggarwal et al., 2004), and anti-inflammatory (Chen, Pu, Liu, 2016).
It has been observed that fruit ripening processes in mulberry result in varying bioactive compositions due to physiological, biochemical, and metabolic changes. Which leads to changes in antioxidant properties of the fruits (Lee, Hwang 2017; Tahir Mahmood et al., 2017; Belwal et al., 2019). Studies have been conducted to examine the total phenols, flavonoids, sugars, anthocynins and antioxidant capacity of mulberry fruits at particular stages either young or ripe fruit (Lou et al., 2012; Oki et al., 2006; Lin, Lay, 2013). Hence, for the present study we have selected Mulberry fruits at two different stages i.e. Young and Ripe. Qualitative phytochemical screening, total phenolic content, antioxidant activity of Young and Ripe fruit extracts (named MAF-Y and MAF-R respectively) were evaluated. The aim of the study was to evaluate the effect of bioactive composition of extracts for their ability to inhibit growth of lung cancer in vitro. The cytotoxic activity of was evaluated against human lung adenocarcinoma cells (A549). Most cancers are treated by targeting apoptosis; therefore, the ability of mulberry fruits was checked for induction of the apoptosis in lung cancer cells by performing differential staining assays (AO-EtBr, DAPI, Giemsa) and DNA band pattern was checked after treatment of fruit extracts on A549 cells.
MATERIAL AND METHODS
Collection and Extraction of the Fruits
Fruits of Morus alba L. were collected during March- May 2022 from Bakarol, District. Anand, Gujarat, India (22°34’53.2”N 72°55’03.7”E). The herbarium was submitted to the P.G. Department of Biosciences, Sardar Patel University, Gujarat, India (Specimen No. PNB03). The fruits were washed three times under tap water to remove the dirt. Young red fruits were segregated from dark blue ripe fruits. The cold maceration method was adopted to carry out the extraction procedure, in which 90% methanol and 10% water were used as solvent. Fruits were crushed to get the pulp. 150 g of fruit pulp was dissolved in 300 ml of solvent and kept for 24 h. The mixture was filtered by Whatman filter paper No. 1, filtrate was transferred to evaporating dishes and kept at room temperature till the solvent gets evaporated. Extracts were labeled MAF-Y (Morus alba L. fruit-young) and MAF-R (Morus alba L. fruit-ripe) and stored in the refrigerator at 4 °C for further use.
Qualitative Screening of Secondary Metabolites
Extracts were dissolved in DMSO to get a final concentration of 1 mg/ml and used for qualitative screening for the presence of various phytochemicals, as described by (Brain, Turner, 1975; Evans, 1996). The detailed process is given below.
Test for Alkaloids
Mayer’s test: 2ml of the fruit extract underwent treatment with a few drops of Mayer’s reagent. The formation of cream precipitates denotes the presence of alkaloids. [Composition of Mayer’s reagent: Dissolve 1.358g of mercuric chloride in 60ml of water and combine it with a solution of 5g of potassium iodide in 10ml of water and adjust to 100 ml with water.]
Dragendorff’s Test: A few drops of Dragendorff reagent were added to 2ml of the fruit extract. The emergence of a reddish-brown precipitate suggests the presence of alkaloids. [Composition of Dragendorff reagent: Solution A - Dissolve 1.7g of bismuth nitrate in 100ml (4:1 ratio of distilled water to acetic acid). Solution B - Combine 40g of potassium iodide with 100ml of distilled water. Mix 5ml of Solution A with 5ml of Solution B, add 20ml of acetic acid, and adjust to 100ml with distilled water.]
Iodine Test: 2ml of fruit extract underwent treatment with iodine solution drop by drop. The appearance of a blue color precipitate indicates the presence of alkaloids. (The precipitates disappear upon boiling and reappear upon cooling).
Test for Glycosides
Keller Killiani Test: In a 2ml of fruit extract, 0.5 ml glacial acetic acid containing 2-4 drops of ferric chloride was added. Later 1ml concentrated H2SO4 was poured into the test tube carefully. At the junction of two liquids formation of dark bluish colour indicates the presence of cardiac glycosides.
Detection of Terpenoids
Horizon test: 2ml of fruit extract was treated with 2ml of trichloroacetic acid. Formation of a red precipitate indicates the presence of terpenoids.
Test For Tannins
Ferric chloride test: 2ml of fruit extract was treated with a few drops of ferric chloride solution. The formation of a black color indicates the presence of tannins.
Test For Flavonoids
Lead acetate test: In a 2ml of fruit extract few drops of lead acetate solution were added. The formation of a yellow color precipitate indicates the presence of flavonoids.
Alkaline Test: In a 2ml of fruit extract few drops of sodium hydroxide solution were added. Appearance of deep yellow colour indicates presence of flavonoids and upon adding few drops of diluted HCl the solution become colourless.
Test For Phenols
Ferric chloride test: In a 2ml of fruit extract few drops of ferric chloride solution were added. The formation of a bluish-black color indicates the presence of phenol.
Lead acetate test: In a 2ml of fruit extract few drops of lead acetate solution were added. The formation of a yellow color precipitate indicates the presence of phenol.
Test For Saponins
Foam test: 2ml fruit extract was diluted with 5 ml of distilled water, warmed gently and shaken for 2-4 minutes. Persistent froth indicates presence of saponins.
Test For Phytosteroids
Salkowski s Test: 2ml of the fruit extract was mixed with 2ml of chloroform, followed by addition of 3ml concentrated H2SO4 to form a layered solution. The appearance of reddish-brown color on the interphase indicates the presence of steroids.
Test For Proteins
Biuret test: 2ml of the fruit extract was treated with an equal volume of 5% Sodium hydroxide solution and two drops of 1% copper sulfate solution. The presence of violet color indicates the presence of proteins and free amino acids.
Ninhydrin test: In a 2ml of fruit extract two drops of freshly prepared 0.25% Ninhydrin reagent wear added and heated for a few seconds. The appearance of pink or purple color indicates the presence of proteins, peptides, or amino acids.
Test For Carbohydrates
Molisch’s test: 2ml of the fruit extract was treated with 2 few ml of Molisch’s reagent. 2ml of concentrated H2SO4 was added carefully down the side of the test tube. The formation of a red or dull violet color at the interphase of the two layers indicates the presence of carbohydrates.
Test For Reducing Sugars
Fehlings test: In a 2 ml of fruit extract an equal amount of Fehling solution A & B were added. The appearance of red precipitates of cuprous oxide indicates the presence of reducing sugars.
Determination of Total Phenolic Content (TPC)
The phenolic content of MAF-Y and MAF-R extracts was evaluated by the Folin-Ciocalteu reagent procedure as described by (Makkar, 2003). In brief, 250 µl Folin-Ciocalteu reagent was added to 25 µl fruit extract and incubated in the dark for 5 min. followed by the addition of 750 µl 20% Na2CO3 to stop the reaction. The mixture was then brought to the final volume of 5 ml by the addition of distilled water and incubated in dark conditions for 120 min at room temperature. Gallic acid in DMSO (1mg/ ml) was served as standard. Aliquots of Gallic acid at different concentrations (100-1000 µg/ml) were prepared and remaining procedure was the same as for the sample. After incubation, absorbance was measured at 760 nm using UV-Visible spectrophotometer (Spectramax M2e, Molecular Devices).
Evaluation of Antioxidant Activity
DPPH assay was performed to evaluate antioxidant potential of MAF-Y and MAF-R extracts according to the procedure mentioned by Sivasankarapillai et al., (2022). In brief, 0.5 ml of extract concentration (100-500 µg/ml) was taken in an amber tube, and 1.5 ml of a 0.1 mM DPPH solution prepared in methanol was added to it. Tubes were incubated in the dark at room temperature for 30 min. Ascorbic acid (100 mg/ml) in methanol was taken as standard. Aliquots of Ascorbic acid at different concentrations (10-100 mg/ml) were prepared and remaining procedure was the same as for the sample. After incubation, absorbance at 517 nm was recorded using a micro-plate reader (Spectramax M2e, Molecular Devices). 0.1 mM DPPH in methanol was taken as a blank. The percentage inhibition of DPPH free radicals by the antioxidants present in the crude extract was calculated using the following formula:
Culturing of the Human Lung Adenocarcinoma Cells
Human lung adenocarcinoma cells (A549) were procured from NCCS, Pune, India. Cells were grown in minimum essential medium (MEM) (AL047S Himedia) supplemented with 10% fetal bovine serum along (RM10832Himedia) with 2.5% antibiotics solution (5000 units Penicillin, 5mg Streptomycin and 10mg Neomycin per ml in Citrate buffer) (A028 Himedia). Cells were maintained in monolayer in the incubator at 37 °C with 5% CO2 and 95% air (Thakkar et al., 2022).
Determination of cytotoxic activity of MAF extracts on A549 cells
It was determined through MTT method as mentioned by (Thakkar et al., 2022). A549 cells were seeded in the 96-well plate in such a way that each well contained around 6,000-10,000 cells. After 24 h, once cells regained their morphology, media was removed from the wells and treated with different concentrations of MAF-Y and MAF-R extracts by preparing serial dilutions (8000-3.9 µg/ml). The treatment was given in triplicate, and the plate was incubated for 24 h in a CO2 incubator. After incubation, 5 µl of MTT dye with a concentration of 5 mg/ml in Phosphate buffer saline (PBS) was added to each well. The plate was wrapped in aluminum foil and incubated in an incubator for 90 min. Absorbance was measured at 570 nm and background wavelength at 690 nm in a micro-plate reader (Spectramax M2e, Molecular Devices).
Effects of extracts on cell migration by wound healing assay
To check the effects of extract treatment on migration of the cells wound healing assay was performed (Grada et al., 2017). Cells were seeded into 6 well plate and allowed to grow till it forms monolayer. Wound was created using 20 µl pipette tip and cell derbies were removed by rinsing the wells by PBS. MAF-Y and MAF-R extracts were added into wells at their respective IC50 concentration along with culture medium, and cells with culture medium only were kept as control. Cells were examined using an inverted phase contrast microscope at intervals of 0, 24, and 48 hours and photomicrographs were collected for the reference.
Evaluation of generation of Reactive oxidant species (ROS)
The H2DCFDA probe was used to measure the changes in ROS production in lung cancer cells after 24 h treatment with MAF-Y and MAF-R extracts at their respective IC50 concentration. 50 µl H2DCFDA in PBS was added into the well treated with extracts. After 30 min. incubation amount of ROS was measured in the form of fluorescence as DCFDA is oxidized by ROS of the cells and form DCF which generates the fluorescence. It was measured using microplate reader spectrophotometer reader (Spectramax M2e, Molecular Devices) at Ex/Em 490/530 nm (Mirza et al., 2018).
Mitochondrial membrane potential (MMP)
Mitochondrial function, a key indicator of cell health, can be assessed by monitoring changes in mitochondrial membrane potential (MMP) (Sakamuru, Attene-Ramos, Xia, 2016). Cationic fluorescent dye JC-10 (Sigma Aldrich, MAK159) was used to assess MMP. A549 Cells in 96 well plate treated with MA fruit extract at their respective IC50 and after incubation of 24 h cells were treated with JC-10 dye according to the procedure provided by manufacturer and decrease in red to green fluorescence was measured of treated cells at excitation / emission wavelengths 540 / 590 nm and 490 / 525 nm respectively, using Microplate reader spectrophotometer reader (Spectramax M2e, Molecular Devices).
Effects of MAF-Y and MAF-R extracts on induction of apoptosis
The effects of Morus alba L. fruit extracts on lung carcinoma cells were evaluated by differential staining methods. AO-EtBr, DAPI, and Giemsa staining were performed for to identify the morphological changes by the treatment of MAF-Y and MAF-R extracts on A549 cells.
AO-EtBr staining
Following a 24-hour treatment with extracts AO-EtBr staining of A549 cells was performed as described by Ittiyavirah et al. (2014). After adding 5 µl of acridine orange (100 µg/ml) and 5 µl of ethidium bromide (100 µg/ml) to each well, the cells were incubated for 15 minutes, then washed with PBS and examined under the fluorescent microscope (Axio observer A1, Carl Zeiss).
DAPI staining
It was performed to observe the morphological alteration of cell nucleus after 24 h treatment with MAF-Y and MAF-R extracts at their respective IC50 concentration. Cells were washed with PBS and 50 µl DAPI dye was added to the each well. After 30 min of incubation cells were washed with PBS twice and then observed under the DAPI filter of phase contrast microscope (Axio observer A1, Carl Zeiss) (Mirza et al., 2018).
Giemsa staining
10 µl Giemsa staining solution (1% in Methanol) was added to the A549 cells treated with MAF-Y and MAF-R extracts at their respective IC50 concentration. After 30 min of incubation, cells were washed twice with PBS and observed under 40x objective lens of light microscope (Axio observer A1, Carl Zeiss) (Mani et al., 2019).
DNA Fragmentation Analysis
DNA isolation was carried out by high salt method (DNA Preparation from Adherent Cells, 2006). Cells were treated with Morus alba L. fruit extracts at their respective IC50 concentration for 24 h and cells with media were served as control. Briefly, 5 × 105 cells were lysed in a DNA buffer consisting of 1 M Tris-HCl (pH 8.0), 0.5 M EDTA (pH 8.0), 10% SDS, 5M NaCl, and dH2O, followed by the addition of Proteinase K (10 mg/ml), it was incubated overnight at 45°C. The lysate was then centrifuged (3000 rpm, 10 min), and an equal volume of phenol (1.8 ml) and chloroform/isoamylalcohol (24:1) was added to the supernatant. After centrifugation (3000 rpm, 10 min), the DNA precipitated was washed with 70% ethanol. Extracted DNA from A549 cells were electrophoresed in 0.8 % agarose gel. DNA fragments were visualized under a Gel-Doc instrument.
RESULTS AND DISCUSSION
Percentage Yield of Extracts
Percentage yield recovery of young and ripe extracts showed variation. After the extraction procedure, 4.59% MAF-Y and 14.76% MAF-R extract was recovered. An increased concentration of total carbohydrates, fatty acids, volatile chemicals and protein may be the cause of the high yield in ripe fruit extract (Jelled et al., 2017).
Presence of Phytochemicals in MAF Extracts
The diverse array of phytochemicals identified in both fruit extracts includes alkaloids, flavonoids, saponins, phenols, glycosides, terpenoids, tannins, phytosteroids, proteins, carbohydrates, and reducing sugars (Table I). However, the quantity of these compounds varies, it evidenced by the differing color intensities observed in each extract. Compared to Young fruit extract (MAF-Y), the ripe fruit extract showed higher amount of Tannins and Phenols which was represented by higher colour intensity. Plant phenolics have been reported to have action against microbial diseases, hypertension, inflammation, oxidant, carcinogenesis, mutagenesis, and acidity (Espín, González-Sarrías, Tomás-Barberán, 2017; Michałowicz et al., 2018, Rasouli, Mazinani, Haghbeen, 2021). Numerous phyto-metabolites comprising polyphenols, alkaloids, flavonoids, tannins, glycosides, gums, resins and oils have been recognized for their potential in exerting anticancer effects, either in their natural states or as modified derivatives (Singh et al., 2013).
Total Phenolic Content in MAF Extracts
Plant phenolics have drawn interest in the management and therapy of cancer (Wani et al., 2023). In the current study, Total Phenolic Content of MAF extracts were determined by standard curve of Gallic acid (Figure 1). The comparison between the young fruit extract (MAF-Y) and the ripe fruit extract (MAF-R) revealed notable differences in phenolic content. Specifically, MAF-Y exhibited a phenolic content of 29.38 ± 0.99 mg/g GAE, while MAF-R demonstrated a higher phenolic content of 100.51 ± 1.91 mg/g GAE. The observed increase in TPC content in mature black fruits is theorized to result from changes in the mechanical and structural characteristics of the cell wall during the ripening process. This intriguing correlation underscores the dynamic nature of phenolic content in fruits during different stages of development, shedding light on potential factors influencing their bioactive properties. Such insights contribute to a deeper understanding of the complex interplay between plant physiology and the bioavailability of bioactive compounds offering valuable information for harnessing the therapeutic potential of these natural substances. The similar observations have been reported by Chen et al. (2022), where red mulberry fruit extract had a lower total phenolic content (TPC) compared to mature black mulberry fruit extract.
Anti - Oxidant Potential of MAF Extracts
The DPPH free radical scavenging method is widely accepted for evaluating antioxidant activity (Huang, Ou, Prior, 2005). Antioxidants influence DPPH by donating hydrogen (Baumann, Wurn, Bruchlausen, 1979). In the current study, calibration curve of concentration verses percentage inhibition was plotted for the standard ascorbic acid (Figure 2) as well as of MAF extracts and IC50 concentration was calculated. Ripe fruit extract exhibited notably higher antioxidant activity at lower concentrations compared to the young fruit extract (Table II). MAF-Y demonstrated an IC50 of 455 ± 0.02 µg/ml, while MAF-R exhibited a lower IC50 of 217.6 ± 0.03 µg/ ml. Both the extracts showed good antioxidant potential comparable with standard ascorbic acid which showed IC50 at 12.89 ± 0.02 mg/ml. It demonstrates that naturally occurring phytochemicals in fruit extracts can neutralize free radicals effectively at a very low concentration in micrograms which is lower than the antioxidant activity displayed by the standard. It further confirms that natural phytochemicals are antioxidants which can scavenge free radicals much more effectively, mitigating the detrimental effects of free radicals in conditions such as cancer (Rohman, Riyanto, Utari, 2006).
The results of antioxidant potential of MAF extracts exhibited a positive correlation with the total phenolic content of the respective fruit extract. MAF-R having higher phenolic content showed good antioxidant activity. The observed enhancement in DPPH scavenging potential during the ripening of mulberry fruits, mirroring the trend associated with total phenolic content, suggests that total phenols may play a pivotal role in contributing to antioxidant activity (Lou et al., 2012). This aligns with findings from studies by Lee & Hwang (2017) and Chen et al. (2022), which demonstrated that the antioxidant capacity of mulberry fruits elevates as they become mature. Polyphenols have the ability to scavenge free radicals created internally. Most of the pharmacological actions of polyphenols have been found to be based on this capacity. Due to their structural resemblance to chemopreventive medicines, plant polyphenols have an anticancer effect (Singh et al., 2013; Hadi et al., 2010; Elbling et al., 2005). D’urso et al. (2020) reported that the antioxidant activity of the mulberry fruit was mostly attributed to two compounds: flavonols and phenylpropanoids. Therefore, the biological activity displayed by mature fruit extracts in present study suggests that they may contain important bioactive chemicals which represented by higher free radical scavenging capacity than extracts from young fruit.
Identification of MAF Extracts Activity on Lung Adenocarcinoma Cells (A549)
Cytotoxic effects of MAF extracts on A549 cells
In the present study, after 24 h of treatment with both the extracts, morphological changes were observed which is shown in Figure 3. Compared to control cells after treatment of the MAF extracts rounding of the cells was observed, indicating dead cells. Standard drug Cisplatin was taken as positive control which showed IC50 at 7.305 ± 1.13 μg/ml. MTT assay unveiled that ripe fruit extract (MAF-R) displayed pronounced cytotoxicity against lung carcinoma cells at lower concentrations, yielding an IC50 value of 18.4 ± 3.01 μg/ml, while the IC50 of the young fruit extract (MAF-Y) was found to be 29.41 ± 3.6 μg/ml.
Morphological changes observed in A549 cells after treatment with MAF extracts. A) Control cells B) Cells treated with IC50 concentration of Cisplatin (Positive control) C) Cells treated with IC50 concentration of MAF-Y extract D) Cells treated with IC50 concentration of MAF-R extract; Arrow indicates dead cells with spherical appearance; Scale bar is representing 10µm.
In a report by El-Baz et al. (2017), the dichloromethane fraction and ethyl acetate fraction of M. alba fruit extract demonstrated potent activity against the colon cancer cell line (HCT116) with IC50 values of 32.3 μg/ml and 56.5 μg/ml, respectively. Moreover, against the breast cancer cell line (MCF-7), the IC50 values were found to be 43.9 μg/ml and 54.6 μg/ml, respectively. The outcomes of our current investigation underscore the substantial potential of mulberry fruits, particularly in the context of lung cancer cells. Cytotoxic activity demonstrated by ripe fruit extract (MAF-R) is good compared to young fruit extract (MAF-Y). Phytochemicals of MAF-R contributed effectively for cytotoxic effect against lung cancer cells. Whole extracts consist diverse group of compounds which collectively exert synergistic effects in effectively preventing complex diseases such as cancer (Neergheen et al., 2010). Along with that, anthocyanin content of fruit increases during the ripening process. In addition to its colouring effects during fruit ripening, anthocyanins have been reported to have anticancer activity both in vivo and in vitro (Huang et al., 2008; Koide et al., 1997). Therefore, it could be the reason of increased cytotoxic action of ripe fruit against lung cancer cells.
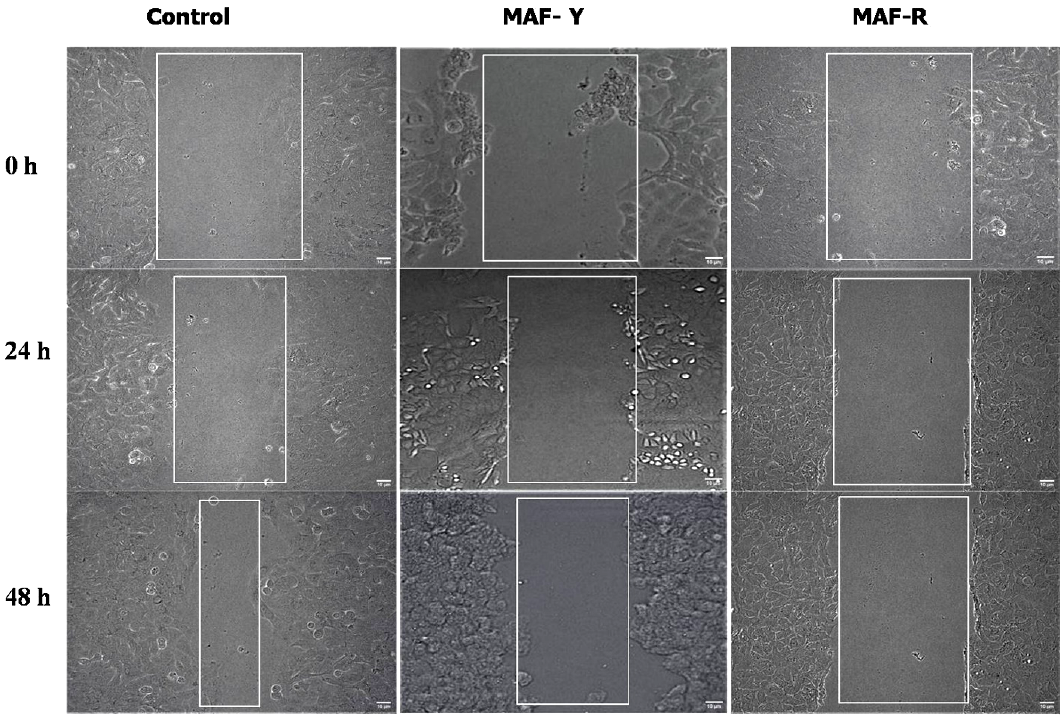
Effects of MAF extracts on migration of A549 cells
Cell migration plays a pivotal role in numerous biological processes, encompassing tissue development, immunological responses, wound healing and metastasis of cancer (Onuki-Nagasaki et al., 2008). In the current investigation, cells treated with MAF extracts demonstrated suppression of lung cancer cell migration. Cells grown in medium alone in a control group were shown to move more quickly, filling the scratch after 24 hours, and nearly filling it after 48 hours as a result of the cell migration and proliferation. In contrast to control cells, cells treated with MAF-Y & MAF-R extracts migrated at very low rate after 24 h and 48 h (Figure 4). Understanding how phytochemical of extracts influence cell migration is crucial for identifying new treatment approaches involving phytochemicals for managing invasive tumor cells.
Anti-migratory effect of MAF extracts. MAF-Y and MAF-R extracts were checking the migration of lung carcinoma cells (A549) compared to control. Images were taken at 0, 24 and 48 h in phase contrast microscope with 10X objective lens. In a control cells after 24h and 48h the cells started migrating and filled the scratch. The migration of lung cancer cells was prevented in cells treated with MAF-Y and MAF-R, as evidenced by a bigger spacing between the cells after treatment of 24 and 48 hours in comparison to the control cells.
In a prior study (Chen et al., 2006), mulberry anthocyanins were reported to exhibit anti-migratory effects against lung carcinoma cells. Another investigation (Huang et al., 2008) demonstrated that the treatment of murine melanoma cells (B16-F1) with mulberry anthocyanins not only inhibited their growth but also impeded their metastatic potential.
These results demonstrate the ability of mulberry fruit extracts, which include a variety of phytochemicals including anthocyanins, to modify cell migration-a crucial process when it comes to cancer metastasis. Further investigation into the therapeutic potential of mulberry extracts in controlling invasive tumour cells might be based on the observed anti-migratory actions, which can provide insightful information about the diverse advantages of using mulberry extracts.
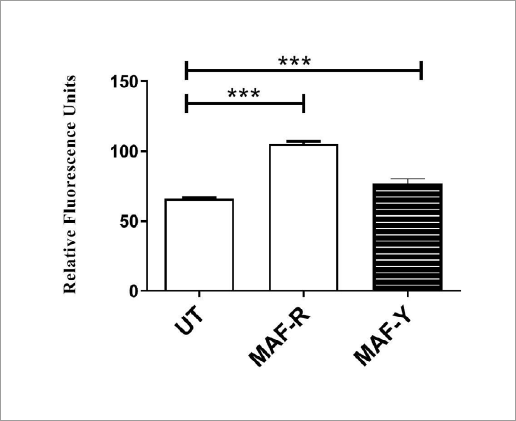
ROS generation in A549 cells by MAF extract treatment
In the current investigation, lung adenocarcinoma cells treated for 24 hours with MAF extracts showed a substantial increase in ROS production as compared to control cells (Figure 5). Intracellular ROS production was assessed using 2,7-dichlorofluorescein diacetate (DCFH-DA). The association between oxidative stress and the development and behavior of cancer cells is well-established, as these cells generate increased reactive oxygen species (ROS) due to their elevated metabolic rates leading to oxidative stress-induced cell death (Trachootham et al., 2009). Consequently, various chemotherapeutic strategies have been designed to effectively elevate ROS levels, triggering the death of tumor cells. Numerous phytochemicals present in plant foods have been identified for their ability to arrest cell growth and induce apoptosis in cancerous cells (Wang, Yi, 2008; Nakagawa et al., 2001).
Effect of MAF extracts treatment on generation of reactive oxygen species (ROS) in A549 cells. After 24 h of treatment, both the MAF-Y and MAF-R extract showed elevated amount of ROS generation compared to the untreated control cells.
These results not only confirm the potential of mulberry fruit extracts in inducing oxidative stress in lung adenocarcinoma cells but also highlight a mechanism that could contribute to the observed cytotoxic and anti-migratory effects. This finding aligns with previous studies conducted by Thakkar et al. (2022; 2023), wherein the treatment with hydromethanolic leaf extracts of Acorus calamus L. and Dalbergia sissoo Roxb. induced oxidative stress in lung cancer cells (A549). This induction of apoptosis was stimulated by an elevated formation of reactive oxygen species. The ability of phytochemicals to modulate intracellular ROS levels underscores their potential as therapeutic agents in targeting cancer cells, providing a foundation for further investigations into their precise mechanisms of action.
Effect of MAF extracts treatment on mitochondrial membrane potential in A549 cells
Mitochondria play a pivotal role in apoptosis, releasing death signals that activate caspases and ultimately leading to cell death. The increase in JC-10 aggregates within mitochondria, indicates loss of membrane integrity, allows for the distinct differentiation between viable and apoptotic cells (Mirza et al., 2018).
Our observations revealed a collapse in the mitochondrial membrane potential (MMP) of lung adenocarcinoma cells after 24 h of treatment with mulberry (MA) fruit extracts, compared to control cells without extract treatment (Figure 6). This observation strongly suggests that cells treated with the extracts undergo apoptosis, likely due to excessive ROS production leading to the loss of mitochondrial membrane potential. Numerous phytochemicals demonstrating anticancer effects have been shown to reduce the potential of the mitochondrial membrane. Our findings align with a prior study (Villarini et al., 2014) that investigated the apoptotic-inducing action of estragole extracted from fennel.
Effect of MAF extracts treatment on mitochondrial membrane potential (MMP) in A549 cells. MMP of the A549 cells after 24 h treatment with MAF-Y and MAF-R extract showed remarkable decrease due to the loss of membrane integrity of mitochondria compared to untreated control cells.
These results contribute to the understanding of the mechanistic aspects of the anticancer effects of mulberry fruit extracts, particularly in inducing apoptosis through the disruption of mitochondrial membrane potential. Further investigation can be made using these insights to check the pathway activated by phytochemicals of mulberry fruit extracts on lung cancer cells.
Apoptosis induction by the treatment of MAF extracts in A549 cells
The most prevalent kind of programmed cell death, apoptosis, serves as a therapeutic target for a various cancer. Therefore, differential staining techniques were employed to observe the morphological alterations in lung cancer cells induced by the 24-hour treatment with MAF extracts. The observations revealed that, in contrast to control cells, treated cells exhibited the distinguishing characteristics of apoptosis. This highlights the significance of the natural phytochemicals found in mulberry fruits and suggests a safer substitute for including them into a diet in addition to other treatments for cancer. Below is an in-depth examination of different staining techniques.
AO-EtBr staining
It was evident from the morphological observations that the treatment with MAF extracts reduced the number of viable cells. Numerous cells were found to go through the apoptotic process, which was distinguished from cells that were not apoptotic. The control cells were evenly stained green and appeared with round undamaged nuclei (Figure 7A), while the cells treated with MAF extracts showed cells undergoing early apoptosis and some of the cells were necrotic. Early apoptotic cells showed distinctive characteristics such as nucleus condensation, fragmented chromatin and bright green fluorescence, late apoptotic cells were stained orange, while necrotic cells exhibited bright orange red staining in the nuclei without chromatin degradation (Figure 7B and C). Maximum number of MAF treated cells showed nuclei giving bright green and orange fluorescence, there were very few cells which showed characteristic of necrotic cells, suggesting that apoptosis was the primary cause of cell death in the majority of MAF treated cells.
AO-Etbr staining. A) Untreated control cells B) treated with MAF-Y extract for 24 h at its IC50 concentration C) treated with MAF-R extract for 24 h at its IC50 concentration. Control cells were stained uniformly green without exhibiting florescence. Cells were observed under 20X objective lens. Early apoptotic cells were green in colour with bright green dots in nucleus, late apoptotic cells were stained orange with condensed & fragmented nuclei while few necrotic cells were showing bright orange to red nucleus which was brighter compared to late apoptotic cells. Arrows indicates cells undergoing process of apoptosis. Scale bar is representing 10 μm.
DAPI staining
Apoptosis is characterized by the fragmentation and shrinking of cell as well as nucleus along with the chromosome degradation (Saraste, Pulkki, 2000). The treated cells with MAF extracts showed constricted chromatin and nuclear fragmentation, both of which indicate the activation of the apoptotic process, in contrast to the control cells, which exhibited distinct spherical nuclei (Figure 8). The examination of the morphology of extract-treated cells in comparison to control cells revealed that mulberry fruit extracts are a rich source of phytochemicals that were inducing apoptosis in lung cancer cells. The previous studies conducted by Thakkar et al. (2022; 2023), wherein the treatment with hydromethanolic leaf extracts of Acorus calamus L. and Dalbergia sissoo Roxb. induced apoptosis in A549 cells also reported the morphology changes such as chromatin and nuclear fragmentation due to the extract activity on cancer cells compare to control cells.
DAPI staining. A) Untreated control cells B) treated with MAF-Y extract for 24 h at its IC50 concentration C) treated with MAF-R extract for 24 h at its IC50 concentration. Control cells showing uniformly stained nucleus with clear margin, Nucleus of the cells treated with MAF-Y & MAF-R extracts are showing abnormality in margin and condensed chromosomes represented by arrows which are the characteristics of Apoptosis. Scale bar is representing 10 μm.
Giemsa staining
Observation revealed that cells which were treated with extracts were showed changes in morphology compared to control cells (Figure 9). MAF extract treated cells going through the process of apoptosis showed characteristic features like masses of condensed chromatin, cell shrinkage, nuclear fragmentation and development of apoptotic bodies, while nuclear and cytoplasmic enlargement was observed in some necrotic cells.
Giemsa Staining. A) Untreated control cells B) treated with MAF-Y extract for 24 h at its IC50 concentration C) treated with MAF-R extract for 24 h at its IC50 concentration. Treated cells were showing morphological features of apoptosis such as condensed chromatin and cell shrinkage which is shown by the arrows while the control cells were showing normal morphological features. Scale bar is representing 10 μm.
MAF Extracts Induces DNA Fragmentation in A549 Cells
The detection of the DNA fragmentation is a common hallmark of cells undergoing late-stage apoptosis (Jiao et al., 2017). In order to determine if M. alba L. fruit extract could induce DNA fragmentation and thus whether apoptosis occurred, human lung cancer cells exposed to M. alba L. fruits extracts treatment were assessed for DNA laddering and visualized by agarose gel electrophoresis (Figure 10). It was found that the human lung cancer cells incubated with M. alba L. fruit extracts showed apoptotic DNA fragmentation profiles. It was similar to doxorubicin, which is known to induces apoptosis (Tacar, Sriamornsak, Dass, 2013). No nucleic acid fragmentation was observed in untreated control cells represented by intact DNA band, while the DNA of extract treated cells was forming a smear. Khan et al. (2023) observed similar DNA smearing pattern in Ehrlich ascites carcinoma (EAC) cells after the treatment with Lucus indica bark extract. The results indicate due to the MAF extract treatment degradation of DNA is observed, which confirmed the apoptosis induction.
DNA fragmentation pattern visualization by gel electrophoresis. DNA of untreated A549 cells was showing intact band while the DNA of the cells treated with MAF-Y and MAF-R extracts for 24 h with their respective IC50 concentrations showed smear formation.
Finally, the isolation of the active principles of the methanolic extract of M. alba L. fruits is currently being undertaken to investigate their cytotoxic, molecular and genetic action mechanisms, which could provide meaningful perspectives for biomedical and biotechnological research.
CONCLUSION
Mulberry fruits are well recognized for their variety of phytochemicals, known to have various health benefits when consumed directly. In this study, we evaluated the potential of Morus alba L. fruit extracts at two different stages of maturity: MAF-Y (young) and MAF-R (ripe). Our findings revealed that both extracts contain phytochemicals, but in varying amounts corresponding to the fruit’s maturity. Notably, ripe fruit extract (MAF-R) exhibited a high phenolic content of 100.51 ± 1.91 mg/g GAE, resulting in superior antioxidant activity compared to the young fruit extract.
Remarkably, both extracts demonstrated potent cytotoxicity against lung cancer cells, with MAF-R displaying a lower IC50 value of 18.4 ± 3.01 µg/ml compared to MAF-Y (29.41 ± 3.6 µg/ml). This suggests a promising potential for mulberry fruit phytochemicals in lung cancer management. Furthermore, both extracts effectively inhibited cell migration and induced apoptosis in lung cancer cells, underscoring their therapeutic potential. Moreover, extract treatment led to an increase in ROS production, causing the loss of mitochondrial membrane potential, a hallmark of apoptosis activation. This was supported by the observation of distinctive morphological alterations associated with apoptosis in A549 cells post-extract treatment, along with DNA fragmentation evidenced by smear formation compared to intact bands in control cells.
Overall, our results suggest that MAF-Y and MAF-R induce programmed cell death in lung cancer cells via a ROS-dependent apoptotic pathway. Future research should focus on identifying and characterizing the specific phytochemicals responsible for inhibiting lung cancer growth in Morus alba L. fruit extracts.
ACKNOWLEDGMENT
The Authors are thankful to the P. G. Department of Biosciences and P. G. Department of Applied and Interdisciplinary Sciences (IICISST), Sardar Patel University for the infrastructure facilities. The authors express their gratitude to Dr. Vasudev Thakkar of P. G. Department of Biosciences, Sardar Patel University and Dr. Parth Thakor from Charotar University of Science and Technology (CHARUSAT) for his invaluable assistance in troubleshooting throughout this research. We extend our sincere thanks to Mr. Harsh Joshi from P. G. Department of Biosciences, Sardar Patel University for his invaluable assistance in diligently refining the manuscript draft.
REFERENCES
- Aggarwal BB, Bhardwaj A, Aggarwal RS, Seeram NP, Shishodia S, Takada Y. Role of resveratrol in prevention and therapy of cancer: preclinical and clinical studies. Anticancer Res. 2004;24(5A):2783-2840.
- Aghajanpour M, Nazer MR, Obeidavi Z, Akbari M, Ezati P, Kor NM. Functional foods and their role in cancer prevention and health promotion: a comprehensive review. Am J Cancer Res. 2017;7(4):740-769.
- Baumann J, Wurn G, Bruchlausen FV. Prostaglandin synthetase inhibitingO2 radical scavenging properties of some flavonoids and related phenolic compounds. Deutsche pharmakologische gesellschaft abstracts of the 20th spring meeting. Arc Pharmacol. 1979;307:R1-R77. Naunyn-Schmiedebergs Abstract No: R27 cited in.
-
Belwal T, Pandey A, Bhatt ID, Rawal RS, Luo Z. Trends of Polyphenolics and anthocyanins accumulation along ripening stages of wild edible fruits of Indian Himalayan region. Sci Rep. 2019;9(1):5894. doi: 10.1038/s41598-019-42270-2
» https://doi.org/10.1038/s41598-019-42270-2 - Brain KR,Turner TD. The Practical Evaluation of Phytopharmaceuticals. Wright-Scientechnica, Bristol; 1975.
-
Budiman A, Aulifa DL, Kusuma ASW, Sulastri A. Antibacterial and antioxidant activity of black mulberry (Morus nigra L.) extract for acne treatment. Pharmacogn J. 2017;9(5):611-614. doi: 10.5530/pj.2017.5.97
» https://doi.org/10.5530/pj.2017.5.97 -
Chang BY, Kim SB, Lee MK, Park H, Kim SY. Improved chemotherapeutic activity by Morus alba fruits through immune response of toll-like receptor 4. Int J Mol Sci. 2015;16(10):24139-24158. doi: 10.3390/ijms161024139.
» https://doi.org/10.3390/ijms161024139 -
Chen C, Kong AN. Dietary cancer-chemopreventive compounds: from signaling and gene expression to pharmacological effects. Trends Pharmacol Sci. 2005 June 1;26(6):318-326. doi: 10.1016/j.tips.2005.04.004.
» https://doi.org/10.1016/j.tips.2005.04.004 -
Chen C, Mokhtar RAM, Sani MSA, Noor NQIM. The effect of maturity and extraction solvents on bioactive compounds and antioxidant activity of mulberry (Morus alba) fruits and leaves. Molecules. 2022;27(8):2406. doi: 10.3390/molecules27082406.
» https://doi.org/10.3390/molecules27082406 -
Chen H, Pu J, Liu D, Yu W, Shao Y, Yang G, et al. Anti-inflammatory and antinociceptive properties of flavonoids from the fruits of black mulberry (Morus nigra L.). PLOS ONE. 2016;11(4):e0153080. doi: 10.1371/journal.pone.0153080
» https://doi.org/10.1371/journal.pone.0153080 -
Chen PN, Chu SC, Chiou HL, Kuo WH, Chiang CL, Hsieh YS. Mulberry anthocyanins, cyaniding 3-rutino-side and cyaniding 3-glucoside, exhibited and inhibitory effect on the migration and invasion of a human lung cancer cell line. Cancer Lett. 2006;235(2):248-259. doi: 10.1016/j.canlet.2005.04.033.
» https://doi.org/10.1016/j.canlet.2005.04.033 -
D’Urso G, Mes JJ, Montoro P, Hall RD, de Vos RCH. Identification of bioactive phytochemicals in mulberries. Metabolites. 2020;10(1):7. doi: 10.3390/metabo10010007
» https://doi.org/10.3390/metabo10010007 -
DNA preparation from adherent cells. Accessed December 04 2023. https://ccr.cancer.gov/sites/default/files/dna_prep_from_adherent_cells.pdf ; 2006 Section of Cancer Genomics, Genetics Branch, NCI. National Institutes of Health.
» https://ccr.cancer.gov/sites/default/files/dna_prep_from_adherent_cells.pdf -
El-Baz FK, Hassan AZ, Abd-Alla HI, Aly HF, Mahmoud K. Phytochemical analysis, assessment of antiproliferative and free radical scavenging activity of Morus alba and Morus rubra fruits. Asian J Pharm Clin Res. 2017;10(6):189-199. doi: 10.22159/ajpcr.2017.v10i6.18029.
» https://doi.org/10.22159/ajpcr.2017.v10i6.18029 -
Elbling L, Weiss RM, Teufelhofer O, Uhl M, Knasmueller S, Schulte-Hermann R, et al. Green tea extract and (-)-epigallocatechin-3-gallate, the major tea catechin, exert oxidant but lack antioxidant activities. FASEB J. 2005;19(7):807-809. doi: 10.1096/fj.04-2915fje.
» https://doi.org/10.1096/fj.04-2915fje -
Eo H, Lim Y. Combined mulberry leaf and fruit extract improved early stage of cutaneous wound healing in high-fat diet-induced obese mice. J Med Food. 2016;19(2):161-169. doi: 10.1089/jmf.2015.3510.
» https://doi.org/10.1089/jmf.2015.3510 -
Espín JC, González-Sarrías A, Tomás-Barberán FA. The gut microbiota: A key factor in the therapeutic effects of (poly) phenols. Biochem Pharmacol. 2017;139:82-93. doi: 10.1016/j.bcp.2017.04.033
» https://doi.org/10.1016/j.bcp.2017.04.033 - Evans WC. Trease Evans Pharmacognosy. 14th ed. W B Saunders Ltd; 1996:119-159.
-
Ferlay J, Colombet M, Soerjomataram I, Parkin DM, Piñeros M, Znaor A, et al. Cancer statistics for the year 2020: an overview. Int J Cancer. 2021;149(4):778-789. doi: 10.1002/ijc.33588.
» https://doi.org/10.1002/ijc.33588 -
Grada A, Otero-Vinas M, Prieto-Castrillo F, Obagi Z, Falanga V. Research techniques made simple: analysis of collective cell migration using the wound healing assay. J Invest Dermatol. 2017;137(2):e11-e16. doi: 10.1016/j.jid.2016.11.020.
» https://doi.org/10.1016/j.jid.2016.11.020 -
Hadi SM, Ullah MF, Azmi AS, Ahmad A, Shamim U, Zubair H, Khan HY. Resveratrol mobilizes endogenous copper in human peripheral lymphocytes leading to oxidative DNA breakage: a putative mechanism for chemoprevention of cancer. Pharm Res. 2010;27(6):979-988. doi: 10.1007/s11095-010-0055-4.
» https://doi.org/10.1007/s11095-010-0055-4 -
Huang D, Ou B, Prior RL. The chemistry behind antioxidant capacity assays. J Agric Food Chem. 2005;53(6):1841-1856. doi: 10.1021/jf030723c.
» https://doi.org/10.1021/jf030723c -
Huang HP, Shih YW, Chang YC, Hung CN, Wang CJ. Chemoinhibitory effect of mulberry anthocyanins on melanoma metastasis involved in the Ras/PI3K pathway. J Agric Food Chem . 2008;56(19):9286-9293. doi: 10.1021/jf8013102.
» https://doi.org/10.1021/jf8013102 -
Ittiyavirah SP, Muraly A, Rajiv P, Raveendran R. Inhibition of growth and induction of apoptosis in PC3 and A549 cell lines by hydro alcoholic fruit extract of Morinda tinctoria roxb: in vitro analysis. J Sci Innov Res. 2014;3(3):303-307. doi: 10.31254/jsir.2014.3305.
» https://doi.org/10.31254/jsir.2014.3305 -
Jelled A, Hassine RB, Thouri A, Flamini G, Chahdoura H, El Arem A, et al. Immature mulberry fruits richness of promising constituents in contrast with mature ones: A comparative study among three Tunisian species. Ind Crops Prod. 2017;95:434-443. doi: 10.1016/j.indcrop.2016.10.053.
» https://doi.org/10.1016/j.indcrop.2016.10.053 -
Jiang DQ, Guo Y, Xu DH, Huang YS, Yuan K, Lv ZQ. Antioxidant and anti-fatigue effects of anthocyanins of mulberry juice purification (MJP) and mulberry marc purification (MMP) from different varieties mulberry fruit in China. Food Chem Toxicol. 2013;59:1-7. doi: 10.1016/j.fct.2013.05.023
» https://doi.org/10.1016/j.fct.2013.05.023 -
Jiao Y, Wang X, Jiang X, Kong F, Wang S, Yan C. Antidiabetic effects of Morus alba fruit polysaccharides on high-fat diet-and streptozotocin-induced type 2 diabetes in rats. J Ethnopharmacol. 2017;199:119-127. doi: 10.1016/j.jep.2017.02.003.
» https://doi.org/10.1016/j.jep.2017.02.003 -
Jung HW, Kang SY, Kang JS, Kim AR, Woo ER, Park YK. Effect of kuwanon G isolated from the root bark of Morus alba on ovalbumin-induced allergic response in a mouse model of asthma. Phytother Res. 2014;28(11):1713-1719. doi: 10.1002/ptr.5191.
» https://doi.org/10.1002/ptr.5191 -
Kadam RA, Dhumal ND, Khyade VB. The Mulberry, Morus alba (L.): the medicinal herbal source for human health. Int J Curr Microbiol Appl Sci. 2019;8(4):2941-2964. doi: 10.20546/ijcmas.2019.804.341.
» https://doi.org/10.20546/ijcmas.2019.804.341 -
Khan MMR, Susmi TF, Miah M, Reza MA, Rahi MS. Morphological alteration and intracellular ROS Generation Confirm apoptosis induction on EAC cells by Leucas indica Bark extract. J Herbs Spices Med Plants. 2023;29(1):84-97.doi: 10.1080/10496475.2022.2103765
» https://doi.org/10.1080/10496475.2022.2103765 -
Kim SB, Chang BY, Hwang BY, Kim SY, Lee MK. Pyrrole alkaloids from the fruits of Morus alba. Bioorg Med Chem Lett. 2014;24(24):5656-5659. doi: 10.1016/j.bmcl.2014.10.073.
» https://doi.org/10.1016/j.bmcl.2014.10.073 -
Koide T, Hashimoto Y, Kamei H, Kojima T, Hasegawa M, Terabe K. Antitumor effect of anthocyanin fractions extracted from red soybeans and red beans in vitro and in vivo. Cancer Biother Radiopharm. 1997;12(4):277-280. doi: 10.1089/cbr.1997.12.277
» https://doi.org/10.1089/cbr.1997.12.277 -
Lee D, Yu JS, Lee SR, Hwang GS, Kang KS, Park JG, et al. Beneficial effects of bioactive compounds in mulberry fruits against cisplatin-induced nephrotoxicity. Int J Mol Sci . 2018;19(4):1117. doi: 10.3390/ijms19041117.
» https://doi.org/10.3390/ijms19041117 -
Lee Y, Hwang KT. Changes in physicochemical properties of mulberry fruits (Morus alba L.) during ripening. Sci Hortic. 2017;217:189-196. doi: 10.1016/j.scienta.2017.01.042.
» https://doi.org/10.1016/j.scienta.2017.01.042 -
Li M, Li T, Hu X, Ren G, Zhang H, Wang Z, et al. Structural, rheological properties and antioxidant activities of polysaccharides from mulberry fruits (Murus alba L.) based on different extraction techniques with superfine grinding pretreatment. Int J Biol Macromol. 2021;183:1774-1783. doi: 10.1016/j.ijbiomac.2021.05.108.
» https://doi.org/10.1016/j.ijbiomac.2021.05.108 -
Li Y, Zhang JJ, Xu DP, Zhou T, Zhou Y, Li S, et al. Bioactivities and health benefits of wild fruits. Int J Mol Sci . 2016;17(8):1258. doi: 10.3390/ijms17081258.
» https://doi.org/10.3390/ijms17081258 - Lin CY, Lay HL. Characteristics of fruit growth, component analysis and antioxidant activity of mulberry (Morus spp). Sci Hortic . 2013;162:285-292.
- Lou H, Hu Y, Zhang L, Sun P, Lu H. Nondestructive evaluation of the changes of total flavonoid, total phenols, ABTS and DPPH radical scavenging activities: and sugars during mulberry (Morus alba L.) fruit development by chlorophyll fluorescence and RGB intensity values. Food Sci Technol. 2012;47:19-24.
- Makkar HP. Quantification of Tannins in Tree and Shrub Foliage: a Laboratory Manual. Springer Science+Business Media; November 30; 2003.
-
Mani S, Natesan K, Shivaji K, Balasubramanian MG, Ponnusamy P. Cytotoxic effect induced apoptosis in lung cancer cell line on Ageratina adenophora leaf extract. Biocatal Agric Biotechnol. 2019;22:101381. doi: 10.1016/j.bcab.2019.101381.
» https://doi.org/10.1016/j.bcab.2019.101381 -
Michałowicz J, Włuka A, Cyrkler M, Maćczak A, Sicińska P, Mokra K. Phenol and chlorinated phenols exhibit different apoptotic potential in human red blood cells (in vitro study). Environ Toxicol Pharmacol. 2018;61:95-101. doi: 10.1016/j.etap.2018.05.014
» https://doi.org/10.1016/j.etap.2018.05.014 -
Mirza MB, Elkady AI, Al-Attar AM, Syed FQ, Mohammed FA, Hakeem KR. Induction of apoptosis and cell cycle arrest by ethyl acetate fraction of Phoenix dactylifera L.(Ajwa dates) in prostate cancer cells. J Ethnopharmacol . 2018;218:35-44. doi: 10.1016/j.jep.2018.02.030.
» https://doi.org/10.1016/j.jep.2018.02.030 - Nakagawa H, Tsuta K, Kiuchi K, Senzaki H. Growth inhibitory effects of diallyl disulfide on human breast cancer cell lines. Carcinogenesis. 2001;32(6):891-897.
-
Neergheen VS, Bahorun T, Taylor EW, Jen LS, Aruoma OI. Targeting specific cell signaling transduction pathways by dietary and medicinal phytochemicals in cancer chemoprevention. Toxicology. 2010 December 5;278(2):229-241. doi: 10.1016/j.tox.2009.10.010.
» https://doi.org/10.1016/j.tox.2009.10.010 -
Oki T, Kobayashi M, Nakamura T, Okuyama A, Masuda M, Shiratsuchi H, et al. Changes in radical-scavenging activity and components ofmulberry fruit during maturation. J Food Sci. 2006;71(1):C18-C22. doi: 10.1111/j.1365-2621.2006.tb12382.x
» https://doi.org/10.1111/j.1365-2621.2006.tb12382.x -
Onuki-Nagasaki R, Nagasaki A, Hakamada K, Uyeda TQ, Fujita S, Miyake M, et al. On-chip screening method for cell migration genes based on a transfection microarray. Lab Chip. 2008;8(9):1502-1506.doi: 10.1039/b803879a.
» https://doi.org/10.1039/b803879a -
Ozen AE, Pons A, Tur JA. Worldwide consumption of functional foods: a systematic review. Nutr Rev. 2012;70(8):472-481. doi: 10.1111/j.1753-4887.2012.00492.x.
» https://doi.org/10.1111/j.1753-4887.2012.00492.x -
Pan MH, Ghai G, Ho CT. Food bioactives, apoptosis, and cancer. Mol Nutr Food Res. 2008;52(1):43-52. doi: 10.1002/mnfr.200700380.
» https://doi.org/10.1002/mnfr.200700380 - Rasouli H, Mazinani MH, Haghbeen K. Benefits and challenges of olive biophenols: a perspective. In: Olives and Olive Oil in Health and Disease Prevention. Academic Press; 2021:489-503.
- Rohman A, Riyanto S, Utari D. Antioxidant activities, total phenolic and flavonoid contents of ethyl acetate extract of Mengkudu (Morinda citrifolia, L) fruit and its fractions. Indones J Pharm. 2006;17:136-142.
-
Rom WN, Hay JG, Lee TC, Jiang Y, Tchou-Wong KM. Molecular and genetic aspects of lung cancer. Am J Respir Crit Care Med. 2000;161(4 Pt 1):1355-1367. doi: 10.1164/ajrccm.161.4.9908012.
» https://doi.org/10.1164/ajrccm.161.4.9908012 -
Sakamuru S, Attene-Ramos MS, Xia M. Mitochondrial membrane potential assay. In: High-Throughput Screening Assays in Toxicology; 2016:17-22. doi: 10.1007/978-1-4939-6346-1_2.
» https://doi.org/10.1007/978-1-4939-6346-1_2 -
Saraste A, Pulkki K. Morphologic and biochemical hallmarks of apoptosis. Cardiovasc Res. 2000;45(3):528-537. doi: 10.1016/s0008-6363(99)00384-3.
» https://doi.org/10.1016/s0008-6363(99)00384-3 -
Seidel DV, Azcárate-Peril MA, Chapkin RS, Turner ND. Shaping functional gut microbiota using dietary bioactives to reduce colon cancer risk. Semin Cancer Biol. 2017;46:191-204. doi: 10.1016/j.semcancer.2017.06.009.
» https://doi.org/10.1016/j.semcancer.2017.06.009 -
Seo KH, Lee DY, Jeong RH, Lee DS, Kim YE, Hong EK, et al. Neuroprotective effect of prenylated arylbenzofuran and flavonoids from Morus alba fruits on glutamate-induced oxidative injury in HT22 hippocampal cells. J Med Food . 2015;18(4):403-408. doi: 10.1089/jmf.2014.3196.
» https://doi.org/10.1089/jmf.2014.3196 -
Shin BR, Kim HS, Yun MJ, Lee HK, Kim YJ, Kim SY, et al. Promoting effect of polysaccharide isolated from Mori fructus on dendritic cell maturation. Food Chem Toxicol . 2013;51:411-418. doi: 10.1016/j.fct.2012.10.018.
» https://doi.org/10.1016/j.fct.2012.10.018 -
Sidhu JS, Kabir Y, Huffman FG. Functional foods from cereal grains. Int J Food Prop. 2007;10(2):231-244. doi: 10.1080/10942910601045289.
» https://doi.org/10.1080/10942910601045289 -
Singh K, Mhatre V, Bhori M, Marar T. Vitamin E and C reduces oxidative stress and mitochondria permeability transition generated by camptothecin-an in vitro study. Toxicol Environ Chem. 2013;95(4):646-657. doi: 10.108 0/02772248.2013.805013.
» https://doi.org/10.108 0/02772248.2013.805013 -
Sivasankarapillai VS, Krishnamoorthy N, Eldesoky GE, Wabaidur SM, Islam MA, Dhanusuraman R, et al. One-pot green synthesis of ZnO nanoparticles using Scoparia dulcis plant extract for antimicrobial and antioxidant activities. Appl Nanosci. 2022:1-11. doi: 10.1007/s13204-022-02610-7.
» https://doi.org/10.1007/s13204-022-02610-7 -
Tacar O, Sriamornsak P, Dass CR. Doxorubicin: an update on anticancer molecular action, toxicity and novel drug delivery systems. J Pharm Pharmacol. 2013;65(2):157-170. doi: 10.1111/j.2042-7158.2012.01567.x.
» https://doi.org/10.1111/j.2042-7158.2012.01567.x - Mahmood T, Anwar F, Afzal N, Kausar R, Ilyas S, Shoaib M. Influence of ripening stages and drying methods on polyphenolic content and antioxidant activities of mulberry fruits. J Food Meas Charact. 2017 Dec;11:2171-9.
-
Tao J, Li Y, Li S, Li HB. Plant foods for the prevention and management of colon cancer. J Funct Foods. 2018;42:95-110. doi: 10.1016/j.jff.2017.12.064.
» https://doi.org/10.1016/j.jff.2017.12.064 -
Thakkar AB, Sargara P, Subramanian RB, Thakkar VR, Thakor P. Induction of apoptosis in lung carcinoma cells (A549) by hydromethanolic extract of Acorus calamus L. Process Biochem. 2022;123:1-10. doi: 10.1016/j.procbio.2022.10.028.
» https://doi.org/10.1016/j.procbio.2022.10.028 - Thakkar AB, Subramanian RB, Thakkar VR, Thakor P. Hydromethanolic leaves extract of Dalbergia sissoo Roxb. Process Biochem . 2023;October 6 ex DC. Induces Apoptosis in Lung Adenocarcinoma Cells.
-
Trachootham D, Alexandre J, Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat Rev Drug Discov. 2009;8(7):579-591. doi: 10.1038/nrd2803.
» https://doi.org/10.1038/nrd2803 -
Villarini M, Pagiotti R, Dominici L, Fatigoni C, Vannini S, Levorato S, et al. Investigation of the cytotoxic, genotoxic, and apoptosis-inducing effects of estragole isolated from fennel (Foeniculum vulgare). J Nat Prod. 2014;77(4):773-778. doi: 10.1021/np400653p.
» https://doi.org/10.1021/np400653p -
Wang D, Li H, Li B, Ma R, Zhang N, Zhang X, et al. Systematic fractionation and immunoenhancement of water-soluble polysaccharides isolated from fruit of Morus alba L. Int J Biol Macromol . 2018;116:1056-1063. doi: 10.1016/j.ijbiomac.2018.05.106.
» https://doi.org/10.1016/j.ijbiomac.2018.05.106 -
Wang J, Yi J. Cancer cell killing via ROS: to increase or decrease, that is the question. Cancer Biol Ther. 2008;7(12):1875-1884. doi: 10.4161/cbt.7.12.7067.
» https://doi.org/10.4161/cbt.7.12.7067 - Wani TA, Bhat IA, Guleria K, Fayaz M, Anju T, Haritha K, et al. Phytochemicals: diversity, sources and their roles. In: Phytochemical Genomics: Plant Metabolomics and Medicinal Plant Genomics. Springer Nature Singapore; 2023:3-33.
-
Wilson DW, Nash P, Buttar HS, Griffiths K, Singh R, De Meester F, et al. The role of food antioxidants, benefits of functional foods, and influence of feeding habits on the health of the older person: an overview. Antioxidants (Basel). 2017;6(4):e81. doi: 10.3390/antiox6040081.
» https://doi.org/10.3390/antiox6040081 -
Xu DP, Zheng J, Zhou Y, Li Y, Li S, Li HB. Extraction of natural antioxidants from the Thelephora ganbajun mushroom by an ultrasound-assisted extraction technique and evaluation of antiproliferative activity of the extract against human cancer cells. Int J Mol Sci . 2016;17(10):171664. doi: 10.3390/ijms17101664.
» https://doi.org/10.3390/ijms17101664 -
Yuan Q, Zhao L. The mulberry (Morus alba L.) fruit: a review of characteristic components and health benefits. J Agric Food Chem . 2017;65(48):10383-10394. doi: 10.1021/acs.jafc.7b03614.
» https://doi.org/10.1021/acs.jafc.7b03614
Data availability
Data will be made available on request.
Publication Dates
-
Publication in this collection
20 Jan 2025 -
Date of issue
2025
History
-
Received
21 Feb 2024 -
Accepted
12 June 2024

 Hydromethanolic extracts of Morus alba L. (Mulberry) young and ripe fruit induces apoptosis in lung carcinoma cells (A549)
Hydromethanolic extracts of Morus alba L. (Mulberry) young and ripe fruit induces apoptosis in lung carcinoma cells (A549)