Abstract
Novel anti-cholinesterase inhibitors including pyrazolone merging chalcone derivatives have been developed and molecular docking has been performed. From the docking results, compound 48 (-8.3 k/cal) shows the highest binding affinity toward the AChE enzyme compared to the standard drug donepezil (-12.76k/cal). This compound produced two conventional hydrogen bonds between the carbonyl oxygen and residue of Asn 491. Every synthetic chemical underwent screening to determine its ability to cause in vitro cytotoxicity activity against Human SH-SY5Y neuroblastoma cell lines. The cells of SH-SY5Y were preincubated for 30 minutes with varying doses of the chosen compounds like 8, 9, 10, 11, 31, 37, 44, 47, 48, and 50 to induce neurotoxicity. They were then grown in Aβ25-35 (25 mol/L) for 48 hr. Cell viability was determined by MTT assay. Compounds 8, 11, and 48 exhibited a better binding score -8.2k/cal -8.2k/cal and -8.3k/cal respectively compared to other analogs. Synthesized compounds 8, 11, and 48 inhibited Aβ25-35-induced apoptosis in SH-SY5Y cells and protected neural cells from damage. The MTT assay confirmed compound 48 significantly reduced Aβ25-35-induced toxicity among human neuroblastoma SH-SY5Y cells, demonstrating its neuroprotective properties.
Keywords:
Anti-cholinesterase; (E)-2-(4-chlorobenzoyl)-5-(2-fluorostyryl)-2,4-dihydro-3Hpyrazol-3-one; Molecular docking; Human SH-SY5Y neuroblastomacelllines; ADMET.
INTRODUCTION
The majority of old age people are affected by a destructive brain disorder, which is called Alzheimer’s disease (AD) (Carvalho, Moreira, 2023). The main pathophysiological factor that deals with AD is the accumulation of beta-amyloid proteins and tau proteins in neurons. Patients with AD commonly associate with neuron loss in the hippocampus region (Sharma, Mehdi, 2023). During this disease, all the neurotransmitters like serotonin, dopamine, glutamate, acetylcholine (ACh), and noradrenaline levels were reduced. This may cause cognition, memory loss functional loss, and motor function loss in affected humans (Hippius, Neundorfer, 2003; García Ayllón et al., 2011). Due to the increase in the aging population, the low number of healthcare supporters, and less personal care, this disease may increase the death rate in the future. Among the various neurotransmitters, ACh plays a central role in the development of AD, and previous research studies showed that the level of Ach is decreased intensely in the hippocampus and brain cortex region of AD patients (Ramsay et al., 2018). The main goal for the treatment of AD is to prevent the action of acetylcholinesterase (AChE), for this currently we have several drugs such as galantamine, rivastigmine, and donepezil (Ansari et al., 2017). These drugs inhibit the action of AChE action and increase the level of Ach in the brain in a temporary manner. Therefore, the developments of new drugs for the treatment of AD through the inhibition of AChE are diversely increased in recent days (Reddy et al., 2022).
Among the nitrogen-containing heterocyclic molecules, the pyrazolone derivatives were the most popular derivative due to their wide biological activity such as anti-inflammatory (Benazzouz Touami et al., 2022), anti-cancer (Hassan et al., 2023), anti-microbial (Nidhar et al., 2022), anti-malarial (Elgiushy et al., 2022), anti-AD (Mukhtar et al., 2021), analgesic (Baashen, Abdel-Wahab, El Hiti, 2017), anti-depressant (Naglah et al., 2020), MAO inhibitory activities. Currently, various research teams working on the biological activity of different substituted pyrazole derivatives. Based on recent studies, pyrazolone leads derivatives to block the action of several enzymes like MAO, AChE, and carbonic anhydrase (Hassan et al., 2017). In addition to this, the chalcone derivative was considered an important pharmacophoric group for various biological activities such as anti-cancer (Hassan et al., 2020), anti-microbial (Rampa et al., 2017), anti-malarial (Mukhtar et al., 2022), anti-AD (Liu et al., 2014), analgesic (Liu et al., 2015), and antidepressant (Ucar et al., 2005). From the above view, the aim of this research work deals with the designing of pyrazolone compounds with chalcone moiety, and molecular docking studies were performed for these compounds, and their cytotoxicity was evaluated towards the neuroblastoma cell line.
MATERIAL AND METHODS
Molecular Docking
Designing of molecules
Ucar et al. (2005) revealed that the various substituted 1-thiocarbamoyl-3-phenyl-5-thienyl-2pyrazoline (1) derivatives are active against both MOA and AChE enzymes at the microgram level. Inspired by this work, we planned to modify the parent molecules and evaluate their anti-AChE activity through the insilico method (Figure 1).
This study proved that the presence of sp2 carbon increases molecular recognition by the AChE active site (Vogel, 2002). This idea will encourage our team to increase the aromaticity of the pyrazole ring with bioisosterism and replace the thionyl ring with chalcone moiety and it may interact with the AChE enzyme (Wang et al., 2006). The presence of amide linkage has shown promising activity against AChE without any potential side effects associated with old irreversible inhibitors. So the amide function is also introduced in the newly designed compound. Based on this we designed various pyrazolone derivatives and it was shown in Figure 2.
Devices and materials
There are various bioinformatics tools are available for the in-silico drug design process. From these, we are using the following software such as Marvin Sketch, Chemdraw, online programs like Protein Data Bank (PDB), and Pyrx docking software for our current work. Protein preparation
The targeted proteins were downloaded from the online tool PDB. We retrieved the targeted enzyme AChE ((PDB ID:4EY6)) from the PDB website. The downloaded protein should contain crystallographic data and it was validated by Ramachandran plot. The protein preparation begins with the removal of crystal water and the water has a distance of more than 5A° followed by the addition of missing hydrogen to the amino acid residue, protonation, ionization, and energy minimization. The force field was applied for the energy minimization of protein.
Identification of active sites
The active amino acid residue for the docking process was found utilizing the Protein-Ligand Interaction Profile (PLIP) online tools at https://pliptool.biotec.tu-dresden.de/plipweb/plip/index.
Preparation of Ligands
Marvin sketch tools were used for drawing the designed molecules in both 2D and 3D models. After drawing the structure was optimized and saved as .pdb format.
Identification of Activities
Since PyRx’s virtual screening tool outperformed the other docking choices (MVD: 89%, Glide: 83%, Surflex: 76%, FlexX: 59%), we decided to employ it. The receptor file was cleared of the nonpolar H atoms, and the matching C-atoms received their partial charges. The docking was done using the Molecular docking engine of PyRx software (https://sourceforge.net/projects/pyrx/). A spherical area including every protein atom within 15.0 Ao of the bound crystallographic ligand atom was designated as the binding site. The default settings were used for all computations. A grid resolution was used for docking.
In silico ADMET prediction
The Swiss ADME (www.swissadme.ch) prediction was used in a computer analysis to evaluate the pharmacokinetic properties of suggested medications (Daina, Michielin, Zoete, 2017). We determined the molecular weight, molecular volume, total CNS activity, percentage of oral absorption in humans, polar surface area, 1-octanol-water distribution constant, number of donors and acceptors of hydrogen bonds, and BBB penetration. The characteristics listed above aid in improving comprehension of the ADME characteristics of any medication or artificial substance. The infractions under the rule of three, rule of five, and drug similarity were all identified. A molecule can only be eaten orally if it meets all of the requirements listed above, which include having a molecular mass of 500, five H-bond donors, ten H-bond acceptors, and a distribution constant of 5.
Chemistry
All the solvents and chemicals are used in reagent grade without any purification and were purchased from Merck.
Step 1. Synthesis of 5-methyl-2,4-dihydro-3H-pyrazol-5one (A)
To ethyl acetoacetate (0.5 mmol) add hydrazine hydrate (0.5 mmol) in 40 mL of ethanol drop-wise with constant string and maintain the temperature around 60OC. The reaction mixture was allowed to stir for 1h at 600C and the crystalline product was separated out and more crystals of the product were obtained after cooling the reaction mixture. The product is filtered and recrystallized with cold ethanol.
Step 2. Synthesis of 2-benzoyl-5-methyl-2,4-dihydro-3Hpyrazole-3-one (B)
The compound A (0.01 mmol) is dissolved in 40 mL of dimethyl formamide and this solution is added to the various substituted acid chloride (0.01 mmol) in triethylamine (0.01 mmol) and it was refluxed for 1h at 151o - 154oC until the disappearance of starting materials, which was checked with TLC by utilizing ethyl acetate and h-hexane (4:6) as mobile phase. The reaction mixture was allowed to cool after the completion of the reaction, and the precipitate of 2-benzoyl-5-methyl-2,4-dihydro-3Hpyrazol-3-one (B) was separated. The compound B was purified by recrystallized by ethanol (Dube et al., 2015).
Step 3. Synthesis of (E)-5-(2-substituted)-2-(substituted2-carbonyl)-2,4-dihydro-3H-pyrazole-3-one (C)
The pyrazolone derivatives of (E)-5-(2-substituted)2-(substituted-2-carbonyl)-2,4-dihydro-3H-pyrazol3-one prepared by the condensing compound B (0.1 mmole) with various substituted aromatic aldehyde (0.1 mmole) in presence of 10% alcoholic NaOH (10 mL) and stirred at 25OC. The reaction mixture was kept aside for 24h at room temperature and HCl to neutralize unreacted NaOH and the precipitate was separated and washed with cold water (Nalini, et al., 2010).
Characterizations
Using a Veegomelting point device, the synthesized compounds’ melting points were measured which were found to be precise and correct. Utilizingethyl acetate, n-hexane, methanol, and chloroform as the mobile phase and pre-coated silica gel as the stationary phase, thin layer chromatography (TLC) were performed to confirm whether the reaction was completed. IR spectroscopy was recorded with Perkin Elmer IR spectroscopy with KBr pellet. The nuclear magnetic spectra were obtained from 500 MHz FTNMR, Bruker DRX-300 spectrophotometer. DMSO-d6 and CDCl3 were used as solvents for NMR and TMS was used as internal standard. The carbon NMR was recorded from 126 MHz and the mass was calculated with Shimadzu LC-MS.
MTT assay
Cell culture
Human SH-SY5Y neuroblastoma cells were procured from Sigma Aldrich and cultivated at 37 °C in a humidified atmosphere with 5.0% CO2 in DMEM supplemented with 10% fetal bovine serum (FBS). The trials were run at 80% confluence.
Determination of cell viability
A tweaked version of the previously published standard MTT test was deployed to assess cell viability. Three replicates of the experiment were conducted. 100 μL of fresh medium supplemented with 10% FBS was added to 96-well plates containing cells at a density of 2.0 x 104 cells per well. After a day of stabilization, the cells were incubated for two hours with five different doses of test chemicals (25, 50, 100, 250, and 500 μg/mL) dissolved in DMEM containing 10% FBS. After two hours, the drug was mixed with 10 μM of Aβ25-35, and it was incubated for an additional twenty-four hours at 37.0˚C and 5.0% CO2. A solvent control condition (10% FBS + DMEM) was applied as a statistical control. After the treatment period, remove the culture material and add 100 μL of MTT (500 μg/mL) to each well. Let the plates incubate for four hours. After the MTT solution was removed, 100 μL of DMSO-d6 was added to each well to dissolve the dark blue crystals. The plates were shaken for a few minutes before being read at 540 nm using a Thermo Plate Reader. Following analysis, the findings were shown as percentages concerning the control group (Wang et al., 2010; Yu et al., 2014).
RESULTS
Molecular docking
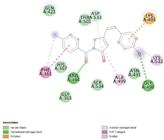
Using PyRx molecular docking software, the suggested compounds’ molecular docking tests were conducted to ascertain their free energy binding to the intended enzymes. Using Discovery Studio, the docking pose for the ligand-enzyme interaction was displayed and the interaction profile of ligands with the protein such as van der Walls interaction, conventional hydrogen Bond interaction, and carbon-hydrogen bond interaction was identified in the given figures for all the compounds. The developed library of compounds was shown to have an uncompetitive-type inhibitory, motivating additional research into its potential interaction with the active site of AChE. Redocking the co-crystallized ligands into the AChE precisely served as the first step in validating the docking processes. Each ligand’s binding free energy was listed in a table. The data unequivocally demonstrate that every chemical interacts with the targeted enzyme AChE in a promising manner. The presence of the lipophilic component of the aromatic heterocyclic ring is the primary cause of the contact. Compound 48 (-8.3 kcal/ mol) exhibits the highest binding affinity for the AChE enzyme when compared to the normal control drug donepezil, according to the docking data (Table I). The remaining investigated chemical exhibits good to moderate affinity for binding to the chosen enzymes. These amino acids are crucial in inhibiting the ligandbinding domain of acetylcholinesterase inhibitors and have been frequently linked to ligand interaction with AChE inhibitors. Figure 3 to 12 shows the docking image of the compounds. Based on the docking score the following derivatives 8, 9, 10, 11, 31, 37, 44, 47, 48, and 50 were selected for the conventional synthesis and it was further evaluated for the cytotoxicity studies against the neuroblastoma cells.
In-silico ADMET prediction
The SWISS ADME program was utilized to assess the in-silico ADME properties of the ligands. The compounds ranged in molecular weight from 298 to 359. There were no hydrogen bond donors except compound 44. Between two and five hydrogen bond acceptors are thought to be present. The estimated number of probable metabolic processes was between one and three, while the anticipated octanol/water partition coefficient ranged from two to 2.8. There were zero instances of breaking Lipinski’s rule of five (Table II).
Chemistry
The final derivatives of chalcone hybrid pyrazolone derivatives were achieved by a three-step process. In the first step, compound A was obtained from the reaction between ethyl acetoacetate and hydrazine hydrate in ethanol. Further, compound A reacted with various acid chlorides in the presence of triethylamine in DMF and then refluxed the reaction mixture under 151o - 154oC for 1h to yield compound B. The final chalcone derivatives were obtained from the reaction from compound B and various aromatic aldehydes. Melting point analysis was performed on the synthesized derivatives after the reaction was completed and the purity of the produced compounds was assessed using TLC with n-hexane and ethyl acetate serving as the mobile phase. Through the use of several spectral investigations, the structure of the produced molecules was clarified. The produced compounds are examined using Mass spectra, 1H NMR, 13C NMR, and Infrared spectra. A single signal was detected in the 1H NMR spectra of the target compounds, chalcone carrying pyrazole scaffold, namely around 6.61 - 6.49 ppm for the -CH=CH- proton. The remaining aromatic protons are present between 7.86 to 7.10. From the spectral analysis, it is evident that all the compounds showed corresponding signals in all the spectral data. The spectral data for all the compounds are given below:
(E)-5-(2-chlorostyryl)-2-picolinoyl-2,4-dihydro-3Hpyrazol-3-one (8)
C17H12ClN3O2; yield: 81%; MP: 151 - 155OC; Rf: 0.81; IR (KBr) cm-1: 3353 (CH str, CH=CH); 1585 (C=O str, amide); 1455 (C=O str, ketone), 851 ( aromatic ring); 718 (C-Clstr); 1H NMR (500 MHz, DMSO-d6) ppm δ: 7.86 - 7.62 (m, 2H), 7.45 (t, J = 13.9 Hz, 6H), 7.10 (t, J = 15.0 Hz, 6H), 7.10 (t, J = 15.0 Hz, 6H), 6.61 - 6.49 (m, 4H), 6.41 (s, 1H), 4.23 - 4.13 (m, 4H), 3.61 - 3.54 (m, 4H). 13C NMR (126 MHz, DMSO-d6) δ: 171.01, 133.99, 133.50, 130.01, 128.96, 128.47, 128.01, 127.56, 126.99, 126.46, 126.12, 125.96, 124.45, 123.71, 119.40, 119.01, 20.89. Mass: Actual: 325; Found: 326 (M+1).
(E)-5-(2-fluorostyryl)-2-picolinoyl-2,4-dihydro-3Hpyrazol-3-one (9)
C17H12FN3O2; yield: 79%; MP: 125 - 128OC; Rf: 0.84; IR (KBr) cm-1: 3194 (CH str, CH=CH); 2479 (CH str, aromatic); 1554 (C=O str, amide); 1374 (C=O str, ketone), 851 ( aromatic ring); 761 (C-F str); 1H NMR (500 MHz, DMSO-d6) δ 7.84 - 7.72 (m, 4H), 7.49 (s, 2H), 7.46 - 7.40 (m, 4H), 7.32 - 7.29 (m, 3H), 7.11 (s, 3H), 7.02 - 6.96 (m, 4H), 6.57 (s, 2H), 3.60 - 3.56 (m, 4H). 13C NMR (126 MHz, DMSO-d6) δ: 170.41, 134.69, 134.46, 130.12, 129.86, 129.32, 128.96, 128.33, 127.91, 127.35, 127.09, 126.87, 124.45, 123.71, 119.40, 119.01, 20.89, 17.16. Mass: Actual: 309; found: 311 (M+2).
(E)-5-(3-fluorostyryl)-2-picolinoyl-2,4-dihydro-3Hpyrazol-3-one (10)
C17H12FN3O2; yield: 83%; MP: 117 - 119OC; Rf: 0.81; IR (KBr) cm-1: 3194 (CH str, CH=CH); 2479 (CH str, aromatic); 1554 (C=O str, amide); 1374 (C=O str, ketone), 851 ( aromatic ring); 761 (C-F str); 1H NMR (500 MHz, DMSO-d6) δ 7.84 - 7.72 (m, 4H), 7.49 (s, 2H), 7.46 - 7.40 (m, 4H), 7.32 - 7.29 (m, 3H), 7.11 (s, 3H), 7.02 - 6.96 (m, 4H), 6.57 (s, 2H), 3.60 - 3.56 (m, 4H). 13C NMR (126 MHz, DMSO-d6) δ: 171.02, 156.01, 155.57, 135.92, 131.98, 128.86, 127.93, 127.38, 126.94, 124.45, 119.01 21.49. Mass: Actual: 309; found: 310 (M+1).
(E)-5-(4-fluorostyryl)-2-picolinoyl-2,4-dihydro-3Hpyrazol-3-one (11)
C17H12FN3O2; yield: 81%; MP: 125 - 127OC; Rf: 0.88; IR (KBr) cm-1: 3353 (CH str, CH=CH); 2985 (CH str, aromatic); 1585 (C=O str, amide); 1455 (C=O str, ketone), 851 ( aromatic ring); 718 (C-F str); 1H NMR (500 MHz, DMSO-d6) δ 7.84 - 7.72 (m, 4H), 7.49 (s, 2H), 7.46 - 7.40 (m, 4H), 7.32 - 7.29 (m, 3H), 7.11 (s, 3H), 7.02 - 6.96 (m, 4H), 6.57 (s, 2H), 3.60 - 3.56 (m, 4H). 13C NMR (126 MHz, DMSO-d6) δ: 170.30, 134.50, 129.99, 129.31, 128.03, 127.26, 127.03, 124.45, 119.01, 64.86, 21.49. Mass: Actual: 309; found: 307 (M-2).
(E)-5-(2-fluorostyryl)-2-(furan-2-carbonyl)-2,4dihydro-3H-pyrazol-3-one (31)
C13H11ClN2O2; yield: 81%; MP: 125 - 127OC; Rf: 0.83; IR (KBr) cm-1: 2916 (CH str, aromatic); 1698 (C=O str, amide); 1546 (C=O str, ketone), 862 ( aromatic ring); 725 (C-F str); 1H NMR (500 MHz, DMSO-d6) δ 7.37 - 7.21 (m, 3H), 7.19 - 7.07 (m, 3H), 3.62 (s, 2H), 2.39 (s, 3H); 13C NMR (126 MHz, DMSO-d6) δ: 170.26, 156.96, 156.43, 153.87, 135.02, 133.95, 130.09, 129.56, 128.86, 127.99, 127.42, 127.12, 126.76, 127.12, 126.73, 124.55, 123.71. Mass: Actual: 298; found: 297 (M-1).
(E)-2-(furan-2-carbonyl)-5-(4-methoxy-3methylstyryl)-2,4-dihydro-3H-pyrazol-3-one (37)
C18H16N2O4; yield: 81%; MP: 134 - 137OC; Rf: 0.85; IR (KBr) cm-1: 2917 (CH str, aromatic); 1602 (C=O str, amide); 1512 (C=O str, ketone), 832 ( aromatic ring); 1H NMR (500 MHz, DMSO-d6) δ 8.31 (s, 1H), 7.51 (s, 1H), 7.25 - 7.09 (m, 3H), 6.99 (s, 1H), 6.79 (d, J = 18.2 Hz, 2H), 6.43 (s, 1H), 3.83 - 3.79 (m, 3H), 3.62 - 3.58 (m, 2H), 2.35 - 2.31 (m, 3H). 13C NMR (126 MHz, DMSO-d6) δ: 170.82, 156.02, 155.86, 135.05, 131.14, 130.81, 129.03, 128.36, 127.73, 124.57, 118.94, 71.55, 64.86, 22.62. Mass: Actual: 324; found: 325 (M-1).
(E)-2-(4-chlorobenzoyl)-5-(4-hydroxystyryl)-2,4dihydro-3H-pyrazol-3-one (44)
C18H13ClN2O3; yield: 81%; MP: 114 - 117OC; Rf: 0.97; IR (KBr) cm-1: 2917 (CH str, aromatic); 1602 (C=O str, amide); 1512 (C=O str, ketone), 832 ( aromatic ring); 1H NMR (500 MHz, DMSO-d6) δ 7.73 (d, J = 7.5 Hz, 2H), 7.45 (d, J = 7.5 Hz, 2H), 7.18 (dd, J = 21.9, 11.3 Hz, 3H), 6.74 (dd, J = 11.3, 3.8 Hz, 3H), 4.04 (s, 1H), 3.58 (s, 2H). 13C NMR (126 MHz, DMSO-d6) δ: 171.82, 155.82, 154.63, 135.90, 131.04, 130.07, 129.03, 128.56, 126.03, 123.86, 22.62. Mass: Actual: 340; found: 341 (M+1).
(E)-2-(4-chlorobenzoyl)-5-(2-chlorostyryl)-2,4dihydro-3H-pyrazol-3-one (47)
C18H12Cl2N2O2; yield: 81%; MP: 114 - 117OC; Rf: 0.97; IR (KBr) cm-1: 2856 (CH str, aromatic); 1569 (C=O str, amide); 912 ( aromatic ring); 746 (C-Clstr); 1H NMR (500 MHz, DMSO-d6) δ 7.80 - 7.66 (m, 2H), 7.44 (t, J = 8.2 Hz, 3H), 7.27 (d, J = 8.9 Hz, 2H), 7.13 (d, J = 6.2 Hz, 2H), 6.64 (s, 1H), 3.61 - 3.57 (m, 2H).13C NMR (126 MHz, DMSO-d6) δ: 171.82, 154.43, 154.17, 133.95, 131.13, 130.71, 129.03, 128.76, 127.91, 125.94, 22.62. Mass: Actual: 358; found: 359 (M+1).
(E)-2-(4-chlorobenzoyl)-5-(2-fluorostyryl)-2,4dihydro-3H-pyrazol-3-one (48)
C18H12Cl2FN2O2; yield: 81%; MP: 136- 138OC; Rf: 0.86; IR (KBr) cm-1: 2849 (CH str, aromatic); 1628 (C=O str, amide); 1512 (C=O str, ketone), 912 ( aromatic ring); 746 (C-Clstr); 1H NMR (500 MHz, DMSO-d6) δ 7.80 - 7.66 (m, 2H), 7.44 (t, J = 8.2 Hz, 3H), 7.27 (d, J = 8.9 Hz, 2H), 7.13 (d, J = 6.2 Hz, 2H), 6.64 (s, 1H), 3.61 - 3.57 (m, 2H). 13C NMR (126 MHz, DMSO-d6) δ: 171.82, 156.42, 155.17, 135.31, 131.82, 130.64, 129.43, 126.98, 126.43, 124.16, 109.01, 22.62. Mass: Actual: 342; found: 342 (M-1).
(E)-2-(4-chlorobenzoyl)-5-(4-fluorostyryl)-2,4dihydro-3H-pyrazol-3-one (50)
C18H12ClFN2O2; yield: 81%; MP: 132- 134OC; Rf: 0.86; IR (KBr) cm-1: 2855 (CH str, aromatic); 1695 (C=O str, amide); 1567 (C=O str, ketone), 856 ( aromatic ring); 729 (C-Clstr); 1H NMR (500 MHz, DMSO-d6) δ 7.80 - 7.66 (m, 2H), 7.44 (t, J = 8.2 Hz, 3H), 7.27 (d, J = 8.9 Hz, 2H), 7.13 (d, J = 6.2 Hz, 2H), 6.64 (s, 1H), 3.61 - 3.57 (m, 2H). 13C NMR (126 MHz, DMSO-d6) δ: 171.79, 155.57, 153.99, 135.89, 131.14, 130.45, 129.03, 128.67, 127.84, 123.96, 109.01, 22.62. Mass: Actual: 342; found: 342.
MTT assay for cell viability
The MTT test was utilized to gauge cell viability. The cells were arranged in a 96-well plate, with two times the number of cells per well. The concentration of the sample (25, 50, 100, 250, and 500µg/ml) was compared to normal control and the percentage of cell viability. The concentration of 100µg/ml that gives the highest viability was found by averaging the results of the triple trials. Aβµ25-35 (10-50 mol/L) treatment to SH-SY5Y cells for 48 hours led to a dose-dependently substantial reduction in cell viability in the medium. The following research investigated the protective effects of synthetic pyrazole derivatives against Aβ2535 neurotoxicity in SH-SY5Ycells using the 25 µmol/L Aβ25-35. To choose a synthetic drug concentration that won’t cause cytotoxicity when studying how synthetic compounds affect Aβ25-35 neurotoxicities, we looked at how different synthetic chemical concentrations such as 25, 50, 100, 250, and 500 µg/ml affected the viability of neuroblastoma cell SH-SY5Y. Pretreatment of SHSY5Y cells with 25-500g/ml synthetic compounds for 30 minutes dramatically reduced the cytotoxicity caused by Aβ25-35 and increased cell survival (Table III).
DISCUSSION
The outcome unequivocally demonstrates that every chemical interacts with the targeted enzyme AChE in a promising manner. The presence of the lipophilic component of the aromatic heterocyclic ring is the primary cause of the contact. Compound 48 (-8.3 kcal/mol) exhibits the highest binding affinity for the AChE enzyme in the docking data (Table I) when compared to the normal control. Two typical hydrogen bonds between the carbonyl oxygen and the Asn 491 residue were formed by this molecule. Hydrophobic bonds allow amino acids like Lys 493, Gly 490, Thr 501, Cys 504, Gly 531, Tyr 537, and Asn 631 to interact with ligands. Because ligands are aromatic, this interaction occurs. The same kind of work was carried out by the author (Pourtaher, Hasaninejad, Iraji, 2022) and his group reported that the compound substituted with the amide group shows competitive inhibition activity towards AChE and does not produce any interaction with BuChE, similarly, the compound 48 have amide moiety in its structure and it also shows the highest interaction with the selected enzyme and may show the competitive inhibition activity towards AChE. The remaining compounds show good to moderate binding affinities to the selected enzymes. These amino acids have been consistently implicated in the interaction of ligands with AChE inhibitors, as well as in the suppression of Acetylcholinesterase inhibitors’ ligand binding domain. Figure 3 to 12 shows the docking image of the compounds. Based on the docking score of the following derivatives 8, 9, 10, 11, 31, 37, 44, 47, 48 and 50 were selected for the conventional synthesis and it was further evaluated for the cytotoxicity studies against the neuroblastoma cells.
The in-silico ADMET properties of the ligands were assessed using SWISS ADME software (Daina, Michielin, Zoete, 2017). The molecular weight of the compounds ranged from 298 to 359. The predicted number of hydrogen bond donors is zero. The expected number of hydrogen bond acceptors ranges from two to five. The anticipated octanol/water partition coefficient ranged from 2 to 2.8, with 1 to 3 probable metabolic activities. The number of infractions of Lipinski’s rule of five was 0. All of the compounds are readily absorbed in the human mouth and enter the BBB. Almost the majority of the compounds’ properties are within permissible limits (Lipinski et al., 2001; Gleeson, 2008). Table II shows data on the compounds’ in-silico ADMET properties.
The final derivatives of chalcone hybrid pyrazolone derivatives were obtained by a three-step method. TLC and melting point determination were used to evaluate the reaction’s completeness and the purity of the produced compounds. Several spectrum analyses were used to elucidate the structure of produced molecules. The IR, 1H NMR, 13C NMR, and Mass spectra are employed to analyze the produced molecules. The 1H NMR spectra of the target compounds, chalcone-bearing pyrazole scaffolds were distinguished by a single signal at 6.61 - 6.49 ppm for the -CH=CH- proton. The remaining aromatic protons range between 7.86 and 7.10 (Hassan et al., 2022; Alkahtani et al., 2023). The spectrum analysis revealed that all substances produced corresponding signals in every spectral data.
Cell viability was assessed using the MTT assay. A 96-well plate was used for plating the cells, with 2.0 x 104 cells per well. The concentration of the cells (25, 50, 100, 250, and 500µg/ml) was compared to the control and the percentage of cell viability. The concentration of synthetic chemicals at 100µg/ml yields the greatest viability of cells, as determined by averaging the results of the triple trials. Human SH-SY5Y neuroblastoma cells were subjected to the MTT assay method for the in vitro cytotoxicity assessment. For instance, excessive exposure of neurons to the amino acid glutamate causes neuronal damage and apoptosis (Crupi, Impellizzeri, Cuzzocrea, 2019). The neuroblastoma SH-SY5Y cell culture has been widely employed as an in vitro model for AD research (de Medeiros et al., 2019), including the examination of the Aβ1-42 inhibitory action of several chemical substances (Suarez-Montenegro et al., 2021). This work investigated the neuroprotective effects of pyrazolone derivatives pre-treatment in Aβ and l-glutamate-induced SH-SY5Y cells. Treatment of SH-SY5Y cells with Aβ25-35 (10-50 mol/L) for 48 hours significantly reduced cell viability in the medium in a dose-dependent manner. This study shows that the synthetic pyrazolone derivatives can protect SH-SY5Y cells from Aβ25-35 neurotoxicity at a concentration of 25 µmol/L. To avoid cytotoxicity when evaluating the effects of synthetic substances on Aβ25-35 neurotoxicity, we tested the viability of neuroblastoma cell SH-SY5Y at various concentrations (25, 50, 100, 250, and 500 µg/ml). Pretreatment of SH-SY5Y cells with 25-500g/ml synthetic chemicals for 30 minutes significantly reduced cytotoxicity from Aβ25-35 and boosted cell survival (Table III).
Among the synthesized compounds, (E)-2-(4chlorobenzene)-5-(2-fluoro styryl)-2,4-dihydro-3Hpyrazol-3-one (48) was discovered to be the most notable for their invitro cytotoxicity activity against Human SH-SY5Y neuroblastoma cell lines, while the rest of the synthesized derivatives were determined to be moderate. Many researchers show that Aβ induces apoptosis in various cell types in vitro (Feng, Zhang, 2004). Aβ25-35 is commonly used in in-vitro models of Alzheimer’s disease because of its neurotoxic effects, which are similar to those of Aβ1-40/42. These effects include learning and memory deficits, neuronal death, cholinergic nerve dysfunction, and oxidative damage (Olariu et al., 2001; Tohda, Tamura, Konatsu, 2003).
CONCLUSION
Data from spectroscopy, chemistry, and physical analysis were used to validate the structure of recently created compounds. The synthesized medicines showed a comparable manner of protein binding to the acetylcholinesterase protein’s active region (PDB ID: 4EY6) in molecular docking tests. Consequently, the anticipated docking energies demonstrate a promising binding energy with the enzyme cholinesterase. The viability of each material was examined in vitro using Human SH-SY5Y neuroblastoma cell lines. It was discovered that Compound 48(-8.3k/cal) was the most successful against the tested cell lines. The ADMET prediction also revealed that the compound is less hazardous and has better pharmacokinetic properties. Based on the binding score, the computational investigation concluded that compound 48 exhibited better binding energy than other analogs. The synthesized compounds 8, 11, and 48 considerably prevented Aβ2535 neuron toxicity and lowered the proportion of SHSY5Y cells that are apoptotic. The quest for novel cholinesterase inhibitors is being advanced by work being done. More derivatives and comprehensive, indepth studies on in vivo activity may be conducted to construct an SAR for rational exploration. As a promising approach for Alzheimer’s disease, chalcone merging pyrazolone derivatives appears to require further investigation, according to the current study.
ACKNOWLEDGMENTS
The authors express their gratitude to the faculties of Kamalakshi Pandurangan College of Pharmacy and Dr. T. Saraswathy, Assistant Professor, Department of Pharmacy in Madras Medical College for their valuable suggestions and timely help.
-
Funding: The study was carried out without funding from any external source.
Ethics Approval and Consent to Participate:
Since no animal activity and clinical studies are involved in our work, the question of ethics approval does not arise.
Abbreviations:
REFERENCES
- Alkahtani HM, Almehizia AA, Al-Omar MA, Obaidullah AJ, Zen AA, Hassan AS, et al. In Vitro evaluation and bioinformatics analysis of schiff bases bearing pyrazole scaffold as bioactive agents: Antioxidant, antidiabetic, anti-alzheimer, and anti-arthritic. Molecules. 2023; 28:7125.
- Ansari A, Ali A, Asif M, Shamsuzzaman. Review: biologically active pyrazole derivatives. New J Chem. 2017;41( 1):16-41.
- Baashen MA, Abdel-Wahab BF, ElHiti GA. A simple procedure for the synthesis of novel 3-(benzofuran2-yl)pyrazole-based heterocycles. Chem Pap. 2017; 71:2159-2166.
- Benazzouz Touami A, Chouh A, Halit S, Terrachet Bouaziz S, MakhloufiChebli M, IghilAhrizK, et al. New Coumarin-Pyrazole hybrids: Synthesis, Docking studies and Biological evaluation as potential cholinesterase inhibitors. J Mol Struct. 2022;1249:131591.
- Carvalho C, Moreira PI. Metabolic defects shared by Alzheimer’s disease and diabetes: A focus on mitochondria. Curr Opin Neurobiol. 2023;79:102694.
-
Crupi R, Impellizzeri D, Cuzzocrea S. Role of metabotropic glutamate receptors in neurological disorders. Front Mol Neurosci. 2019;12:20. https://doi.org/10.3389/fnmol.2019.00020
» https://doi.org/10.3389/fnmol.2019.00020 - Daina A, Michielin O, Zoete V. Swiss ADME: A free web tool to evaluate pharmacokinetics, druglikeness and medicinal chemistry friendliness of small molecules. Sci Rep. 2017;7:42717.
-
de Medeiros LM, De Bastiani MA, Rico EP, Schonhofen P, Pfaffenseller B, Wollenhaupt-Aguiar B, et al. Cholinergic differentiation of human neuroblastoma SH-SY5Y cell line and its potential use as an in vitro model for Alzheimer’s disease studies. Mol Neurobiol. 2019;56:7355-67. https://doi.org/10.1007/S12035-019-1605-3
» https://doi.org/10.1007/S12035-019-1605-3 - Dube PN, Bule SS, Ushir YV, Kumbhare MR, Dighe PR. Synthesis of novel 5-methylpyrazol-3-one derivatives and there in vitro cytotoxic evaluation. Med Chem Res 2015;24(3):1070-6.
- Elgiushy HR, Mohamed SH, Taha H, Sawaf H, Hassan Z, AbouTaleb NA, et al. Identification of a promising hit from a new series of pyrazolo[1,5-a] pyrimidinebased compounds as a potential anticancer agent with potent CDK1 inhibitory and pro-apoptotic properties through a multistep in vitro assessment. Bioorg Chem. 2022; 120:105646.
- Feng Z, Zhang JT. Melatonin reduces amyloid b-induced apoptosis inpheochromeocytoma (PC12) cells. J Pineal Res. 2004;37:257-66.
- García Ayllón MS, Small DH, Avila J, Sáez Valero J. Revisiting the role of acetylcholinesterase in Alzheimer’s disease: cross-talk with P-tau and β-amyloid. Front Mol Neurosci. 2011;4:22.
- Gleeson MP. Generation of a set of simple, interpretable ADMET rules of thumb. J Med Chem. 2008;51:817-834.
- Hassan AS, Masoud DM, Sroor FM, Askar AA. Synthesis and biological evaluation of pyrazolo[1,5-a] pyrimidine-3-carboxamide as antimicrobial agents. Med Chem Res. 2017;26(11):2909-2919.
- Hassan AS, Morsy NM, Aboulthana WM, Ragab A. In vitro enzymatic evaluation of some pyrazolo[1,5-a] pyrimidine derivatives: design, synthesis, antioxidant, anti-diabetic, anti-Alzheimer, and anti-arthritic activities with molecular modelling simulation. Drug Dev Res. 2023;84(1):3-24.
- Hassan AS, Morsy NM, Awad HM, Ragab A. Synthesis, molecular docking, and in silico ADME prediction of some fused pyrazolo [1,5-a] pyrimidine and pyrazole derivatives as potential antimicrobial agents. J Iran Chem Soc. 2022;19:521-545.
- Hassan AS, Moustafa GO, Morsy NM, Abdou AM, Hafez TS. Design, Synthesis and antibacterial activity of N-aryl-3-(arylamino)-5-(((5-substituted furan-2yl) methylene) amino)-1H-pyrazole-4-carboxamide as Nitrofurantoin® analogues. Egypt J Chem. 2020;63( 11):4469-4481.
- Hippius G, Neundorfer, The discovery of Alzheimer’s disease. Dialog Clin Neurosci. 2003;5:101-108.
- Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 2001;46:3-26.
- Liu HR, Liu XJ, Fan HQ, Tang JJ, Gao XH, Liu WK. Design, synthesis and pharmacological evaluation of chalcone derivatives as acetyl cholinesterase inhibitors. Bioorg Med Chem. 2014;22:6124-6133.
- Liu HR, Zhou C, Fan HQ, Tang JJ, Liu LB, Gao XH, et al. Novel potent and selective acetylcholinesterase inhibitors as potential drugs for the treatment of Alzheimer’s disease: Synthesis, pharmacological evaluation, and molecular modelling of amino-alkylsubstituted fluoro-chalcones derivatives. Chem Biol Drug Des. 2015;86:517-522.
- Mukhtar SS, Hassan AS, Morsy NM, Hafez TS, Hassaneen HM, Saleh FM. Overview on Synthesis, Reactions, Applications, and Biological Activities of Schiff Bases. Egypt J Chem. 2021;64(11):6541-6554.
- Mukhtar SS, Morsy NM, Hassan AS, Hafez TS, Hassaneen HM, Saleh FM. A Review of Chalcones: Synthesis, Reactions, and Biological Importance. Egypt J Chem. 2022;165(8):973-395.
- Nalini N, Rao S, Manikandan, Murali K, Kavitha. Synthesis and anti-microbial screening of some novel sulphonamide containing heterocyclic derivatives. J Pharm Res. 2010:3(9):2258-2261.
- Naglah AM, Askar AA, Hassan AS, Khatab TK, Al -Omar MA, Bhat MA. Biological evaluation and molecular docking with in silico physicochemical, pharmacokinetic and toxicity prediction of pyrazolo[1,5-a]pyrimidines. Molecules. 2020;25(6):1431.
- Nidhar M, Khanam S, Sonker P, Gupta P, Mahapatra A, Patil S, et al. Click inspired novel pyrazole-triazolepersulfonimide & pyrazole-triazole-aryl derivatives; Design, synthesis, DPP-4 inhibitor with potential antidiabetic agents. Bioorg Chem. 2022;120:105586.
- Olariu A, Tran MH, Yamada K, Mizuno M, Hefco V, Nabeshima, T. Memory deficits and increased emotionality induced b-amyloid (25-35) are correlated with the reduced acetylcholine release and altered phorbol dibutyrate binding in the hippocampus. J Neural Transm 2001;108:1065-79.
- Pourtaher H, Hasaninejad A, Iraji A. Design, synthesis, in silico and biological evaluations of novel poly substituted pyrroles as selective acetylcholinesterase inhibitors against Alzheimer’s disease. Sci Rep. 2022;12( 1):15236.
- Rampa A, Montanari S, Pruccoli L, Bartolini M, Falchi F, Feoli A, et al. Chalcone-based carbamates for Alzheimer’s disease treatment, Future Med Chem. 2017; 9:749-764.
- Ramsay RR, PopovicNikolic MR, Nikolic K, Uliassi E, Bolognesi ML. A perspective on multi-target drug discovery and design for complex diseases. Clin Transl Med. 2018;7(1).
- Reddy GM, Zyryanov GV, Basha NM, Munagapati VS, Wen JC, Gollakota ARK, et al. Synthesis of pyrazole tethered oxadiazole and their analogs as potent antioxidant agents. J Heterocycl Chem. 2022;59(11):1879-1887.
- Sharma V, Mehdi MM. Oxidative stress, inflammation and hormesis: The role of dietary and lifestyle modifications on aging. Neurochem Int. 2023;164:105490.
-
Suarez-Montenegro ZJ, Alvarez-Rivera G, SanchezMartinez JD, Gallego R, Valdes A, Bueno M, et al. Neuroprotective effect of terpenoids recovered from olive oil by-products. Foods. 2021;10(7):1507. https://doi.org/10.3390/foods10071507
» https://doi.org/10.3390/foods10071507 - Tohda C, Tamura T, Konatsu K. Repair of amyloid b(25-35)- induced memory impairment and synaptic loss by a Kampo formula, Zokumei-to. Brain Res. 2003;990:141-7.
- Ucar G, Gokhan N, Yesilada A, Bilgin A. 1-Nsubstituted thiocarbamoyl-3-phenyl-5-thienyl-2-pyrazolines: a novel cholinesterase and selective monoamine oxidase B inhibitors for the treatment of Parkinson’s and Alzheimer’s diseases. Neurosci Lett. 2005;382:327-31.
- Vogel WH, Schokens BA, Sandow J, Muller G, Vogel WF. Drug discovery and evaluation - pharmacological assays, 2nd ed. New York: Springer-Verlag, 2002, pp 599-601.
- Wang HQ, Sun XB, Xu YX, Zhao H, Zhu QY, Zhu CQ. Astaxanthin up regulates heme oxygenase-1 expression through ERK1/2 pathway and its protective effect against beta-amyloid-induced cytotoxicity in SH-SY5Y cells. Brain Res. 2010;1360:159-167.
-
Wang J, Wang W, Kollman PA, Case DA. Automatic atom type and bond type perception in molecular mechanical calculations. J Mol Graph Model. 2006 Oct;25(2):247-60. doi: 10.1016/j.jmgm.2005.12.005. Epub 2006 Feb 3. PMID: 16458552.
» https://doi.org/10.1016/j.jmgm.2005.12.005. - Yu H, Yao L, Zhou H, Qu S, Zeng X, Zhou D, et al. Neuroprotection against Aβ25-35-induced apoptosis by Salvia miltiorrhiza extract in SH-SY5Y cells. Neuro Chem Int. 2014;75:89-95.
Edited by
-
Associated Editor: Gabriel Araújo
Publication Dates
-
Publication in this collection
05 Dec 2025 -
Date of issue
2025
History
-
Received
16 Apr 2024 -
Accepted
13 Nov 2024

 Design, synthesis, in-silico, and in-vitro assessment of pyrazolone-merged chalcone derivatives as anti-alzheimer agents
Design, synthesis, in-silico, and in-vitro assessment of pyrazolone-merged chalcone derivatives as anti-alzheimer agents