Abstract
Sjögren’s syndrome (SS) damages exocrine glands, and Lilium brownii var. viridulum Baker (Lilii Bulbus, LB) shows potential as a therapeutic agent. This study evaluated LB’s efficacy in alleviating xerostomia using non-obese diabetic/severe combined immunodeficiency (NOD/SCID) mice and human salivary gland acinar (NS-SV-AC) cells. In vitro, NS-SV-AC cells were treated with LB (1, 5, 10, 50, and 100 µg/mL) and 5-aza-2′-deoxycytidine (2 µM/mL) for 48 h. Cell viability, fluid secretion, and aquaporin-5 (AQP-5) expression were assessed. In vivo, thirty 20-week-old NOD/SCID mice received LB orally (100, 500, and 1,000 mg/kg) for 4 weeks, with salivary secretion rates measured. AQP-5 and M3 muscarinic acetylcholine receptor (M3R) expression and inflammatory mediator levels were determined using western blotting and enzyme-linked immunosorbent assay. Histopathological examination of salivary glands was also performed. LB significantly increased NS-SV-AC cell proliferation, fluid secretion, and AQP-5 expression. In NOD/SCID mice, LB reduced anti-SSA/Ro and anti-SSB/La antibodies, tumor necrosis factor-α, interferon-γ, and interleukin-6, while increasing AQP-5 and M3R expression. This resulted in increased salivary secretion and reduced glandular inflammation. LB extract appears promising for managing oral health by enhancing salivation, upregulating AQP-5, and modulating immune-inflammatory responses.
Keywords:
Sjögren’s syndrome; Salivary; Dry mouth; Lilii; Lilium
INTRODUCTION
Sögren’s syndrome (SS) is a persistent systemic autoimmune disorder associated with the production of autoantibodies in the blood. It damages tissues by allowing lymphocytes to invade exocrine glands, including the salivary and lacrimal glands and other organs (Vivino, 2017). Patients primarily experience issues, such as dry eyes, dry mouth, fatigue, musculoskeletal pain, and anemia (Vivino et al., 2019). SS is categorized into primary and secondary subtypes depending on the presence of other systemic connective tissue diseases, such as rheumatoid arthritis and systemic lupus erythematosus (Sebastian, Szachowicz, Wiland, 2019). SS diagnosis is generally based on the subjective symptoms of ocular and oral dryness, objective signs of histopathological changes in the minor salivary glands, and the presence of serum autoantibodies (Patel, Shahane, 2014).
To date, no drug has ameliorated SS progression (Kwok, 2015). SS treatment aims to relieve symptoms, prevent complications, and suppress aberrant immune responses (Vivino, 2017). Local lubricants, including artificial tears and saliva, and stimulants, such as cevimeline and pilocarpine are used to relieve dryness. Additionally, corticosteroids and immunosuppressants, such as mizoribine and methotrexate can be used for systemic treatment (Sumida et al., 2018). However, long- term administration of these therapies can induce renal and hematologic toxicities, as well as various side effects, such as weight gain, hypertension, and hyperglycemia (Bamoulid et al., 2015).
Botanical drugs and their extracts have traditionally been used to manage SS and alleviate xerostomia symptoms. In the SS mouse model, polysaccharides from dried Ophiopogon japonicus (Thunb.) Ker Gawl. root tuber [Asparagaceae; Liriopis Tuber] improved the histological changes of salivary gland and reduced serum interferon-γ (IFN-γ) (Wang et al., 2007), and total glucosides from the dried Paeonia lactiflora Pall. roots [Paeoniaceae; Paeoniae Radix] reduced the autoantigen and inflammatory cytokine levels (Li et al., 2020). Unlike the single active components in modern drugs targeting a single target, the active ingredients and mechanisms of botanical drugs remain obscure. However, they are emerging as potential therapeutic approaches for SS, as they can act as multi-component and multi-target therapies, helping to restore and maintain the overall balance and function with less toxicity (Kim et al., 2015).
Lilii Bulbus (LB) is the dried fleshy scale leaf of Lilium brownii var. viridulum Baker [Liliaceae] and has been mainly used as an antidepressant and anti-tussive, as well as to treat cough, hemostasis, and abdominal pain, and to regulate immunity in traditional Chinese medicine. Belonging to the genus Lilium, LB contains various chemical compounds, including steroidal saponins, sterols, polysaccharides, and alkaloids, and possesses pharmacological effects, such as antioxidant, antitumor, and immunomodulatory activities (Zhou, An, Huang, 2021). Moreover, LB extracts exert anti-inflammatory effects by inhibiting the nuclear factor kappa B and mitogen-activated protein kinase pathways (Han et al., 2018). In addition, LB polysaccharides improved non- specific immune reactions in mice immunosuppressed with cyclophosphamide (Hu et al., 2007). It is reasonable to assume that LB positively affects oral health through its immunomodulatory activity, in combination with its antioxidant and anti-inflammatory effects, despite the current lack of research on the effects of LB on SS.
This study aimed to examine the potential therapeutic effects of LB on SS by assessing salivary flow rates, evaluating water channel protein aquaporin-5 (AQP-5) expression, measuring serum autoimmune-related factor (anti-SSA/Ro and anti-SSB/La autoantibodies) and pro- inflammatory cytokine (tumor necrosis factor-α [TNF-α], IFN-γ, and interleukin [IL]-6) levels, and histologically analyzing salivary glands.
MATERIAL AND METHODS
Preparation of materials
Water extract of LB
The botanical drug LB was purchased from Bonchomaru (Seoul, Republic of Korea). The dried LB sample (50 g) was boiled in 1 L distilled water at 100 °C for 2 h and evaporated under pressure (98 kPa) using an electric extractor (COSMOS-660; Kyungseo Machine Co., Incheon, Republic of Korea). The suspension was filtered using a standard sieve (No. 270, 53 μm; Chung Gye Sang Gong Sa, Seoul, Republic of Korea). The extract solution was freeze-dried to obtain a 14.1 g powder sample. The dried extract was dissolved in distilled water (Millipore, Billerica, MA, USA) and vortexed at 20-25 °C for 2 min.
Human salivary gland acinar cells
Human salivary gland acinar (NS-SV-AC) cells were purchased from the American Type Culture Collection (Manassas, VA, USA) and cultured in Dulbecco’s minimal essential medium (Gibco-BRL, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (Gibco-BRL) and penicillin (100 U/mL)/streptomycin (100 μg/mL) at 37 °C in a 5% CO2 incubator.
Animals and experimental groups
We used 20-week-old non-obese diabetic/severe combined immunodeficiency (NOD/SCID) mice as the SS mouse model and BALB/c female mice as normal controls (SAERONBIO INC., Seoul, Republic of Korea). The experimental animals were bred in a pathogen-free controlled environment with a 12-h light/dark cycle at the Center for Laboratory Animal Care and Use at Kyung Hee University, Republic of Korea. All animal care and experimental procedures were performed in compliance with the “Guide for the Care and Use of Laboratory Animals,” and the protocol was approved by the Institutional Animal Care and Use Committee of Kyung Hee University, Republic of Korea (KHUASP (SE)-19-204). The mice were randomly divided into five groups (n=6 per group): normal (N: BALB/c mice), control (C: NOD/SCID mice), and 100, 500, and 1,000 mg/kg oral LB treatment 5 d/week for 4 weeks. The animals were weighed weekly to adjust the gavage volume and to evaluate their overall health.
Pharmacological studies
Cell viability measurement using
3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide assay
Cell viability was determined by quantifying the formation of blue formazan obtained from the yellowish 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide (MTT; Sigma-Aldrich Corp., St. Louis, MO, USA) using mitochondrial dehydrogenases, which are functional enzymes exclusively present in viable cells. Acinar cells (5×105 cells/mL) were seeded in a 96-well plate and simultaneously treated with 1, 5, 10, 50, or 100 μg/mL LB and 2 µM/mL 5-aza-2′-deoxycytidine (5′ Aza; Sigma-Aldrich) for 48 h at 37 ºC. The normal and control cells were treated with 10 μL/well phosphate- buffered saline (DPBS, LB001-02; Welgene, Inc., Gyeongsan, Republic of Korea) and only 2 µM/mL 5′ Aza, respectively. The culture medium was then replaced with fresh medium, and the cells were incubated with 0.5 mg/mL MTT solution for 4 h. The supernatant was removed, and the formazan blue formed in the cells was dissolved in dimethyl sulfoxide (Sigma-Aldrich). Finally, the optical density of the formazan crystals at 540 nm was determined using an enzyme-linked immunosorbent assay (ELISA) plate reader (MRX; Dynatech Laboratories, Chantilly, VA, USA).
Fluid secretion measurement
Net fluid secretion rates were measured in both normal and control acinar cells cultured in six-well Transwell-Col culture chambers (Coster, Cambridge, MA, USA) until confluency. The apical fluid and basolateral chamber medium were replaced with 0.4 mL hyperosmotic (400 mOsm) and isosmotic (300 mOsm) media, respectively. After 4 h, the volume of liquid collected from the apical surface was measured using a calibrated pipette and the net fluid secretion rate was calculated.
For the in vivo study, each experimental mouse was anesthetized via intraperitoneal urethane administration (1.3 g/kg). Saliva was collected from the floor of the mouth for 10 min using a capillary paper plug and its weight was measured before and after collection. The amount of salivary fluid was normalized to time (10 min) and body weight (1 g).
Protein extraction and western blot analysis
To extract total protein, NS-SV-AC cells and NOD/ SCID mice salivary glands were treated with lysis buffer at 4 °C for 1 h. After centrifugation at 13,000× g using a Smart R17 refrigerated microcentrifuge (Hanil BioMed, Inc., Seoul, Republic of Korea) at 4 °C for 20 min, the insoluble substances were discarded. Protein concentration in the cell lysate was measured using a protein assay. Equal protein quantities were separated using 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis at 120 V for 2 h, transferred onto a membrane, and subsequently blocked with 5% skim milk in Tris-buffered saline (10 mM Tris-HCl, pH 7.4) with 0.5% Tween-20 at 20-25 °C for 1 h. The membranes were then incubated with rabbit anti-AQP-5 (1:1,000; Santa Cruz Biotechnology, Inc., Dallas, TX, USA), anti-M3 muscarinic acetylcholine receptor (M3R, 1:500; Abcam, Cambridge, England), and anti-β-tubulin (1:1,000, Santa Cruz Biotechnology).
Immunofluorescence staining
Acinar cells smeared on glass slides were cultured with 5′ Aza and then treated with LB for 48 h. The cells were fixed in 2% paraformaldehyde and incubated with an anti-AQP-5 antibody (Santa Cruz Biotechnology) for 4 h at 20-25 °C. A cyanine dye-conjugated anti-rabbit immunoglobulin G (IgG) antibody (Invitrogen, Carlsbad, CA, USA) was used as the secondary antibody. The immunostained cells were treated with mounting medium containing 4′,6-diamidino-2-phenylindole and observed using an Olympus immunofluorescence microscope (Olympus Optical Co., Ltd., Tokyo, Japan).
Autoantibody profiling
IgG was detected in 1:50 diluted serum samples using mouse anti-SSA/Ro and anti-SSB/La ELISA kits (Signosis, Inc., Santa Clara, CA, USA) according to the manufacturer’s instructions.
Pro-inflammatory cytokine measurement
A mouse TNF-α, IFN-γ, and IL-6 ELISA kit (R&D Systems, Inc., Minneapolis, MN, USA) was used for analyzing the serum TNF-α, IFN-γ, and IL-6 levels in the NOD/SCID mouse model, according to the manufacturer’s instructions.
Histopathological observation of the salivary glands
Salivary glands were extracted from euthanized mice, fixed using 4% paraformaldehyde, and embedded in optimal cutting temperature compound. Each dissected serial section was 5 µm thick. Hematoxylin staining was used to examine the histological changes in the salivary glands.
Statistical analysis
Statistical analyses were performed using GraphPad Prism 4.0.3 (GraphPad Software, Inc., San Diego, CA, USA) and the Statistical Package for the Social Sciences software (version 12.0; SPSS, Chicago, IL, USA). Data are expressed as the mean ± standard deviation (SD). One-way analysis of variance followed by Dunnett’s test was performed to compare the mean differences between groups. Statistical significance was set at P<0.05.
RESULTS
In vitro evaluation of cell protective effects
LB provided protection against 5' Aza-induced toxicity, promoting the proliferation rate of acinar cells
The effects of LB on the NS-SV-AC cell proliferation rate were measured using the MTT assay, which evaluates cellular metabolic activity as an indicator of cell viability. Before the experiment, growth-controlled acinar cells were incubated in a starvation medium for 24 h and cultured with LB and 2 µM/mL 5′ Aza for 48 h. The viability of cells co-treated with 5′ Aza and 1, 5, 10, 50, or 100 µg/mL LB was significantly enhanced than that of only 5′ Aza- treated cells (52.4±2.7%, P<0.01; 49.0±2.5%, P<0.05; 52.1±2.1%, P<0.05; 49.1±5.1%, P<0.05; 56.3±2.5%, P<0.01 vs. 39.2±6.4%, respectively; Figure 1).
LB increased the fluid secretion rate in acinar cells
The effect of LB on the secretion rate of acinar cells was assessed. The net fluid secretion rate of 2 µM/mL 5′ Aza-treated cells was lower than that of untreated normal acinar cells (1.2±0.3 vs. 1.5±0.5 µL/cm2·h); however, the difference was not statistically significant. In contrast, salivary flow after 10 µg/mL LB after 5′ Aza treatment was significantly higher than that after 5′ Aza treatment alone (1, 10, and 100 µg/mL LB; 1.6±0.4 µL/cm2·h; 2.3±0.5 µL/ cm2·h, P<0.05; 2.0±0.3 µL/cm2·h, respectively; Figure 2).
In vitro evaluation of AQP-5 expression
LB increased the expression level of AQP-5 protein in NS-SV-AC cells
Western blotting and immunofluorescence were performed to confirm whether LB affects AQP-5 protein expression. AQP-5 protein expression in the 5′ Aza- only treated cells was significantly decreased than that in untreated normal cells (60.1±15.1% vs. 100.0%, P<0.05). However, AQP-5 expression in cells treated with 1, 10, and 100 µg/mL LB with 2 µM/mL 5′ Aza was significantly increased than that in 5′ Aza-only treated cells (82.5±12.9%, P<0.05; 70.4±17.8%, 62.7±11.8%, respectively; Figure 3).
Immunofluorescence analysis of AQP-5 protein expression in acinar cells confirmed the western blotting findings. The AQP-5 protein expression in 5′ Aza-only treated cells was lower than that in untreated normal cells.
However, the AQP-5 expression in 100 µg/mL LB-treated cells was more positive than that in 5′ Aza-only treated cells (Figure 4).
In vivo evaluation of cell protective effects
LB increased the salivary secretion in NOD/SCID mice
An NOD/SCID mouse model of SS was used to confirm whether LB increased salivary secretion in vivo. The net fluid secretion rate in the control group was significantly lower than that in the normal group (5.7±1.3 vs. 16.0±4.6 µL/10 min/g, P<0.05). Moreover, salivary secretion significantly improved in 100, 500, and 1,000 mg/kg LB-treated group than that in the control group (21.6±3.2 µL/10 min/g, P<0.001; 10.8±2.5 µL/10 min/g, P<0.01; 10.3±2.5 µL/10 min/g, P<0.05, respectively; Figure 5).
In vivo evaluation of AQP-5 and M3R expressions
LB increased the AQP-5 and M3R protein detection in NOD/SCID mice
Salivary secretion is associated with membrane channel proteins and AQP-mediated mechanisms. AQP- 5 is a major water transport channel protein present in acinar cells (Agre, 2004). Additionally, M3R, a major receptor subtype, stimulates salivary secretion from acinar cells (Zuo et al., 2016). Accordingly, the effect of LB on AQP-5 and M3R protein expression in NOD/SCID mice was confirmed using western blotting.
AQP-5 protein expression level in the control group was significantly decreased than that in the normal group (69.0±7.2% vs. 100.0%, P<0.05). However, AQP-5 expression level in the 1,000 mg/kg LB-treated group was significantly higher than that in the control group (97.6±9.7% vs. 69.0±7.2%, P<0.05). Additionally, M3R protein expression level in the control group was significantly reduced than that in the normal group (73.0±5.7% vs. 100.0%, P<0.05). Meanwhile, M3R expression level in the 500 and 1,000 mg/kg LB-treated groups was significantly higher than that in the control group (93.6±8.7 and 103.2±10.4%, respectively, P<0.05; Figure 6).
In vivo evaluation of inflammatory mediators
LB reduced anti-SSA/Ro and anti-SSB/La autoantibody production in NOD/SCID mice
Anti-SSA/Ro and anti-SSB/La autoantibodies are autoimmune biomarkers associated with severe lymphocyte infiltration-mediated salivary gland damage and are detected in approximately 60-70% of patients with primary SS (Hernández-Molina, Leal-Alegre, Michel- Peregrina, 2011). Accordingly, we confirmed whether LB reduced anti-SSA/Ro and anti-SSB/La production in the serum of NOD/SCID mice using ELISA.
Anti-SSA/Ro and anti-SSB/La production was higher in the control group than that in the normal group. However, anti-SSA/Ro and anti-SSB/La production in the 100, 500, and 1,000 mg/kg LB-treated groups was lower than that in the control group.
LB reduced the serum levels of the inflammatory cytokines TNF-α, IFN-γ, and IL-6 in NOD/SCID mice
The balance between T-helper 1 (Th1) and T-helper 2 cells plays an important role in the development of autoimmune diseases (Roescher, Tak, Illei, 2009). IFN-γ, an inflammatory cytokine secreted by Th1 cells, is known to be overexpressed in patients with SS (Mavragani, Crow, 2010). TNF-α and IL-6 activate B cells and induce an inflammatory response, with IL-6 being particularly associated with B-cell hyperactivity (Rieckmann, Tuscano, Kehrl, 1997). To evaluate the inhibitory effect of LB treatment on inflammatory response in NOD/SCID mice, we analyzed each serum level of TNF-α, IFN-γ, and IL-6 using ELISA. The results revealed that the TNF-α level in the control group was 116.6±19.0 pg/mL, which was significantly increased than that in the normal group (P<0.001). However, the TNF-α levels decreased significantly in the 100 and 500 mg/kg LB-treated groups than that in the control group (21.8±5.9 pg/mL, P < 0.01; 19.5±17.6 pg/mL, P<0.001, respectively).
IFN-γ levels significantly increased to 75.7±20.3 pg/ mL in the control group compared with that in the normal group (P<0.01). However, the IFN-γ levels in 100, 500, and 1,000 mg/kg LB-treated groups significantly decreased compared with that in the control group (30.3±5.9 pg/ mL, P<0.01; 16.1±10.7 pg/mL, P<0.01; 33.9±8.9 pg/mL, P<0.05, respectively).
The IL-6 level in the control group significantly increased to 203.7±24.6 pg/mL than that in the normal group (P<0.001). The IL-6 level in the 100 and 500 mg/ kg LB-treated group was significantly lower than that in the control group (73.6±32.9 and 59.9±17.7 pg/mL, respectively, P<0.01; Figure 7).
In vivo evaluation of the histologic changes
LB improved the infiltration- and inflammation-induced cellular damage in the salivary gland tissue of NOD/ SCID mice
Hematoxylin and eosin (H&E) staining was used to examine the effect of LB treatment on morphological and histological changes in the salivary glands. Lymphocyte infiltration and inflammatory lesions were observed in the salivary glands of the control group but not in those of the normal group. Meanwhile, infiltration- and inflammation- induced cell damage improved in the 100, 500, and 1,000 mg/kg LB-treated groups compared with the control group (Figure 8).
DISCUSSION
Our results revealed that LB administration for 4 weeks had potential effects on oral health in SS treatment. LB extract enhanced acinar cell proliferation and protected them from 5′ Aza-induced cytotoxicity. LB also increased salivary fluid secretion and AQP-5 protein expression. In addition, treating NOD/SCID mice with LB reduced anti-SSA/Ro and anti-SSB/La production; inhibited the secretion of inflammatory cytokines TNF-α, IFN-γ, and IL-6; and restored cellular damage in salivary gland tissue.
SS evaluation involves eye and mouth dryness assessment, histological examination of the minor salivary glands, and serological tests (Park, Gauna, Cha, 2015). Xerostomia and keratoconjunctivitis sicca are representative SS-related diseases caused by chronic inflammation and tissue breakdown in exocrine glands, such as the salivary and lacrimal glands. In particular, xerostomia detrimentally affects quality of life by causing difficulty in swallowing, discomfort during conversation, changes in taste perception, increased tooth decay, and other negative effects on oral health (Hopcraft, Tan, 2010).
B and T cells are considered significant contributors to SS development. B lymphocyte activation has been associated with a notable increase in cytokines, such as IL-6 and IL-10 (Halse et al., 1999). Moreover, B-lymphocyte stimulator levels are significantly elevated not only in the serum, where they correspond to autoantibody levels, but also in the salivary glands (Mariette et al., 2003). In relation to T cells, Th1-related cytokines, particularly IFN- γ and IL-12, marked increased in SS mouse models (Cha et al., 2004). Furthermore, epithelial cells may play a significant role in SS development, as they serve as immune process targets and initiate immune activation (Selmi, Gershwin, 2017). Therefore, treatment that resolves the complex immune-inflammatory response may be an important strategy for symptomatic relief in SS.
LB, a medicinal lily called “Bai-he” in Chinese, has traditionally been used in clinical settings to alleviate various symptoms, including persistent cough, sleeping difficulty, and anxiety. This is attributed to its traditional Chinese medicinal properties of moistening the lungs to suppress cough and clearing the heart for tranquilization. To date, many studies have revealed various beneficial effects of genus Lilium extracts or components that align with their traditional uses, including those that utilize their anti-inflammatory, antidepressant, and sedative effects. Moreover, recent studies have reported additional pharmacological benefits, such as antioxidant, immunomodulatory, antibacterial, and antitumor activities, of genus Lilium (Zhou, An, Huang, 2021).
In this study, LB was administered to both acinar cells and NOD/SCID mice to determine whether it restores fluid secretion and inhibits inflammatory response. NOD- derived strains, created as spontaneously developing type 1 diabetes mouse models, are characterized by lymphocyte infiltration into exocrine glands and reduced salivary secretion (Hu et al., 1992). As NOD/SCID mice share certain physiological, histopathological, and immunological characteristics with autoimmune salivary gland diseases from 8 weeks of age, they are commonly used as SS models (Yamano et al., 1999). Previously, this model exhibited decreased reactions to parasympathetic nerve and muscarinic receptor stimulation compared with BALB/c mice (Berggreen et al., 2009). Additionally, various autoantibodies related to SS, including anti-SSA/ Ro, anti-SSB/La, and anti-M3R, have been detected in the sera of NOD mice (Delaleu et al., 2008).
Salivary secretion occurs in salivary gland acinar cells and is modulated by the autonomic nervous system. The parasympathetic nervous system (PNS) is involved in water and electrolyte secretion, whereas the sympathetic nervous system is involved in protein secretion. Acetylcholine released from the PNS activates M3R and M1 muscarinic acetylcholine receptors in ductal and acinar cells, thereby increasing the Ca2+ concentration. Increased Ca2+ levels activate AQP water channel proteins, leading to the movement of water through the apical membranes of acinar cells (Proctor, Carpenter, 2007). Using MTT assay, we observed that all LB doses significantly increased acinar cell viability. In addition, the impaired salivary secretion was recovered after treatment with LB, both in vitro and in vivo. This indicates that LB enhances acinar cell proliferation and protects them from 5′ Azainduced cytotoxicity, confirming its beneficial effects on the manifestations of SS.
M3R is a muscarinic receptor subtype mainly responsible for stimulating salivary secretion in response to acetylcholine (Zuo et al., 2016). Antibodies against M3R inhibit exocrine function in NOD/SCID mice (Nguyen et al., 2000). AQPs are a group of transmembrane channel proteins that facilitate osmotic gradient-driven water transfer across cellular membranes (Li et al., 2008). The three AQP subtypes are classified according to their permeability. AQP-1, AQP-2, AQP-4, AQP-5, AQP-6, and AQP-8 are only permeable to water, while AQP-3, AQP-7, AQP-9, and AQP-10 are permeable to water and small solutes, such as glycerol and urea. However, the permeability of AQP-11 and AQP-12 remains unclear (Ishibashi, Morishita, Tanaka, 2017). AQP-5 is mainly expressed in the apical membrane and plays a crucial role in maintaining salivary and lacrimal gland functions (Agre, 2004). Animal studies have demonstrated that AQP- 5 suppression decreases salivary secretion by regulating water permeability in acinar cells (Ma et al., 1999). The western blotting and immunofluorescence analyses performed during this study showed that AQP-5 protein expression was significantly increased in LB-treated cells compared with controls in vitro. Additionally, both AQP-5 and M3R protein expression were significantly increased in LB-treated mice compared with controls in vivo. This increase in protein expression improved water transfer in the salivary gland acinar cells. These findings confirm that LB exerts a therapeutic effect on xerostomia by increasing AQP-5 and M3R protein expression.
Antibodies against SSA/Ro and SSB/La are commonly recognized markers of autoimmune diseases (Franceschini, Cavazzana, 2005). Anti-SSA/Ro and anti-SSB/La antibodies have been detected in 33-74% and 23-52% of patients with SS, respectively, and their presence is a common diagnostic criterion for SS (Patel, Shahane, 2014). They are commonly located inside the cells and are inaccessible to the immune system. However, during apoptosis or exosome release, these antibodies are transported to the cell surface and become immunogenic (Ohlsson, Jonsson, Brokstad, 2002), thereby contributing to tissue damage (Franceschini, Cavazzana, 2005). Using ELISA, we were able to confirm that the levels of anti- SSA/Ro and anti-SSB/La antibodies in NOD/SCID mice sera decreased after LB treatment. Thus, LB exerts a therapeutic effect on SS by reducing autoantibody production in animal models of SS.
TNF-α, IFNs, ILs (IL-1β, IL-6, IL-12, and IL-18), and B-cell activating factors are crucial in SS pathology. TNF-α, IFN-γ, and IL-6 levels increase in the serum of patients with SS, increasing lymphocytic infiltrates of the salivary gland (Roescher, Tak, Illei, 2009). TNF-α promotes apoptosis of salivary gland cells and impairs salivary secretion by downregulating AQP-5 expression (Zhou, Kawai, Yu, 2017). IFN-γ induces inflammatory reactions in salivary glands and directly inhibits salivary secretion. Both IFN-γ and IL-6 levels are linked to the extent of lymphocytic infiltration in salivary glands (Roescher, Tak, Illei, 2009). In this study, ELISA results showed that LB significantly decreased TNF-α, IFN-γ, and IL-6 levels in NOD/SCID mice serum and improved inflammatory lesions in the histopathological study of salivary glands. Therefore, our findings suggest that LB administration could regulate inflammatory responses in experimental autoimmune diseases, thereby improving histological findings in the salivary glands.
A limitation of our study is that our in vitro and in vivo studies did not demonstrate a clear dose-response relationship. This may be due to the simplified environment of in vitro experiments, which often fail to replicate the complex interactions that occur in vivo, thereby obscuring straightforward dose-response relationships. In the in vivo experiments, variations in results with different dosages could be attributed to differences in the absorption, distribution, metabolism, and excretion of the botanical drug at varying doses. At higher doses, drug interactions, side effects, and toxic effects may alter outcomes. Future research should aim to identify the minimum effective and maximum doses of LB that yield the greatest efficacy with the least potential for adverse effects. In future studies, we aim to explore the therapeutic potential of the optimal LB dose and evaluate its effectiveness and safety as an adjuvant treatment, along with conventional therapies, for patients with SS. We also intend to study the specific signaling pathways responsible for the anti-inflammatory response to SS and investigate the chemical composition and activities of LB. While acknowledging these future endeavors, it is important to note that this study provides novel perspectives on LB as an immunomodulatory drug. These findings suggest that LB administration could be beneficial in future nutritional approaches aimed at preventing and treating SS.
CONCLUSION
To the best of our knowledge, this is the first study to investigate the therapeutic effects of LB in SS. In this study, we revealed the significant immunomodulatory effects of LB in an animal model of SS and explored its anti-inflammatory mechanism in vitro. LB not only halted the progression of oral manifestations of SS but also improved serologic indicators. Specifically, LB reduced pro-inflammatory cytokine and autoimmune antibody levels, promoted acinar cell proliferation, enhanced salivary secretion by upregulating AQP-5 expression, and restored infiltration-induced salivary gland tissue damage. These findings suggest that LB could serve as an alternative treatment for SS.
ACKNOWLEDGMENTS
This research was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare, Republic of Korea (grant number: HI20C0865) and by the Korea Institute of Oriental Medicine (grant number: KSN2212010).
REFERENCES
- Agre P. Aquaporin water channels (Nobel lecture). Angew Chem Int Ed. 2004;43(33):4278-90.
- Bamoulid J, Staeck O, Halleck F, Khadzhynov D, Brakemeier S, Dürr M, et al. The need for minimization strategies: current problems of immunosuppression. Transpl Int. 2015;28(8):891-900.
- Berggreen E, Nyløkken K, Delaleu N, Hajdaragic- Ibricevic H, Jonsson MV. Impaired vascular responses to parasympathetic nerve stimulation and muscarinic receptor activation in the submandibular gland in nonobese diabetic mice. Arthritis Res Ther. 2009;11:1-12.
- Cha S, Brayer J, Gao J, Brown V, Killedar S, Yasunari U, et al. A dual role for interferon-γ in the pathogenesis of Sjögren’s syndrome-like autoimmune exocrinopathy in the nonobese diabetic mouse. Scand J Immunol. 2004;60(6):552-65.
- Delaleu N, Immervoll H, Cornelius J, Jonsson R. Biomarker profiles in serum and saliva of experimental Sjögren’s syndrome: associations with specific autoimmune manifestations. Arthritis Res Ther . 2008;10:1-14.
- Franceschini F, Cavazzana I. Anti-ro/ssa and la/ssb antibodies. Autoimmunity. 2005;38(1):55-63.
- Halse A, Tengner P, Wahren-Herlenius M, Haga H, Jonsson R. Increased frequency of cells secreting interleukin-6 and interleukin-10 in peripheral blood of patients with primary Sjögren’s syndrome. Scand J Immunol . 1999;49(5):533-8.
- Han SY, Yi Y-S, Jeong S-G, Hong YH, Choi KJ, Hossain MA, et al. Ethanol extract of lilium bulbs plays an anti- inflammatory role by targeting the IKK α/β-Mediated NF-κ B pathway in macrophages. Am J Chin Med. 2018;46(06):1281-96.
- Hernández-Molina G, Leal-Alegre G, Michel-Peregrina M. The meaning of anti-Ro and anti-La antibodies in primary Sjögren’s syndrome. Autoimmun Rev. 2011;10(3):123-5.
- Hopcraft M, Tan C. Xerostomia: an update for clinicians. Aust Dent J. 2010;55(3):238-44.
- Hu M, Cai B, Zhang Z, Zhang H. Pharmacodynamics research of lilium brownii polysaccharide. Traditional Chinese Drug Research & Clinical Pharmacology. 2007.
- Hu Y, Nakagawa Y, Purushotham KR, Humphreys- Beher MG. Functional changes in salivary glands of autoimmune disease-prone NOD mice. Am J Physiol. 1992;263(4):E607-E14.
-
Ishibashi K, Morishita Y, Tanaka Y. The evolutionary aspects of aquaporin family. Adv Exp Med Biol 2017; doi:10.1007/978-94-024-1057-0_2.35-50.
» https://doi.org/10.1007/978-94-024-1057-0_2.35-50 - Kim HU, Ryu JY, Lee JO, Lee SY. A systems approach to traditional oriental medicine. Nat Biotechnol. 2015;33(3):264-8.
- Kwok S-K. Review of Sjögren’s syndrome for primary physicians. Korean J Med. 2015;89(3):291-4.
- Li B, Liu G, Liu R, He S, Li X, Huang L, et al. Total glucosides of paeony (TGP) alleviates Sjogren’s syndrome through inhibiting inflammatory responses in mice. Phytomedicine. 2020;71:153203.
- Li X, Azlina A, Karabasil MR, Purwanti N, Hasegawa T, Yao C, et al. Degradation of submandibular gland AQP5 by parasympathetic denervation of chorda tympani and its recovery by cevimeline, an M3 muscarinic receptor agonist. Am J Physiol Gastrointest Liver Physiol 2008;295(1):G112-G23.
- Ma T, Song Y, Gillespie A, Carlson EJ, Epstein CJ, Verkman A. Defective secretion of saliva in transgenic mice lacking aquaporin-5 water channels. J Biol Chem. 1999;274(29):20071-4.
- Mariette X, Roux S, Zhang J, Bengoufa D, Lavie F, Zhou T, et al. The level of BLyS (BAFF) correlates with the titre of autoantibodies in human Sjögren’s syndrome. Ann Rheum Dis. 2003;62(2):168-71.
- Mavragani CP, Crow MK. Activation of the type I interferon pathway in primary Sjogren’s syndrome. J Autoimmun. 2010;35(3):225-31.
- Nguyen KHT, Brayer J, Cha S, Diggs S, Yasunari U, Hilal G, et al. Evidence for antimuscarinic acetylcholine receptor antibody-mediated secretory dysfunction in NOD mice. Arthritis Rheum 2000;43(10):2297-306.
- Ohlsson M, Jonsson R, Brokstad K. Subcellular redistribution and surface exposure of the Ro52, Ro60 and La48 autoantigens during apoptosis in human ductal epithelial cells: a possible mechanism in the pathogenesis of Sjögren’s syndrome. Scand J Immunol . 2002;56(5):456- 69.
- Park Y-S, E Gauna A, Cha S. Mouse models of primary Sjogren’s syndrome. Curr Pharm Des. 2015;21(18):2350- 64.
-
Patel R, Shahane A. The epidemiology of Sjögren’s syndrome. Clin Epidemiol. 2014; doi:10.2147/CLEP. S47399.247-55.
» https://doi.org/10.2147/CLEP. S47399.247-55 - Proctor GB, Carpenter GH. Regulation of salivary gland function by autonomic nerves. Auton Neurosci. 2007;133(1):3-18.
- Rieckmann P, Tuscano J, Kehrl J. Tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) in B-lymphocyte function. Methods. 1997;11(1):128-32.
- Roescher N, Tak PP, Illei GG. Cytokines in Sjögren’s syndrome. Oral Dis. 2009;15(8):519-26.
- Sebastian A, Szachowicz A, Wiland P. Classification criteria for secondary Sjögren’s syndrome. Current state of knowledge. Rheumatology. 2019;57(5):277-80.
- Selmi C, Gershwin ME. Chronic autoimmune epithelitis in Sjögren’s syndrome and primary biliary cholangitis: a comprehensive review. Rheumatology Ther. 2017;4:263- 79.
- Sumida T, Azuma N, Moriyama M, Takahashi H, Asashima H, Honda F, et al. Clinical practice guideline for Sjögren’s syndrome 2017. Mod Rheumatol. 2018;28(3):383-408.
- Vivino FB. Sjogren’s syndrome: Clinical aspects. Clin Immunol. 2017;182:48-54).
- Vivino FB, Bunya VY, Massaro-Giordano G, Johr CR, Giattino SL, Schorpion A, et al. Sjogren’s syndrome: An update on disease pathogenesis, clinical manifestations and treatment. Clin Immunol . 2019;203:81-121.
- Wang Y, Yan T, Shen J, Guo H, Xiang X. Preventive effect of Ophiopogon japonicus polysaccharides on an autoallergic mouse model for Sjogren’s syndrome by regulating the Th1/Th2 cytokine imbalance. J Ethnopharmacol. 2007;114(2):246-53.
- Yamano S, Atkinson JC, Baum BJ, Fox PC. Salivary gland cytokine expression in NOD and normal BALB/c mice. Clin Immunol . 1999;92(3):265-75.
- Zhou J, Kawai T, Yu Q. Pathogenic role of endogenous TNF-α in the development of Sjögren’s-like sialadenitis and secretory dysfunction in non-obese diabetic mice. Lab Invest. 2017;97(4):458-67.
- Zhou J, An R, Huang X. Genus Lilium: A review on traditional uses, phytochemistry and pharmacology. J Ethnopharmacol . 2021;270:113852.
- Zuo J, Williams AE, Park Y-J, Choi K, Chan AL, Reeves WH, et al. Muscarinic type 3 receptor autoantibodies are associated with anti-SSA/Ro autoantibodies in Sjögren’s syndrome. J Immunol Methods . 2016;437:28-36.
-
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The animal study protocol was approved by the Institutional Animal Care and Use Committee of Kyung Hee University, Republic of Korea (protocol code KHUASP (SE)-19-204) and was conducted in accordance with the “Guide for the Care and Use of Laboratory Animals.”
Publication Dates
-
Publication in this collection
20 Jan 2025 -
Date of issue
2025
History
-
Received
26 Apr 2024 -
Accepted
30 July 2024

 Salivary stimulatory and anti-inflammatory effects of aqueous Lilium brownii var. viridulum Baker extract for Sjögren’s syndrome: in vitro and in vivo studies
Salivary stimulatory and anti-inflammatory effects of aqueous Lilium brownii var. viridulum Baker extract for Sjögren’s syndrome: in vitro and in vivo studies








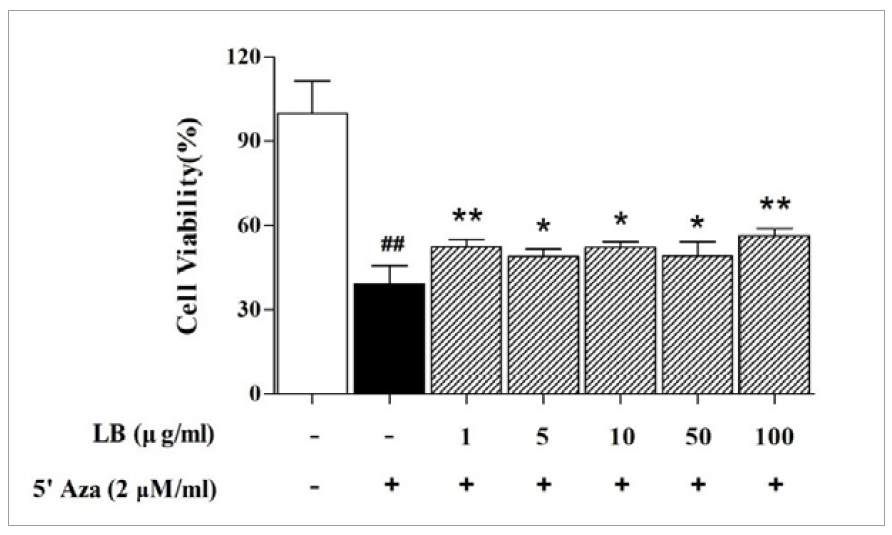 Each column represents the mean ± SD (n=3). ## P<0.01 vs. normal group (white column); * P<0.05, ** P<0.01 vs. control group (black column). LB, Lilii Bulbus; SD, standard deviation; 5′ Aza, 5-aza-2′-deoxycytidine.
Each column represents the mean ± SD (n=3). ## P<0.01 vs. normal group (white column); * P<0.05, ** P<0.01 vs. control group (black column). LB, Lilii Bulbus; SD, standard deviation; 5′ Aza, 5-aza-2′-deoxycytidine.
 Each column represents the mean ± SD (n=3). * P<0.05 vs. control group (black column). LB, Lilii Bulbus; SD, standard deviation.
Each column represents the mean ± SD (n=3). * P<0.05 vs. control group (black column). LB, Lilii Bulbus; SD, standard deviation.
 (A) Western blot analysis of AQP-5 protein levels. (B) Quantification of western blot bands for AQP-5. Each column represents the mean ± SD (n=3). # P<0.05 vs. normal group (white column); * P<0.05 vs. control group (black column). AQP-5, aquaporin-5; LB, Lilii Bulbus; SD, standard deviation.
(A) Western blot analysis of AQP-5 protein levels. (B) Quantification of western blot bands for AQP-5. Each column represents the mean ± SD (n=3). # P<0.05 vs. normal group (white column); * P<0.05 vs. control group (black column). AQP-5, aquaporin-5; LB, Lilii Bulbus; SD, standard deviation.
 A positive reaction for AQP-5 is represented by a red dot (left line), and DAPI nucleic acid staining is shown in blue (right line). (A, B) Normal group. (C, D) Control group (only 5′ Aza-treated cells). (E, F) 100 µg/mL LB- and 5′ Aza-treated cells. Objective magnification: 200×. Bar = 100 µm. AQP-5, aquaporin-5; DAPI, 4′,6-diamidino-2-phenylindole; 5′ Aza, 5-aza-2′-deoxycytidine.
A positive reaction for AQP-5 is represented by a red dot (left line), and DAPI nucleic acid staining is shown in blue (right line). (A, B) Normal group. (C, D) Control group (only 5′ Aza-treated cells). (E, F) 100 µg/mL LB- and 5′ Aza-treated cells. Objective magnification: 200×. Bar = 100 µm. AQP-5, aquaporin-5; DAPI, 4′,6-diamidino-2-phenylindole; 5′ Aza, 5-aza-2′-deoxycytidine.
 N: normal group (BALB/c mice); C: control group (NOD/SCID mice); 100, 500, and 1,000: 100, 500, and 1,000 mg/kg LB-treated groups, respectively. # P<0.05 vs. normal group; * P<0.05, ** P<0.01, *** P<0.001 vs. control group. LB, Lilii Bulbus; NOD/SCID, non-obese diabetic/severe combined immunodeficiency.
N: normal group (BALB/c mice); C: control group (NOD/SCID mice); 100, 500, and 1,000: 100, 500, and 1,000 mg/kg LB-treated groups, respectively. # P<0.05 vs. normal group; * P<0.05, ** P<0.01, *** P<0.001 vs. control group. LB, Lilii Bulbus; NOD/SCID, non-obese diabetic/severe combined immunodeficiency.
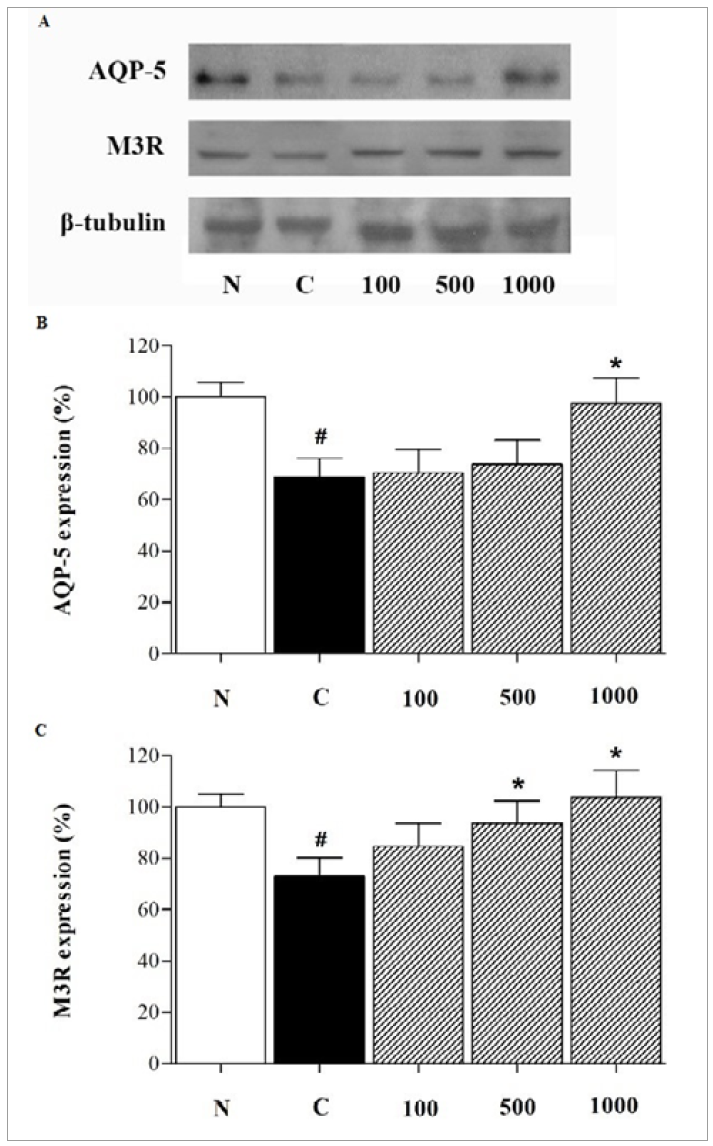 (A) Western blot analysis of AQP-5 and M3R protein levels. Quantification of western blot bands for (B) AQP-5 and (C) M3R. Each column represents the mean ± SD (n=3). N: normal group (BALB/c mice); C: control group (NOD/SCID mice); 100, 500, and 1,000: 100, 500, and 1,000 mg/kg LB-treated groups, respectively. # P<0.05 vs. normal group; * P<0.05 vs. control group. AQP-5, aquaporin-5; LB, Lilii Bulbus; M3R, M3 muscarinic acetylcholine receptor; NOD/SCID, non-obese diabetic/severe combined immunodeficiency; SD, standard deviation.
(A) Western blot analysis of AQP-5 and M3R protein levels. Quantification of western blot bands for (B) AQP-5 and (C) M3R. Each column represents the mean ± SD (n=3). N: normal group (BALB/c mice); C: control group (NOD/SCID mice); 100, 500, and 1,000: 100, 500, and 1,000 mg/kg LB-treated groups, respectively. # P<0.05 vs. normal group; * P<0.05 vs. control group. AQP-5, aquaporin-5; LB, Lilii Bulbus; M3R, M3 muscarinic acetylcholine receptor; NOD/SCID, non-obese diabetic/severe combined immunodeficiency; SD, standard deviation.
 (A) Anti-SSA/Ro antibody. (B) Anti-SSB/La antibody. (C) TNF-α. (D) IFN-γ. (E) IL-6. Each column represents the mean ± SD (n=6). N: normal group (BALB/c mice); C: control group (NOD/SCID mice); 100, 500, and 1,000: 100, 500, and 1,000 mg/kg LB-treated groups, respectively. ## P<0.01, ### P<0.001 vs. normal group; * P<0.05, ** P<0.01, *** P<0.001 vs. control group. IFN-γ, interferon-γ; IL-6, interleukin-6; LB, Lilii Bulbus; NOD/SCID, non-obese diabetic/severe combined immunodeficiency; SD, standard deviation; TNF-α, tumor necrosis factor-α.
(A) Anti-SSA/Ro antibody. (B) Anti-SSB/La antibody. (C) TNF-α. (D) IFN-γ. (E) IL-6. Each column represents the mean ± SD (n=6). N: normal group (BALB/c mice); C: control group (NOD/SCID mice); 100, 500, and 1,000: 100, 500, and 1,000 mg/kg LB-treated groups, respectively. ## P<0.01, ### P<0.001 vs. normal group; * P<0.05, ** P<0.01, *** P<0.001 vs. control group. IFN-γ, interferon-γ; IL-6, interleukin-6; LB, Lilii Bulbus; NOD/SCID, non-obese diabetic/severe combined immunodeficiency; SD, standard deviation; TNF-α, tumor necrosis factor-α.
 (A) Normal group (BALB/c mice). (B) Control group (NOD/SCID mice). (C-E) 100, 500, and 1,000 mg/kg LB-treated groups, respectively. Arrows indicate lymphocytic infiltrates. Objective magnification: 200×. Bar = 100 µm. LB, Lilii Bulbus; NOD/SCID, non-obese diabetic/ severe combined immunodeficiency.
(A) Normal group (BALB/c mice). (B) Control group (NOD/SCID mice). (C-E) 100, 500, and 1,000 mg/kg LB-treated groups, respectively. Arrows indicate lymphocytic infiltrates. Objective magnification: 200×. Bar = 100 µm. LB, Lilii Bulbus; NOD/SCID, non-obese diabetic/ severe combined immunodeficiency.