Abstract
In an alternative medicinal system, Matricaria chamomilla is used for various conditions like inflammation, higher cholesterol, wounds, diabetes and pregnancy-related issues. However, there is contradiction about the use of Matricaria chamomilla at the time of pregnancy. Therefore, the present study is designed to investigate the embryotoxic and developmental effects of Matricaria chamomilla using zebrafish embryos as a model. Matricaria chamomilla's chemical profiling was done using GC-MS/MS analysis. Fertilized embryos were selected and exposed to 0.1% vehicle (alcohol) and test medicine Matricaria chamomilla (mother tincture, 6C and 30C) for 96 hrs. Normal control group embryo exposed to embryo media. All the embryos were observed for mortality, hatching, morphological parameters and heart rate till 96 hours. GC-MS/MS analysis revealed 80 phytochemical compounds in hydro-alcoholic extract of Matricaria chamomilla. It exerted a mild effect on hatching rate and showed mortality than any other group. Whereas, after the exposure of Matricaria chamomilla-6C and 30C, there were no changes observed in all the parameters. Overall, the result reveals that Matricaria chamomilla-6C and 30C (at high dilution) is found safe and indicated its safety at the time of pregnancy. However, further validation in higher animal models is recommended for proper clinical correlation.
Keywords:
Pregnancy; Danio rerio; Development; Embryo; Teratogenic effect.
INTRODUCTION
Chamomile (Matricaria chamomilla L.; M. chamo.), belonging to the family Asteraceae, is an annual plant native to Europe and Asia (Ortiz et al., 2016). It possesses branched, erect, smooth stems and is also known as chamomile, Italian camomilla, German chamomile, wild chamomile and Hungarian chamomile (Mekonnen et al., 2016). The main phytochemical constituents present in chamomile are sesquiterpenes, flavonoids, coumarins, and polyacetylenes (Singh, Khanam, Misra, 2011).
Medical system has used it for the treatment of stomach aches, irritable bowel syndrome and insomnia. It also possess several activities like anti-inflammatory, bactericidal, anxiolytic, anti-mutagenic, cholesterollowering, wound healing, and antidiabetic properties (Amsterdam et al., 2012; Hashempur et al., 2015; Sharifi et al., 2017). Matricaria chamomilla is known to contain flavonoids, including apigenin and quercetin, which has been identified as phytoestrogens (Piersen, 2003). Phytoestrogens are used to modulate estrogenic activity in the body, which may raise concerns about hormonal balance during pregnancy (van Duursen, 2017; Naji, Hossenzadeh Sahafi, Saffari, 2015). There are many studies available regarding the negative effects of M. chamo. in the case of pregnancy. A study in 2010 reported the regular use of M. chamo. resulted in a higher risk of preterm labor or miscarriage (Cuzzolin et al., 2010). At 35 weeks gestation period, women of 32-year intake chamomile tea intermittently during their pregnancy, which results in premature constriction of the fetal ductus arteriosus (Sridharan et al., 2009). According to reports, preterm births and lower birth weights have linked to regular chamomile consumption during pregnancy (Trabace et al., 2015). Therefore it is advised not to take chamomile during pregnancy due to its unsafe and adverse perinatal outcomes.
In contrast to this, M. chamo. is also used in homoeopathic medicine systems for a number of diseases, such as various central nervous system disorders; stress, derangement of the cerebral functions, depression and other conditions. It is frequently used medicine for numerous symptoms in various diseased conditions (Samuel, 1880). In addition to this, at ultrahigh dilution (1012 and 1060) its use is also reported in the case of pregnancy for various symptoms like severe pains following labor, especially if they extend down the thighs, with great nervous excitement (Boericke, 1998). Though at high dilutions, chances of adverse effects are least or negligible however, a report of Posadzki et al., (2012), alarming the scientist to check the safety of such preparations even at high dilution because they reported the adverse effects of homoeopathy in their systemic review. In addition to this, the WHO in 2009 also published a report and showed its concern about the risk evaluation of homoeopathic preparation at high dilution. The evidence regarding the safety of homoeopathic preparation of chamomilla (mother tincture and its potencies) in pregnancy cases has also not been found. Therefore, it is essential to conduct safety tests on the development of embryos to find out whether the homoeopathic preparation of chamomile has any effect on embryonic development or not. There will always be uncertainty about the use of homoeopathic chamomile preparation in pregnancy due to the lack of scientific proof and conflicts on this issue.
Due to the various advantages of the zebrafish model along with the following the principle of RRR, the zebrafish embryo model has become a prominent experimental model to assess the safety, toxicity, and efficacy of a number of drugs due to their rapid development, cost-effective, transparency in embryos and highly-conserved genetic pathway between zebrafish and humans (Brannen et al., 2013; Santoro 2014).
For rapid toxicological assessment for pharmaceuticals, the National Toxicology Program of USA is also trying to establish and validating of zebrafish as a model (Dach et al., 2019). Besides, this model is also used by a number of scientific communities to evaluate the toxic effect of different chemical compounds as well as medicinal plants (Wang et al., 2020; Chahardehi et al., 2020; Sharma et al., 2021a; Sharma et al., 2021b). Therefore, the present study is designed to evaluate the embryotoxic and developmental effects of homoeopathic preparation of M. chamo. mother tincture and its potencies, i.e. 6C and 30C, using zebrafish embryos as a model.
MATERIAL AND METHODS
Collection of plant material
Fresh plant (whole part) of M. chamo. was collected, taxonomically identified, and authenticated, a voucher specimen was deposited at the Centre for Medicinal Plants Research in Homoeopathy (CMPRH), Emerald, Tamil Nadu, India (Voucher specimen No.: 9887).
Preparation of homoeopathic drugs
The raw plant material was collected, sorted, washed, and powdered to various degrees of fineness. The hydro-alcoholic-preparation (mother tincture) was prepared as per standard protocol with 25 g of plant material of M. chamo. to prepare 100 ml of mother tincture (ϕ). The potencies were prepared by adding mother tincture with solvent (dispensing alcohol) in 1:9 ratio and “succussed”. The procedure was repeated to prepare the 6th and 30th potency of chamomilla (having dilution factors of 1012 and 1060 respectively) with the same above procedures as mentioned in Homoeopathic Pharmacopoeia of India (India, 1974).
GC-MS/MS Analysis of Hydro-alcoholic Extract of M. chamo.
GC-MS/MS analysis of the sample was performed by using Trace 1300 GC Triplus RSH auto sampler TSQ 8000 Evo Mass Spectrometer (Thermo, USA) equipped with TG 5MS capillary column (30 m X 0.25mm), 0.25μm film thickness of stationary phase, 5% phenyl and 95% dimethyl polysiloxane. This was operated in split (split ratio 1:20) injection mode with an injector temperature of 80 °C. Helium was used as a carrier gas with a flow rate of 1.0 mL min-1. The GC oven temperature was programmed as follows: 65 °C (hold for 2 min), increased to 210 °C at a rate of 5 °C min-1 (hold for 2.5 min) and finally reached to 300 °C (hold for 11min) at a rate of 12 °C min-1. Total run time was 53 min. The ion source and mass interface temperature were set at 230 °C and 300 °C, respectively. The mass spectrometer was operated at electron energy of 70eV. The samples were derivatized by using BSTFA (1%TMCS) as silylation agent. All the samples were analysed in full scan mode of mass in the range of m/z 40-600. 1µL of sample was injected into chromatographic system.
Maintenance of fish and egg spawning
Adult zebrafish was locally purchased from a vendor and maintained in clean poly-sulphone tanks with UV treated, and filtered water with constant filtration and aeration. The pH of aquarium water was maintained between 7.0 ± 0.2. The zebrafish facility environment was maintained with the temperature at 27-29 °C and a light/ dark cycle of ~14/10 h, respectively. The environment of the zebrafish housing facility was according to the standard protocol (Westerfield, 1995). The protocol of the study was approved by IAEC (DDPR- CRIH/ Pharmacology/CPCSEA/IAEC/2018/006).
For breeding purposes, male and female zebrafish in a ratio of 1:2 were paired in breeding boxes a few hours before the onset of darkness on the day before the test. Spawning was triggered once the light was turned on and was usually completed within 30 min on next morning. After spawning, the fish were returned to their home cage and the eggs were collected and placed in petri plates containing medium with the help of a stereo zoom microscope (DSRi-2, Nikon Corporation, India).
Fish Embryo Acute Toxicity (FET) Test on Zebrafish
Zebrafish embryos exposed to the test medicine were carried out in 24-well plates according to OECD 236 guidelines on fish embryo toxicity tests with some modifications (OECD-236, 2006). Fertilized eggs were divided randomly into five groups namely: Water (N. control), dispensing alcohol (V. control), M. chamo.mother tincture (ϕ), M. chamo.-6C, M. chamo.-30C and each group contained ten eggs in 24 well plates for further experiment and the experiment was performed in 3 independent replicates in a 24-well plate. The fish of group I were exposed to 2 mL of embryo media, and groups II, III, IV and V were exposed to 2 mL of embryo media containing 0.1% of dispensing alcohol, M. chamo.-ϕ, M. chamo.-6C and 30C respectively. The exposure was given in semi-static conditions at 10 embryos per group of exposure medium, and the test solutions were changed every day till 96 hours post fertilization (hpf). During the exposure period, dead embryos were removed immediately to avoid contamination. The parameters that were evaluated, egg coagulation, mortality, somite cell formation, tail detachment, eyes, otolith, heart-beat, blood circulation and skeletal deformities were recorded each day for 96 hpf (Table I). The parameters like eye diameter, body Length pericardial sac and yolk sac area were evaluated on 96 hpf. The malformation of the embryo body in each group for a period of 96 hpf was subsequently examined with the help of a stereo zoom microscope (SMZ-18, Nikon Corporation, India), and the images of embryos were captured with a camera (Nikon model no: DSRi2).
Morphological characteristics evaluated as measures for the normal development at different time points
Evaluation of Survival Rate
The survival rate of embryos was observed from the start of the exposure to 96 hpf. The following formula was used to calculate the survival rate of the embryos.
Survival Rate % = No. of live embryos or larvae / total number of embryos (10) X 100
Evaluation of Hatch Rate
The hatching of embryos was observed as a rupture in the chorion for the release of larvae. Zebrafish embryos of each group were observed for 96 hpf to determine their hatching rates using a stereo zoom microscope.
Evaluation of Heartbeat
The heartbeat was recorded at 48, 72 and 96 hpf after the treatment of M. chamo.-(ϕ), 6C and 30C. The zebrafish cardiac ventricles were directly observed using a stereo zoom microscope to count the heartbeats. The heartbeat was recorded per minute with the help of a stopwatch.
Determination of Body Length
The image of the hatched larvae at 96 hpf was captured using a microscope camera (Nikon model no: DSRi2) and the body length was measured using imaging software (Nikon imaging software NISElements BR-5.30).
Determination of pericardial sac area and yolk sac area
The area of the pericardial sac and yolk sac was determined to quantify the severity of pericardial edema and yolk sac edema respectively (Prasch et al., 2003). The pericardial sac and yolk sac area were outlined, traced and determined the area using imaging software (Nikon imaging software NIS-Elements BR-5.30).
Determination of eye diameter
To find out the effect of M. chamo. on the eye, the diameter of both eyes (left and right) was measured. The area of each eye was outlined, traced and the diameter was measured using imaging software (Nikon imaging software NIS-Elements BR-5.30).
Statistical Analysis
Results are expressed as mean ± SEM. Oneway ANOVA was used to determine the statistical significance and then Tukey’s post hoc test was applied using the Graph Pad Prism ver.5. Differences were considered significant at p < 0.05.
RESULTS
Phytochemical constituents of M. chamo. through GC-MS/MS analysis
The GC-MS/MS analysis of the derivatized M. chamo. hydro-alcoholic extract, prepared according to HPI principles, revealed the presence of 80 phytochemical compounds. The main key phytochemicals among above compounds were primarily dominated by carbohydrates, organic acids, fatty acids, and other biomolecule groups (Table II). The carbohydrate profile was largely dominated by α-D-lactose, D-allopyranose, D-fructofuranose, trehalose, sucrose, and ribofuranose. Additionally, D-gluconic acid, a sugar acid derived from glucose, was also identified. These carbohydrates likely contribute to the plant's energy storage and structural integrity. The fatty acid profile of the extract included palmitic acid, linoleic acid, and stearic acid, which are commonly reported in the essential oil and extract of M. chamo. In addition to carbohydrates and fatty acids, various other compounds were identified in the extract. These included sugar alcohols like glycerol and dulcitol, sugar acids like arabinonic acid, and cyclic polyols like quinic acid. Myo-inositol, a carbocyclic polyol with potential biological activity, was also detected. Moreover, glycolic acid, an alpha hydroxy acid (AHA) known for its exfoliating and moisturizing properties, was also present. The chromatogram of M. chamo. having structure details of key fatty acids and carbocyclic sugar is depicted in Figure 1.
Effect of M. chamo.-ϕ, 6C and 30C on survival rate
Every 24 hours, the survival rate was measured by optical monitoring and illustrated in Figure 2. Throughout the experiment, mortality stayed below 10% in the control group, which followed the validation criteria of OECD 236. After the treatment with M. chamo.-6C and M. chamo.-30C, the survival rate was found normal and within the 10% criteria similar to the normal control group. On the other hand, exposure with M. chamo.-ϕ showed mild effect and showed 20% mortality at 24 hpf in embryos.
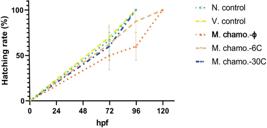
Effect of M. chamo.-ϕ, 6C and 30C on hatching rate
Zebrafish embryos exposed to M. chamo. showed delayed hatching at 6C and 30C in terms of the hatching rate represented in Figure 3. Hatching rates at 48 hpf were 50% in the M. chamo.-30C group and 72% in the M. chamo.-ϕ and 6C group. At 96 hpf, 100% hatching was achieved in normal control, vehicle control and M. chamo.-6C groups, whereas approx. 60% hatching was found in M. chamo.- ϕ group, which was increased in M. chamo.-30C exposure i.e., 87%. The 100% hatching rate was recorded in the M. chamo. ϕ treatment group at 120 hpf, which is comparable to the results seen in the control and vehicle control groups.
Effect of M. chamo.-ϕ, 6C and 30C on morphological characteristics of zebrafish embryos
At 24, 48 and 72 hpf, the embryos were incubated with different M. chamo. potencies (ϕ, 6C and 30C). In the present study, the N. control group exhibited normal embryonic development, similar to a previous study (Kimmel, Ballard, 1995). Figure 4-5 demonstrates that exposure to M. chamo.-ϕ, M. chamo.-6C and M. chamo.30C had no deleterious effects on the development of zebrafish and found similar morphological characteristics as a normal control group. The sharpedged somite formation and tail detachment were observed in all the groups at 24 hpf. An evaluation of the morphological features showed that all study groups had a circular yolk sac, a straight spine, a normal body form and heart rate, and pigmentation on the body and eyes. No coagulation and organ malformation occur during the study period. The effect of exposure to M. chamo.-ϕ, M. chamo.-6C and M. chamo.-30C in larvae at 96 hpf on various parameters like length (Figure 6a), eye diameter (Figure 6b), yolk sac area (YS; Figure 6c), pericardial area (PC; Figure 6d) and heart rate at 48, 72 and 96 hpf shown in Figure-7. Statistical analysis in all the mentioned parameters (larvae length, eye diameter, YS and PC) showed that there was no significant (p≥0.05) difference observed within the groups as compared to the normal control group (Figure 5a-5d). Heart rate was constant throughout the experiment after continuous 96-hour exposure and found same as the normal (E-3 media) control group (Figure 7). From 48 hpf to the end of the experiment (96 hpf) blood circulation was exist and visible under the microscope in all the zebrafish in all groups. From 24 hpf onward the otolith started appearing and at the end of the exposure i.e., 96 hpf, the otolith was fully developed and seen in all the groups. The tail formation was found normal, straight line in all the larvae of the groups. There was no bending or kinking of the tail observed among the larvae throughout the study. The notochord was found normal, and extended from the cranium to the cauda parts in all the larvae among the groups. No abnormality was observed in the notochord structure after exposure to M. chamo. Overall, no physical changes were found in all the groups, after the microscopical observation.
Morphological characteristics assessed as a measure for zebrafish embryo development effect of M. chamo. ɸ, 6C and 30C at different time points.
Larval image at 96 hpf where red colour indicates the length, yellow colour indicates eye area, Green colour for yolk sac area and pink colour for the pericardial area of zebrafish larvae.
Effects of M. chamo. ɸ, 6C and 30C on length (a), eye diameter (b), yolk sac area (c), pericardial area (d) of the zebrafish larvae after 96 hpf. All values are Mean ± SEM (One-way ANOVA followed by Turkey’s post hoc test).
Effects of M. chamo. ɸ, 6C and 30C on heart rate of embryos at 48, 72 and 96 hpf. All values are Mean ± SEM, (One way ANOVA followed by Turkey’s post hoc test).
DISCUSSION
Fetal development is a highly organized process in which complex modifications occur in the specified time, and molecular as well as cellular modifications can manifest throughout the entire organism. It is essential to evaluate the teratogenic and embryotoxic effects of medicinal plants on fetal development must be assessed because several herbal formulations are made from toxic sources or have teratogenic effects. These types of formulations are becoming more popular in the global healthcare system without having their toxicological profiles disclosed. The present study was designed to investigate whether the exposure of M. chamo. showed toxic effects at the early life stage in the embryos of zebrafish.
Overall, the results of this GC-MS/MS analysis provided valuable insights into the chemical constituents of M. chamo. The identification of a wide range of carbohydrates, fatty acids, and other bioactive compounds supported the traditional use of chamomile for various health conditions and suggests potential avenues for future research and development of chamomile-based therapies (Milovanovic et al., 2023; Dai et al., 2022).
Zebrafish are popular models for testing lethality and teratogenicity due to their advantages over other models (Zon, Peterson, 2005; Brannen et al., 2010). Due to the similarity in genetics and physiology of zebrafish embryos and humans, this model gains attention for researching developmental toxicity and using to predict how people may react to different chemicals (Jayasinghe, Jayawardena, 2019; Chahardehi et al., 2020; Cassar et al., 2020). Understanding chamomile's safety profile in zebrafish embryos offers useful information on possible impacts on human development and health (Jarque et al., 2020; Song et al., 2021). There are number of studies available regarding the use of herbal medicines, their benefits, and the risks associated with being a mother. Studies have shown that M. chamo. administration during pregnancy can be safe and effective in decreasing vomiting and depressed reflexes (Modares et al., 2012). Additionally, positive effect is found on spontaneous abortion and histopathological changes in the placenta and liver associated with STZ diabetes in pregnant female rats after the administration of M. chamo (Namjooyan et al., 2011). On the other hand, frequent usage of chamomile during pregnancy is associated with an increased risk of kidney enlargement, cardiac abnormalities, miscarriages, and preterm labor (Cuzzolin et al., 2010; Sarecka-Hujar, Szulc-Musioł, 2022). Regular use of the third trimester is also associated with a lower birth weight and a higher risk of preterm birth (Trabace et al., 2015; Artur et al., 2017). Chamomile use during pregnancy may be harmful and have adverse effects on the fetus, causing significant controversy in the literature. Therefore the present study is designed to investigate the embryotoxic and developmental effects of M. chamo. using zebrafish embryos. So far, our work has shown the first source of evidence regarding M. chamo. and its teratogenic effects as a natural botanical drug utilizing the zebrafish bioassay.
The present study revealed that M. chamo.-ϕ (the highest concentration) showed a mild effect of delaying hatching rate and mortality in zebrafish embryos as shown in a previous study which showed the toxic effect of M. chamo. in larvae of Gulex pipens L (Gayar, Shazli, 1968). In contrast to this, the present study revealed the ultra-high dilution of M. chamo. i.e. 6C and 30C (having dilution factors of 1012 and 1060 respectively) were found safe in case of both mortality as well as hatching rate after 96-hour exposure to zebrafish embryos.
It has been reported that the fundamental body structure of zebrafish is formed at 24 hpf, the hatching occurs between 48 and 72 hpf, and the major organ organogenesis and larval development occur at 96 hpf (Kimmel, Ballard 1995). For evaluating the effect on the development of organisms in early-life stages, the exposure to 96 hpf along with these time points was considered using morphological techniques. Direct or microscopic observation of morphological changes can be done using a variety of model species, including Daphnia magna and zebrafish, whose embryos are completely transparent (Mu et al., 2013; Toumi et al., 2015). In the present study, morphological methods were used to examine the impact of M. chamo. on the early life stages of zebrafish development. Based on these findings, M. chamo. not caused early-life developmental abnormalities in zebrafish, including somite cell formation, tail detachment, yolk sac, pericardial region, eye size, otolith, blood circulation, and tail formation. These all parameters were found normal in all the treatment groups. In addition to these parameters, the heart is the first organ to develop and function in vertebrates (Wang et al., 2006). It is complex, morphogenetic, and functional elements that interact to build mature organs (Matrone et al., 2015). Similarly, cardiac deformities may lead to aberrant growth or even death. It has been reported that significant heart deformity and malfunction caused by acrylamide in zebrafish embryos, led to a compromised cardiovascular system. (Huang et al., 2018).
According to the obtained results, no abnormalities in larvae, including yolk sac edema, pericardial edema, and heart rate were observed after the 96-hour exposure of M. chamo. to zebrafish. The obtained results showed that M. chamo. at high dilution (6C and 30C) exhibited neither lethal nor developmental toxicity in zebrafish. Taken all together, it can be hypothesized that the consumption of M. chamo. 6C and 30C at the time of pregnancy could be found safe and may be used for pregnancy-related complications. The present study on chamomile safety using the zebrafish embryo model enhances our understanding of its potential benefits and risks, providing evidence-based recommendations for clinical practice and public health. However, research using larger animal models is recommended to verify and validate the impact of M. chamo. as well as molecular level analysis may be required in the future.
CONCLUSIONS
This present research has shown that 0.1% M. chamo. mother tincture showed a mild effect on mortality and hatching rate. In contrast to this the 6C and 30C potencies (high dilution) of M. chamo. found safe with no effect on the hatching rate. Additionally, there were no morphological changes that occurred in the early life stage of zebrafish, like effect on eyes, length, area of the yolk sac and pericardial, notochord, otolith, somite cell, etc., after the exposure of M. chamo. Since this herbal medicine is usually consumed at the time of pregnancy-related issues, the present study indicates that M. chamo. at high dilution could be safe at that time. However, to prove that M. chamo. is safe for expectant mothers, a thorough toxicity assessment on a higher animal model together with a molecular investigation would be conducted.
ACKNOWLEDGEMENTS
We are thankful to Dr. Subhash Kaushik, DG, CCRH, Officer In-charge, DDPRCRIH, Noida, and Dr. Anil Khurana, Former Director General, CCRH, for providing administrative support.
REFERENCES
- Amsterdam JD, Shults J, Soeller I, Mao JJ, Rockwell K, Newberg AB. Chamomile (Matricaria recutita) may provide antidepressant activity in anxious, depressed humans: an exploratory study. Altern Ther Health Med. 2012;18( 5):44-9. PMID: 22894890.
- Artur L. Belica, Nenad B. Cetkovic, Natasa B, Natas P. Herbal therapy in pregnancy - what to expect when you expect?. Nat Prod Commun. 2017;12(12):1957-69.
- Boericke W. Boericke’s new manual of homeopathic materia medica with reportory. 3rd revised & augmented edition based on 9th edition, B. Jain Publishers Pct Ltd. 1998;166-8.
-
Brannen KC, Charlap JH, Lewis EM. Zebrafish teratogenicity testing. Methods Mol Biol. 2013;947:383401. doi: 10.1007/978-1-62703-131-8_28.
» https://doi.org/10.1007/978-1-62703-131-8_28. - Brannen KC, Panzica-Kelly JM, Danberry TL, Augustine-Rauch KA. Development of a zebrafish embryo teratogenicity assay and quantitative prediction model. Birth Defects Res B Dev Reprod Toxicol. 2010; 89:66-77.
-
Cassar S, Adatto I, Freeman JL, Gamse JT, Iturria I, Lawrence C, et al.. Use of zebrafish in drug discovery toxicology. Chem Res Toxicol. 2020;33(1):95-118. doi: 10.1021/acs.chemrestox.9b00335.
» https://doi.org/10.1021/acs.chemrestox.9b00335. - Chahardehi A, Arsad H, Lim V. Zebrafish as a successful animal model for screening toxicity of medicinal plants. Plants (Basel, Switzerland). 2020;9(10): 1-35.
-
Cuzzolin L, Francini-Pesenti F, Verlato G, Joppi M, Baldelli P, Benoni G. Use of herbal products among 392 Italian pregnant women: focus on pregnancy outcome. Pharmacoepidemiol Drug Saf. 2010; 19(11):1151-8. doi: 10.1002/pds.2040.
» https://doi.org/10.1002/pds.2040. - Dach K, Yaghoobi B, Schmuck M, Carty D, Morales K, Lein P. Teratological and behavioral screening of the national toxicology program 91-compound library in zebrafish (Danio rerio). Toxicol Sci. 2019;167(1):7791.
- Dai YL, Li Y, Wang Q, Niu FJ, Li KW, Wang YY, et al. Chamomile: a review of its traditional uses, chemical constituents, pharmacological activities and quality control studies. Molecules. 2022;28(1):133.
- Gayar F, Shazli A. Toxicity of certain plants to Culex pipens L.larvae (Diptera: Culicidae) Bull Soc Entomol (Egypt). 1968;52:467-75.
-
Hashempur MH, Lari ZN, Ghoreishi PS, Daneshfard B, Ghasemi MS, Homayouni K, et al.. A pilot randomized double-blind placebo-controlled trial on topical chamomile (Matricaria chamomilla L.) oil for severe carpal tunnel syndrome. Complement Ther Clin Pract. 2015;21( 4):223-8. doi: 10.1016/j.ctcp.2015.08.001.
» https://doi.org/10.1016/j.ctcp.2015.08.001. - Huang M, Jiao J, Wang J, Xia Z, Zhang Y. Characterization of acrylamideinduced oxidative stress and cardiovascular toxicity in zebrafish embryos. J. Hazard Mater. 2018;347:451e460.
-
India. Homoeopathic Pharmacopoeia Committee. Homoeopathic pharmacopoeia of India (H.P.I.). Delhi: Controller of Publications; 1974. Available from: https://books.google.co.in/books?id=83JFAAAAYAAJ
» https://books.google.co.in/books?id=83JFAAAAYAAJ -
Jarque S, Rubio-Brotons M, Ibarra J, Ordoñez V, Dyballa S, Miñana R, et al.. Morphometric analysis of developing zebrafish embryos allows predicting teratogenicity modes of action in higher vertebrates. Reprod Toxicol. 2020;96:337-48. https://doi.org/10.1016/j.reprotox.2020.08.004
» https://doi.org/10.1016/j.reprotox.2020.08.004 -
Jayasinghe CD, Jayawardena UA. Toxicity assessment of herbal medicine using zebrafish embryos: A systematic review. Evidence-Based Complementary Altern Med. 2019; 2019:17. https://doi.org/10.1155/2019/7272808
» https://doi.org/10.1155/2019/7272808 -
Kimmel C, Ballard W. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203(3):253-310. Doi:10.1002/aja.1002030302.
» https://doi.org/10.1002/aja.1002030302 - Matrone G, Wilson K, Mullins J, Tucker C, Denvir M. Temporal cohesion of the structural, functional and molecular characteristics of the developing zebrafish heart. Differ Res. Biol Divers. 2015;89:117e127.
-
Mekonnen A, Yitayew B, Tesema A, Taddese S. In vitro antimicrobial activity of essential oil of Thymus schimperi, Matricaria chamomilla, Eucalyptus globulus, and Rosmarinus officinalis. Int J Microbiol. 2016; 2016:9545693. doi: 10.1155/2016/9545693.
» https://doi.org/10.1155/2016/9545693. - Milovanovic S, Grzegorczyk A, Świątek Ł, Grzęda A, Dębczak A, Tyskiewicz K, et al. a novel strategy for the separation of functional oils from chamomile seeds. Evidence-Based Complementary Altern Med. 2023;16( 8):1806-1821.
- Modares M, Besharat S, Rahimi Kian F, Besharat S, Mahmoudi M, Salehi S. Effect of Ginger and Chamomile capsules on nausea and vomiting in pregnancy. J Gorgan Univ Med Sci. 2012;14:46-51.
- Mu X, Pang S, Sun X, Gao J, Chen J, Chen X, et al.. Evaluation of acute and developmental effects of difenoconazole via multiple stage zebrafish assays. Environ. Pollut. 2013;175:147e157.
- Naji T, Hossenzadeh Sahafi H, Saffari M. The effects of phytoestrogens Matricaria recutita on growth, maturation of oocytes in the three spot gourami (Trichogaster trichopterus). ISFJ. 2015;23:85-94.
-
Namjooyan F, Panahi M, Ahmadpour F, Darvish A, Azemi M, Samaee H, et al.. Effect of Matricaria chamomilla L. extract on fetal absorption, placenta structure and liver of diabetic pregnant rats. Planta Med. 2011;77. PM184 DOI: 10.1055/s-0031-1282942.
» https://doi.org/10.1055/s-0031-1282942. -
Organization for Economic Co-operation and Development. Test No. 236: Fish Embryo Acute Toxicity (FET) Test; 2006. Assessed on April 24, 2023 at: https://www.oecd-ilibrary.org/environment/test-no-423-acute-oral-toxicity-acute-toxicclassmethod_9789264071001-en
» https://www.oecd-ilibrary.org/environment/test-no-423-acute-oral-toxicity-acute-toxicclassmethod_9789264071001-en - Ortiz M, Fernandez-Martínez E, Soria-Jasso L, LucasGómez I, Villagómez-Ibarra R, González-García M,et al.. Isolation, identification and molecular docking as cyclooxygenase (COX) inhibitors of the main constituents of Matricaria chamomilla L. extract and its synergistic interaction with diclofenac on nociception and gastric damage in rats. Biomed. Pharmacother. 2016; 78:248-56.
-
Piersen CE. Phytoestrogens in botanical dietary supplements: Implications for cancer. Integr Cancer Ther. 2003; 2:120-38. doi: 10.1177/1534735403002002004.
» https://doi.org/10.1177/1534735403002002004. -
Posadzki P, Alotaibi A, Ernst E. Adverse effects of homeopathy: a systematic review of published case reports and case series. Int J Clin Pract. 2012;66( 12):1178-88. doi: 10.1111/ijcp.12026.
» https://doi.org/10.1111/ijcp.12026. - Prasch AL, Teraoka H, Carney SA, Dong W, Hiraga T, Stegeman JJ, et al.. Aryl hydrocarbon receptor 2 mediates 2,3,7,8-tetrachlorodibenzo-p-dioxin developmental toxicity in zebrafish. Toxicol Sci. 2003;76(1):138-50.
- Samuel H. Full text of "Materia medica pura". London. 1880; 379-99.
-
Santoro MM. Zebrafish as a model to explore cell metabolism. Trends Endocrinol Metab. 2014;25( 10):546-54. doi: 10.1016/j.tem.2014.06.003.
» https://doi.org/10.1016/j.tem.2014.06.003. -
Sarecka-Hujar B, Szulc-Musioł B. Herbal medicines-are they effective and safe during pregnancy? Pharmaceutics. 2022;14( 1):171. doi: 10.3390/pharmaceutics14010171.
» https://doi.org/10.3390/pharmaceutics14010171. -
Sharifi H, Minaie MB, Qasemzadeh MJ, Ataei N, Gharehbeglou M, Heydari M. Topical use of Matricaria recutita l (chamomile) oil in the treatment of monosymptomatic enuresis in children: a doubleblind randomized controlled trial. J Evid Based Complementary Altern Med. 2017;22(1):12-7. doi: 10.1177/2156587215608989.
» https://doi.org/10.1177/2156587215608989. - Sharma M, Prajapati S, Kumar A, Kumar GVN, Gupta P. Effect of acute exposure of belladonna mother tincture on zebrafish embryonic development, Indian J Pharm Sci. 2021a;83(5):947-54.
- Sharma M, Prajapati S, Kumar A, Tripathi A, Kumar GVN, Gupta P. Safety evaluation of syzygium jambolanum on the development of zebrafish embryos. Indian J Pharm Educ Res. 2021b;55(1):198-204.
- Singh O, Khanam Z, Misra N, Srivastava m. Chamomile (Matricaria chamomilla l.): an overview Pharmacogn Rev. 2011;5(9):82.
-
Song Y-S, Dai M-Z, Zhu C-X, Huang Y-F, Liu J, Zhang C-D, et al. Validation, optimization, and application of the zebrafish developmental toxicity assay for pharmaceuticals under the ICH S5(R3) Guideline. Front Cell Dev Biol. 2021; 9:721130. doi: 10.3389/ fcell.2021.721130.
» https://doi.org/10.3389/fcell.2021.721130 -
Sridharan S, Archer N, Manning N. Premature constriction of the fetal ductus arteriosus following the maternal consumption of camomile herbal tea. Ultrasound Obstet Gynecol. 2009;34(3):358-9. doi: 10.1002/uog.6453.
» https://doi.org/10.1002/uog.6453. - Toumi H, Boumaiza M, Millet M, Radetski C, Camara B, Felten V, et al.. Investigation of differences in sensitivity between 3 strains of Daphnia magna (crustacean Cladocera) exposed to malathion (organophosphorous pesticide). J. Environ. Sci. Health Part B Pestic. Food Contam Agric Wastes. 2015;50:34e44.
-
Trabace L, Tucci P, Ciuffreda L, Matteo M, Fortunato F, Campolongo P, et al.. "Natural" relief of pregnancyrelated symptoms and neonatal outcomes: above all do no harm. J Ethnopharmacol. 2015;174:396-402. doi: 10.1016/j.jep.2015.08.046.
» https://doi.org/10.1016/j.jep.2015.08.046. -
van Duursen MBM. Modulation of estrogen synthesis and metabolism by phytoestrogens in vitro and the implications for women's health. Toxicol Res (Camb). 2017;6( 6):772-94. doi: 10.1039/c7tx00184c.
» https://doi.org/10.1039/c7tx00184c. - Wang R, Liu K, Zhang Y, Chen X, Wang X. Evaluation of the developmental toxicity induced by e804 in zebrafish embryos. Front Pharmacol. 2020;11:32.
- Wang W, Huang C, Lu Y, Hsin J, Prabhakar V, Cheng C, et al.. Heart targeted overexpression of Nip3a in zebrafi sh embryos causes abnormal heart development and cardiac dysfunction. Biochem Biophys Res Commun. 2006;347:979-87.
- Westerfi eld M. The Zebrafi sh book. A guide for the laboratory use of Zebrafi sh (Danio rerio). 3rd Ed on Eugene, OR, University of Oregon Press. 1995;385.
-
WHO (World Health Organization). Safety issues in the preparation of homeopathic medicines. 2009;1-51. https://iris.who.int/bitstream/handle/10665/44238/9789241598842_eng.pdf
» https://iris.who.int/bitstream/handle/10665/44238/9789241598842_eng.pdf - Zon LI, Peterson RT. In vivo drug discovery in the zebrafi sh. Nat Rev Drug Discov 2005;4:35-44.
-
Associated Editor: Luciana Oliveira Cruz
Publication Dates
-
Publication in this collection
05 Dec 2025 -
Date of issue
2025
History
-
Received
20 Mar 2024 -
Accepted
19 Sept 2024

 Safety profile of Matricaria Chamomilla: insights from zebrafish embryo development toxicity testing
Safety profile of Matricaria Chamomilla: insights from zebrafish embryo development toxicity testing