Abstract
Metformin hydrochloride (MTF) has pharmacological properties for managing inflammatory skin conditions. MTF is a hydrophilic medication. Accordingly, embedding MTF into lipid carriers for enhancing skin penetration presents a challenge. The study aims to optimize the loading of MTF into nanostructured lipid carriers (NLCs) using a 22 full factorial design, employing the solvent injection technique. The NLCs were evaluated for encapsulation efficiency, hydrodynamic diameter, zeta potential, and polydispersity index. Alkalinization of the aqueous phase (pH = 12.5) resulted in maximizing the entrapment of MTF within NLCs. Furthermore, the tested solid lipids impacted the encapsulation of MTF based on their hydrophilic-lipophilic balance. The optimized formulation is composed of a lipid phase incorporating beeswax (75 mg), oleic acid (25 mg), and Span 60 (1% w/w), and an aqueous phase comprised of 1% w/w Tween 80, pH 12.5. The selected formula attained an entrapment efficiency of 53.68 ± 0.27%, a particle size of 333.0 ± 6.4 nm, and a negative surface charge, indicating adequate particles` stability. DSC and Molecular docking analyses confirmed the MTF incorporation within the lipid phase. The outcomes emphasize the importance of optimizing investigations in developing a viable delivery system for MTF to boost its permeation across the skin layers.
Keywords:
Lipid nanoparticles; Nanostructured lipid carriers (NLCs); Metformin Hydrochloride; Molecular docking; Factorial design
Graphical abstract

INTRODUCTION
Numerous biotic, chemical, and physical assaults on human skin trigger a variety of skin illnesses. Pharmaceutical experts have faced significant challenges in developing topical drug formulations due to the stratum corneum’s limited permeability, which is the main impediment to the permeation of therapeutics through the skin (Mendes et al., 2019). The merit of applying medications to the inflamed skin site is that the drug can be targeted specifically, avoiding the side effects associated with oral administration (Bawazeer et al., 2020).
Metformin hydrochloride (MTF), (1,1-Dimethylbiguanide hydrochloride), (Figure 1) is a hydrophilic basic biguanide with a pKa of 12.4. MTF is categorized as a BCS class III in the biopharmaceutical classification system, with high aqueous solubility and limited skin permeation (Metry et al., 2021). Although MTF has mainly a glucoregulatory action, the drug has a significant role in numerous inflammatory skin conditions. The mechanism of MTF in controlling inflammatory skin disorders relies on its potential to suppress the mTOR (mammalian target of rapamycin) pathway and to stimulate AMP-activated protein kinase (AMPK) signaling (Stanescu et al., 2021). Blocking the mTOR pathway can suppress various proinflammatory cytokines, particularly IL-6, VEGF, and TNF-α. These cytokines are the key to the development of many skin conditions. Furthermore, MTF plays a significant prophylactic role by limiting IL-1β secretion, the key mediator of many inflammatory diseases. Diverse inflammatory skin conditions, namely psoriasis, acne, hidradenitis suppurative, allergic contact dermatitis, and acanthosis nigricans, have been explored to respond well to MTF (Chang, Choi, 2020).
Nanotechnology is a unique approach that can effectively boost the topical delivery of medications without changing their molecular structure (Álvarez-Trabado, Diebold, Sanchez, 2017; Goyal et al., 2016), making it possible to benefit from MTF’s anti-inflammatory activity on the skin. Several innovative topical drug delivery platforms have been explored to obtain improved therapeutic efficacy of MTF, e.g., bilosomes (Salem et al., 2022), Microneedles (Liu et al., 2018), and niosomes (El-Ridy et al., 2019).
Lipid nanoparticles (LNPs) are biodegradable, lipid-cored systems. They include nanocapsules, nanoemulsions, solid lipid nanoparticles (SLNs), and nanostructured lipid carriers (NLCs). Solvent injection, high-pressure homogenization, high shear homogenization, Hot melt emulsification, and ultrasonication procedures are examples of the techniques used to fabricate LNPs (Battaglia , Gallarate, 2012). SLNs have been widely investigated for topical administration owing to their appropriate association with skin layers and enhanced skin penetration (Mady, Al-Shoubki, Donia, 2021). Although, incorporating water-soluble therapeutics into the hydrophobic lipid core of SLNs are very challenging because of the tendency of hydrophilic medications to partition towards the aqueous phase during the manufacturing stage (El-Ridy et al., 2019). Previously, MTF-loaded SLNs were developed by emulsification and low-temperature solidification methods. However, the drug entrapment efficiency within the produced nanocarriers was relatively low (26.25 ± 2.59%) (Xu et al., 2016). NLCs are colloidal drug delivery systems comprised of a mix of solid lipids and liquid oils. NLCs were developed to surmount the inadequacies of SLNs. The specific configuration of solid and liquid lipids within NLCs constitutes an imperfect matrix structure, hindering the recrystallization process and enabling the nanocarrier to incorporate more drugs (Kenechukwu et al., 2022). Besides, the advantages of NLCs include small particle size, less tendency of medicine expulsion, the capability of encapsulating lipophilic and hydrophilic therapeutics, enhanced in vitro drug release, improved skin permeation, and suitability for many routes of administration. The occlusive effect of NLCs is produced on the skin by forming a hydrophobic monolayer. Subsequently, disruption of the stratum corneum occurs through different mechanisms such as lipid exchange, structural elasticity, polarity change, and stratum corneum fluidization, allowing the drug to permeate the skin (Mendes et al., 2019).
Previous studies have proved the improved skin permeation of water-soluble drugs using lipid nanoparticles. For instance, colchicine, a hydrophilic alkaloid, was loaded in SLNs for transdermal administration using the ultrasonication technique. The drug loaded SLNs provided an enhanced penetration potential across the skin layers compared to the colchicine patch (Joshi et al., 2017). Additionally, according to Charoenputtakun et al.’s (2015) evaluation, iontophoresis conjunction with lipid nanoparticles resulted in improved skin distribution of acyclovir.
The current study aimed to boost the entrapment of MTF within NLCs for maximum therapeutic efficacy. The solvent injection technique was applied to formulate MTF-loaded NLCs. To design an optimized nanocarrier formulation, levels of excipients and the process parameters should be rigorously controlled. A 22-full factorial layout was employed for optimizing variables. The effect of external aqueous phase pH (7 or 12.5) and type of solid lipids (Beeswax or Compritol® 888ATO) on entrapment efficiency percentage, hydrodynamic size, particles` homogeneity, and surface charge were examined. The optimum MTF-laden NLC formulation can serve as a viable platform for future studies on animal models of inflammatory skin disorders.
MATERIAL AND METHODS
Material
Metformin Hydrochloride (MTF) was kindly gifted from Chemical Industries Development (CID Co., Assiut, Egypt). Compritol®888 ATO, Capryol® 90, and Labrasol® were a kind supply from Gattefossée (Saint-Priest Cedex, France). Stearic acid, Tween 80, Acetone, Methanol, and Ethanol were obtained from El Nasr Pharmaceutical Chemicals (Cairo, Egypt). Beeswax was obtained from ISOCHEM fine chemicals (Vert-Le-Petit - France). Oleic acid was bought from Alpha Chemicals Co. (Cairo, Egypt). Jojoba oil was obtained from the Imtenan group (Obour City, Egypt). Spectra/Por® dialysis membrane, 12,000-14,000 molecular weight cut off, was purchased from Spectrum Laboratories Inc. (Rancho Dominguez, CA, USA).
Liquid oil selection
The oil employed in the formulation of MTF-loaded NLCs was chosen according to the saturation solubility of MTF in the respective oils (Rizwanullah, Amin, Ahmad, 2017). 3 mL of various oils (Labrasol, Jojoba, Capryol, or oleic acid) were mixed with an excess of MTF and constantly shaken in a mechanical shaker bath (Gallenkamp, Cambridge, UK) at 25 °C for 72 h to attain equilibrium. Then the MTF-liquid lipid mixtures were centrifuged at 10,000 rpm and 25 °C for 15 min using (Benchtop Low-Speed Centrifuge, Aiken, USA). Subsequently, supernatants were separated and diluted with methanol. The dissolved MTF was measured by UV-Vis Spectrophotometer (Shimadzu, model UV-1601 PC, Kyoto, Japan) at λmax =233 nm (Houacine, Adams, Singh, 2020). The solubility tests were performed in triplicate and outcomes were represented as average ± SD.
Differential scanning calorimetry (DSC)
DSC analysis was performed to detect the interaction between the liquid lipid that showed maximum drug solubility and MTF. The DSC thermograms were generated utilizing a thermal analyzer (Linseis STA PT 1600, Germany). (3-5 mg) of MTF powder or the physical mixture of MTF with liquid oil were precisely weighed and sealed in an aluminum pan. Heating was conducted from 25 to 300°C at a 10°C scanning rate. The nitrogen flow rate was set to 40 mL/min.
Molecular docking
AutoDock Vina 4.2.6 was used to perform a molecular investigation of the possible interactions between the selected liquid lipid and MTF. Energy minimization was performed on each ligand using the MMFF94 force field in Avogadro v1.2 (Morris et al., 2009). Furthermore, the Pred-Rxn utility by IBM was used to predict the likely binding modes between the docked complex (MTF-Oleic acid). The obtained outcomes were used to validate laboratory results to get the predicted sites of interactions.
Assessment of solubility of MTF in solid lipids
MTF saturation solubility was determined in beeswax and Compritol®888 ATO. The test was accomplished by dissolving increasing amounts of MTF in the melted solid lipids (1 g) and then calculating the maximum amount of MTF that dissolved in each lipid (Sharma, Baldi, 2018). The solubility tests were conducted in triplicate.
DSC investigation was used to confirm the probable drug-lipid interactions of MTF and the tested solid lipids (beeswax and Compritol®888 ATO). The analysis was performed by the same method described in the Differential scanning calorimetry (DSC) section.
Preparation of MTF loaded nanostructured lipid carriers
The solvent injection technique was adopted to prepare MTF-laden NLCs as previously stated by Eleraky et al. (2020), with some alterations. In brief, 2 mL of acetone/ethanol solvent mixture (3:1 v/v) was used to dissolve the solid lipid-liquid lipid mix (3:1 w/w), Span 60 (1% w/w), and MTF (50 mg/200 μl dist. water) at 75 °C. The organic solution was rapidly injected into the aqueous phase (30 ml dist. water supplemented with 1% tween 80, 75 °C). The produced nanodispersion was stirred at 1000 rpm for 3 h at room temperature in order to let the organic solvent evaporate. Subsequently, sonication of the prepared MTF-NLCs was done for 20 min at room temperature using a bath sonicator (Ultrasonic bath unit SONOREX™ SUPER RK, Bandelin, Germany).
Experimental layout
A 22-full factorial analysis (Design-Expert version 13, State-Ease Inc, Minneapolis, MN), was employed to formulate MTF-loaded NLC preparations. The independent variables selected for the experimental design were the pH of the external aqueous phase (7 or 12.5) (A) and the type of solid lipids (beeswax or Compritol® 888ATO) (B). Each variable was tested in two distinct levels. The percentage of entrapment efficiency and particle size were the measured responses. Table I illustrates the investigated factors and their levels.
Data optimization
To obtain the optimum response values for EE% and particle size (nm), the levels of experimental parameters (A and B) were optimized using polynomial equations that were produced by applying the experimental layout. Based on the anticipated levels of A and B, an optimized formulation was prepared. The measured responses were compared with the estimated values.
Characterization of the developed MTF-laden nanostructured lipid carriers’ formulations
Entrapment efficacy
To determine the percentage of MTF entrapped within NLCs, the dialysis method was applied with some modifications (Swarnakar, Thanki, Jain, 2014). An accurate volume of a freshly prepared MTF-laden NLC formulation (250 µl containing 0.42 mg MTF) was placed into a glass cylinder fitted at its lower end with a cellulose membrane (Spectra/Por® dialysis membrane, molecular weight cut off 12,000-14,000). After that, the glass cylinder was submerged in a beaker containing 50 ml of distilled water. The dialysis was performed for 15 minutes at room temperature. MTF encapsulation within NLCs was determined indirectly by evaluating the amount of unincorporated drug spectrophotometrically at λmax = 233 nm. Calculations were performed according to the following equation:
Particle size analysis, surface charge, and morphology assessment
The fabricated MTF-NLCs’ hydrodynamic size and polydispersity index were determined utilizing Malvern Zetasizer (Nano Series-based dynamic light scattering (DLS) technology) at 25 °C at a fixed angle of 173°. Each MTF-loaded NLC formulation was sonicated for 2 min, before measurement (Gordillo-Galeano, Mora-Huertas, 2021). The surface charge of MTF-NLCs was computed using electrophoretic mobility assessment (Clogston, Patri, 2011). Measurements were performed in three separate runs. Scanning electron microscope (SEM) (JEOL, JSM-5400LV, Japan) was employed to image the optimum MTF-loaded NLC formulation (F4). The sample was fixed on a carbon holder and stained with a 150°A coat of gold. SEM images were captured by Pentax Z-50P Camera at an accelerating voltage of 15 kV(Abdel-Rahman et al., 2023).
Statistical analysis
Graph Pad software (GraphPad Prism 5.0, GraphPad, San Diego, CA, USA) was applied for the statistical analysis. The differences between experimental groups were analyzed using the Newman-Keuls post-hoc test, one-way analysis of variance (ANOVA). A probability was deemed statistically significant if it was less than 0.05 (p <0.05). Every experiment was conducted in triplicates and the outcomes were presented as means ± SD.
RESULTS AND DISCUSSION
Selection of liquid oil
The aim of this research was to develop MTF-laden NLCs with high entrapment efficiency to enable improved therapeutic efficiency and enhanced drug permeation through the skin. The hydrophilic MTF has limited skin penetration. The choice of the proper lipids to create a solid-liquid lipid matrix based on their ability to dissolve the drug, is one of the key parameters in the production of lipid nanoparticle dispersion (Negi, Jaggi, Talegaonkar, 2014). Consequently, the solubility of MTF was tested in a variety of liquid lipids, to select the most suitable one to entrap high MTF amounts in NLCs. The physicochemical properties of the tested liquid oils are illustrated in Table II.
The best MTF solubility was detected in oleic acid. Oleic acid is a monounsaturated fatty acid derivative (Sharma et al., 2009) (HLB =1). The solubility of MTF in oleic acid reached 12.018 ± 0.026 mg/ml, Figure 2. This finding could be attributed to a proper interaction between the liquid oil and MTF hydrochloride resulting in the formation of lipophilic salt (MTF-oleate). The lipophilic salt formed via a simple metathesis reaction between the MTF hydrochloride salt and the different counterions sodium salt leading to enhancing MTF lipid solubility was previously confirmed by Saeed et al. (2021). This finding does not adhere to the HLB proportionality rule. Oleic acid with a low HLB value has low polarity and a lipophilic nature that enhances the loading of lipophilic drugs. However, due to the basic nature of MTF, it tends to interact with oleic acid forming a salt. On the other hand, the solubility of MTF in Labrasol®, jojoba oil, and capryol® 90 was found to be 2.504 ± 0.07, 2.075 ± 0.106, and 0.812 ± 0.017mg/ml, respectively. The solubility of MTF increased with increasing polarity of the used oils (Table II).
Screening of the solubility of MTF in different liquid lipids. The highest solubility of MTF was observed in oleic acid (12.018 ± 0.026 mg/ml). **Statistically significant difference (p < 0.01), ***Statistically significant difference (p < 0.001).
In agreement with the obtained outcomes, Devani et al. (2010), indicated from the Mefenamic acid solubility study in different oils that Labrasol® showed the highest drug solubility over the other used oils (Labrafac CM 10®, Labrafac Lipophile WL 1349, Labrafil M 1944 CS, Labrafil WL 2609 BS®, and Labrafil M 2125 CS). The results were explained based on the hydrophilic nature of the drug, which has a higher affinity to more polar oils.
DSC Analysis
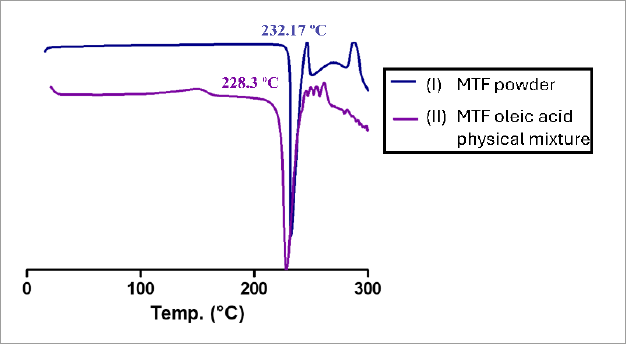
DSC scanning was employed to investigate the possible interactions between MTF and oleic acid. Figure 3A depicts the DSC thermogram of MTF powder, exhibiting a sharp endothermic peak at 232.17 ºC (Tonset=243 ºC, TPeak= 232.17 ºC, Enthalpy= -385.64 J/g) which matches its intrinsic melting point (Acebedo-Martínez et al., 2023). Subsequently, a huge endothermic peak was observed at 247-290 ºC, attributed to the decomposition of the drug (Mady, Al-Shoubki, Donia, 2021). DSC scan of the MTF-oleic acid physical mixture (Figure 3B) displays a sharpened melting endotherm at 228.3 ºC (Tonset= 218 ºC, TPeak= 228.3 ºC, Enthalpy= -575.95 J/g). The slight depression of the melting endotherm and the widening of the peak observed in the physical mixture may be due to drug miscibility in the oil and partial drug conversion to an amorphous form (Kenechukwu et al., 2022).
DSC thermogram of (I) MTF powder, (II) MTF oleic acid physical mixture. The changes observed in the characteristic endothermic peak of MTF when combined with oleic acid, confirm the formation of a new lipophilic salt of MTF with oleic acid.
Molecular docking
The in silico assessment attempted to evaluate the binding affinity of the selected liquid oil (oleic acid) with the drug molecule and examine the stability of the formed mixture (Albasri, Kumar, Rajagopal, 2023). The modeling provides structure-based evidence for the affinity between MTF and oleic acid. Molecular docking revealed favorable binding affinities −1.5 kcal/mol, with two hydrogen bonding interactions at distances 2.41°A and 2.57°A, identified between metformin’s NH2 group and hydroxyl moieties on oleic acid, as represented in Figure 4A. While Figure 4B illustrates the predicted forward reaction by The Pred-Rxn with a prediction score of 2.3.
A) 3D interaction between MTF molecule (gray and blue sticks) and oleic acid (gray and red sticks). The H-bonds formed between MTF and oleic acid are described by dotted green lines. B) The predicted structure from Pred-Rxn software. The observed results confirm the formation of Metformin-oleate lipophilic salt.
Determination of solubility of MTF in solid lipids
The solubility experiment demonstrated that beeswax has a better capability to dissolve MTF (3.67 ± 0.57 mg/g) more than Compritol®888 ATO (0.817 ± 0.76 mg/g). The results correlated well with HLB values of the lipids; beeswax = 12, Compritol®888 ATO = 2 (Hassan, 2018). DSC measurements investigated the effect of the association between MTF and the solid lipids. An observed reduction in the crystallinity of MTF was pointed out after integration with beeswax in the physical mixture, Figure 5A. Lipid crystallinity significantly impacts both the drug release from NLCs and their ability to load drugs (Kiss et al., 2019). On the other hand, when MTF and Compritol®888 ATO were mixed, the melting point of MTF slightly decreased with a peak widening, Figure 5B. The characteristic endothermic peak of MTF changed from 232.17 ºC (Tonset= 243 ºC, TPeak= 232.17 ºC, Enthalpy= -385.64 J/g) to 230.64 ºC (Tonset= 220.46 ºC, TPeak= 230.64 ºC, Enthalpy= 401.57 J/g) in the physical mixture (MTFCompritol®888 ATO). This shift could be related to the drug’s partial transition to the amorphous form with a little miscibility in Compritol®888 ATO.
DSC thermogram of (A) Beeswax (BW), MTF powder and physical mixture of Beeswax and MTF. (B) Compritol®888 ATO, MTF powder and physical mixture of Compritol®888 ATO and MTF. The disappearance of MTF melting endotherm in the MTF-beeswax physical mix denotes the reduction of drug crystallinity. The slight reduction in melting point of MTF after incorporation as a physical mixture with Compritol®888 ATO is possibly due to a partial transition to the amorphous form of the drug.
Factorial design analysis
The “design of experiments” (DOE) approach refers to a process used to set up tests where a range of specific parameters are used to examine how they affect different outcomes to produce a well-designed formulation. From preliminary investigations, it was observed that the external aqueous phase pH and the type of solid lipid exhibited a notable impact on the efficiency of MTF encapsulation (EE%) and the particle size of the prepared NLCs. Therefore, these variables were selected for the following assessments on the meticulous fabrication of NLCs. Utilizing Design-Expert® software (22 full factorial layouts), version 13.0.0, Stat-Ease Inc., Minneapolis, MN, USA, the data generated for each response was investigated. The generated polynomial equations (1-3) display the magnitude and the effect of each factor on the measured responses. Synergistic and antagonistic impacts were denoted by positive and negative signs, respectively. Residual and ANOVA statistical analysis revealed model validation and the reasonable agreement between the anticipated and derived R-squared values. To fulfill the ANOVA presumption, the Box-Cox plot recommended power transformation (lambda= -2.12) for particle size, as applied in equation 3. ANOVA assessment illustrated the statistical significance of the evaluated responses as the Mean square (MS), degree of freedom (df), P-value, and F-value (Table IV). The outcomes indicated that altering the external aqueous phase pH from 7 to 12.5 had a significant agonistic influence on the EE% with a P-value < 0.0001. Moreover, changing pH to alkaline resulted in a significant synergistic impact on the size of NLCs with a P-value < 0.0001. The solid lipid used (beeswax or Compritol®888 ATO) affected the EE% agonistically with a P-value of 0.004 and particle size antagonistically with a P-value of < 0.0001. The interaction of the two independent factors (AB) had a significant antagonistic impact on particle size with a P-value of 0.0066. The computed results are presented in the form of a normal % probability plot and 3D surface graph, in Figures 6 & 7.
Normal plot shows the effect of dependent variables A: pH, B: solid lipid type, and AB on each response; EE% (A), particle size (B). pH (A) showed positive effects on EE% and particle size of MTF-NLCs. The type of solid lipid (B) exhibited positive effects on EE% and negative influence on the particle size of MTF-NLCs. The interaction (AB) displayed negative influence on particle size.
Graphical illustration of the influence of factors A: pH, B: solid lipid type on the evaluated responses EE% (A), particle size (B), and the desirability (C). pH (A) showed positive effects on EE% and particle size of MTF-NLCs. The type of solid lipid (B) exhibited positive effects on EE% and a negative influence on the particle size of MTF-NLCs. The interaction (AB) displayed a negative influence on particle size. The optimum formulation with maximum desirability value (0.7) was demonstrated.
Impact of the parameters on EE%
MTF-loaded NLCs` entrapment efficiency (EE%) varied from 29.187 ± 1.08% to 53.68 ± 0.27% (Table III). Elevating the pH of the external phase from 7 (neutral, distilled water) to 12.5; resulted in a significant positive impact on EE% of the developed formulations (Table IV, Figures 6A & 7A). Altering the pH of the external aqueous phase to 12.5 increased the proportion of the non-ionized form of MTF and subsequently reduced its solubility. The influence of pH adjusting on the encapsulation of hydrophilic drugs within PLGA nanoparticles has previously been investigated by Khalil et al. (2014) and Cohen-Sela et al. (2009). Moreover, Leo et al. (2004) indicated a dramatic elevation in the entrapment efficiency of Cloricromene (AD6) within poly (d, L-lactide) (PLA) nanoparticles, when the aqueous phase pH increased to 11. Besides, the incorporation of beeswax as a solid lipid resulted in a significant rise in the entrapment of MTF within NLCs compared to Compritol®888 ATO (Table IV, Figures 6A & 7A). The evaluation results can be ascribed to the higher affinity of the hydrophilic drug to the more polar solid lipid owing to its better solubility. Consequently, compared to Compritol®888 ATO (HLB = 2), Beeswax (HLB = 9) has a more hydrophilic nature, allowing a better entrapment of the water-soluble drug (Elbahwy et al., 2017).
Although the incorporation of hydrophilic therapeutics in lipoidal nanoparticles is challenging. Some previous studies went over this obstacle. For instance, Xu et al. (2016) loaded 26.25 % and 33.6% of MTF in both SLNs and chitosan-modified SLNs, respectively, using the emulsification solidification technique. Recently, Shete, Deshpande and Shende (2023) maximized the entrapment of MTF within NLCs (60.10 ± 2.23 %), using hot melt emulsification and probe-sonication techniques. Compared to the obtained research findings, using the solvent injection technique provided an increase in MTF encapsulation (53.68 ± 0.27%) via optimizing pH and the type of solid lipid. The solvent injection technique is a simple and rapid method for lipid nanoparticles preparation which represents an advantage over other complicated methods that need sophisticated equipment or utilize unpreferable organic immiscible solvents (Gomaa et al., 2022; Joshi, Adhikari, 2019).
Influence of the parameters on particle size
All formulated MTF-loaded NLCs were in the nano size, ranging from 265.4 ± 13.26 to 637.7 ± 35.75 nm (Table III) which is appropriate for the size range suitable for topical skin delivery (Tekko et al., 2020). Skin layers can be penetrated by particles smaller than 1 µm (Lademann et al., 2011), with molecular weight ≤ 500 Daltons (Bos, Meinardi, 2000). Nevertheless, Agarwal, Katare and Vyas (2001) evaluated earlier the enhanced skin permeability of 5 μm-sized liposomes and niosomes loaded with dithranol. Altering the pH of the aqueous phase led to significant changes in the lipid carriers’ structure according to their composition of the solid lipid. Increasing the pH to 12.5 led to a significant increase in the particle size (Table IV, Figures 6B and 7B). The finding could be explained based on the induced surface tension asymmetry resulting from varying pH. This asymmetry resulted in shape deformation of the lipoidal particles and increases in their hydrodynamic size (Angelova et al., 2018). The fabricated formulations included Span 60 and Tween 80 as surface active agents. The calculated surfactant mixture’s HLB value was 9.85. When the HLB value of the surfactant mixture is close to the HLB value of the solid lipid utilized, the particle size is comparatively lower (Rostamkalaei et al., 2019). This explanation clarifies the size reduction of the NLC preparations formulated using beeswax (HLB = 12) compared to that prepared using Compritol®888 ATO (HLB = 2).
Measurement of surface charge of MTF-loaded NLCs
As observed in (Table III); all fabricated NLCs are negatively charged varying from (-13.1 ± 0.32 to -71.4 ± 0.95 mV). The high values of surface charge denote the stability of the developed nano-lipid carriers. The negative surface charge values resulted from the ionization of glyceryl behenate, a fatty acid composing Compritol® 888 ATO, or the fatty acids composing beeswax. Besides, the ionization of carboxylic groups composing oleic acid increased the negative charge of the fabricated NLCs (Swidan et al., 2016). A similar observation was previously reported (Eleraky et al., 2020)
Regarding the effect of stabilizers on the surface charge of the prepared NLCs, the lipophilic surfactant (Span 60) included in the NLCs formulations tends to disturb the tween film layer surrounding the lipid nanocarriers resulting in exposing the negative charges on the prepared NLC formulations. Previously, Shi et al, (2011) fabricated solid Lipid nanoparticles using different types and ratios of Span & Tween surfactant combination via the double emulsion method. All resulting formulations have acquired negative surface potentials. The apparent high value of the surface charge of the F3 formula (-71.4 ± 0.95 mV) could be ascribed to the difference in the chemical nature and the behavior of the used solid lipids in the basic pH. Compritol® 888 ATO is a synthetic Glyceryl Behenate (C22 fatty acid) and consists of a mix of monobehenate, dibehenate, and tribehenate of glycerol (Brubach et al., 2007). On the other hand, Beeswax is a natural mixture of long-chain fatty alcohols (C24-C38) and fatty acids (Matkin et al., 2006). Compritol® 888 ATO has a shorter carbon chain length, therefore the number of functional groups present could affect their interaction with the alkaline media resulting in an increased negative charge.
Measurement of PDI of MTF-loaded NLCs
The homogeneity of NLCs size distribution could be determined from PDI. Generally, the PDI values differ from zero for the monodispersed NLCs` populations to one for the highly polydispersed lipid vesicles (Danaei et al., 2018). All formulations` PDI varied from 0.473 ± 0.038 to 0.623 ± 0.119 (Table III), indicating a relatively good dispersion of the uniform lipid particles (Moghadam, Zakeri, Samimi, 2019). Employing a size reduction procedure may contribute to the generation of a more homogenous size distribution of the formulated lipid nanocarriers. The external aqueous phase pH (A) and the type of solid lipid (B) had no significant effect on the formulated lipid carriers’ consistency (P-value = 0.248, 0.532, respectively).
Determination of the optimum MTF-loaded NLC formulation
Optimization was done by employing numerical and graphical assessments. The optimum preparation was selected according to the selected criteria for every response. The measured outcome could be converted to a desirability value that rises with increasing its favorability. Based on the developed equations (1-3), optimizing the formula was done by determining the levels of A and B that will maximize EE% and lessen particle size. The optimized formulation lipid phase is comprised of beeswax (75 mg), oleic acid (25 mg), and span 60 (1% w/w), while 1% w/w of tween 80 was added in the aqueous phase, pH 12.5. The selected preparation showed an entrapment efficiency of 53.68 ± 0.27%, a nanometer size range, and a relatively homogenous size distribution. The zeta potential was equal to -13.1 ± 0.32 mV, indicating adequate stability of the nano lipid carriers. The desirability value was equal to 0.7 (Figure 7C).
Morphological analysis
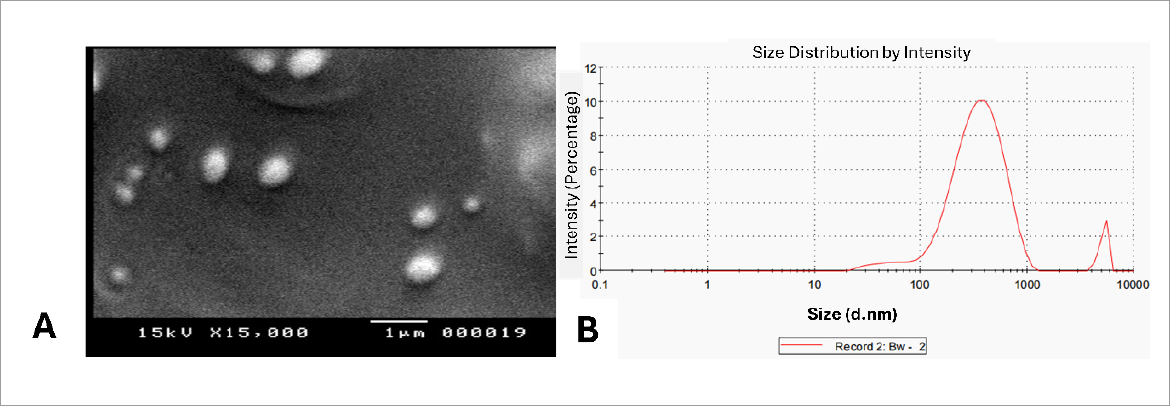
Applying the scanning electron microscope (SEM) indicated the typical morphological aspects of the prepared MTF loaded-NLCs, spherical shape (Figure 8A). The image demonstrated a consistent distribution in the nano-size range. Additionally, the determined size agreed well with that acquired by the DLS technique (Figure 8B). In the representative intensity-weighted size distribution profile of the optimized MTF-NLC formulation (F4), two major lipid particle populations with a mean hydrodynamic diameter of 333.0 nm (intensity 95%) and >1μm (intensity 5%) were identified, Figure 8B. Employing a size reduction procedure such as probe sonication, may contribute to the generation of more uniform lipid nanocarriers. Furthermore, future studies have been planned to enhance the uniformity of NLCs by examining the effect of changing the type & concentration of lipophilic and hydrophilic surfactants, varying drug concentration, modifying the volume of drug solution, or using different ratios of solid lipid to liquid lipid in the oil phase.
A) SEM image of the MTF-loaded NLC preparation (F4), and B) particle size distribution curve of the selected F4 formulation which indicated the formation of spherical distinct nanoparticles with an average size of 333.0 ± 6.4 nm.
CONCLUSION
The main hurdle in this study was to embed the highly hydrophilic drug (MTF) into the lipid nanosystem. The MTF loaded-NLCs formulations were prepared using the solvent injection method, which provides a rapid and effective way to prepare lipid nanocarriers, unlike other intricate procedures that require using expensive equipment or employing improper organic immiscible solvents. The MTF nanoparticles were optimized by using a 22-full factorial analysis model. Notably, the aqueous phase pH and the solid lipid type used in the fabrication of the nano-lipoidal carriers demonstrated a significant impact on the MTF encapsulation and the hydrodynamic size of the developed NLC preparations. The optimized formulation attained a high entrapment efficiency of 53.68 ± 0.27 %, and a particle size of 333.0 ± 6.4 nm, suitable for the potential topical delivery. Future perspectives will focus on examining the in vitro release of MTF from the fabricated NLC formulation and the various techniques employed for enhancing the stability of MTF-NLC preparation to withstand different storage conditions for a prolonged time. Besides, an in-vivo animal model will be applied to evaluate the anti-inflammatory efficacy of the fabricated MTF loaded nano lipid carriers.
REFERENCES
- Abdel-Rahman MA, El-Said WA, Sayed EM, Abdel-Wahab A-MA. Synthesis, characterization of some conductive aromatic polyamides/Fe3O4 NPs/ITO, and their utilization for methotrexate sensing. Surfaces. 2023;6:83-96.
- Acebedo-Martínez FJ, Domínguez-Martín A, Alarcón-Payer C, Garcés-Bastida C, Verdugo-Escamilla C, Gómez-Morales J, et al. Metformin-NSAIDs molecular salts: A path towards enhanced oral bioavailability and stability. Pharmaceutics. 2023;15.
- Agarwal R, Katare OP, Vyas SP. Preparation and in vitro evaluation of liposomal/niosomal delivery systems for antipsoriatic drug dithranol. Int J Pharm. 2001;228:43-52.
- Albasri OWA, Kumar PV, Rajagopal MS. Development of Computational In Silico Model for Nano Lipid Carrier Formulation of Curcumin. Molecules. 2023;28:1833.
- Álvarez-Trabado J, Diebold Y, Sanchez A. Designing lipid nanoparticles for topical ocular drug delivery. Int J Pharm . 2017;532(1):204-217.
- Angelova MI, Bitbol AF, Seigneuret M, Staneva G, Kodama A, Sakuma Y, et al. pH sensing by lipids in membranes: The fundamentals of pH-driven migration, polarization and deformations of lipid bilayer assemblies. Biochim Biophys Acta Biomembr. 2018;1860:2042-2063.
- Battaglia L, Gallarate M. Lipid nanoparticles: state of the art, new preparation methods and challenges in drug delivery. Expert Opin Drug Delivery. 2012;9:497-508.
- Bawazeer S, El-Telbany DFA, Al-Sawahli MM, Zayed G, Keed AAA, Abdelaziz AE, et al. Effect of nanostructured lipid carriers on transdermal delivery of tenoxicam in irradiated rats. Drug Delivery. 2020;27:1218-1230.
- Bos JD, Meinardi MM. The 500 Dalton rule for the skin penetration of chemical compounds and drugs. Exp Dermatol. 2000; Viewpoint 9;165-169.
- Brubach JB, Jannin V, Mahler B, Bourgaux C, Lessieur P, Roy P, et al. Structural and thermal characterization of glyceryl behenate by X-ray diffraction coupled to differential calorimetry and infrared spectroscopy. Int J Pharm . 2007;336:248-256.
- Chang J-E, Choi MS. A molecular perspective on the potential benefits of metformin for the treatment of inflammatory skin disorders. Int J Mol Sci. 2020;21:8960-8972.
- Charoenputtakun P, Li SK, Ngawhirunpat T. Iontophoretic delivery of lipophilic and hydrophilic drugs from lipid nanoparticles across human skin. Int J Pharm . 2015;495:318-328.
- Clogston JD, Patri AK. Zeta potential measurement. Characterization of nanoparticles intended for drug delivery. 2011;63-70.
- Cohen-Sela E, Chorny M, Koroukhov N, Danenberg HD, Golomb G. A new double emulsion solvent diffusion technique for encapsulating hydrophilic molecules in PLGA nanoparticles. JCR. 2009;133:90-95.
- Danaei M, Dehghankhold M, Ataei S, Hasanzadeh Davarani F, Javanmard R, Dokhani A, et al. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics. 2018;10.
- Devani M, Ashford M, Craig DQM. The emulsification and solubilisation properties of polyglycolysed oils in self-emulsifying formulations. JPP. 2010;56:307-316.
- Eleraky NE, Omar MM, Mahmoud HA, Abou-Taleb HA. nanostructured lipid carriers to mediate brain delivery of temazepam: Design and in vivo study. Pharmaceutics. 2020;12(5):451.
- El-Ridy MS, Yehia SA, Elsayed I, Younis MM, Abdel-Rahman RF, El-Gamil MA. Metformin hydrochloride and wound healing: from nanoformulation to pharmacological evaluation. J Liposome Res. 2019;29:343-356.
- Elbahwy IA, Ibrahim HM, Ismael HR, Kasem AA. Enhancing bioavailability and controlling the release of glibenclamide from optimized solid lipid nanoparticles. J Drug Delivery Sci Technol. 2017;38:78-89.
- Gomaa E, Fathi HA, Eissa NG, Elsabahy M. Methods for preparation of nanostructured lipid carriers. Methods. 2022;199:3-8.
- Gordillo-Galeano A, Mora-Huertas CE. Hydrodynamic diameter and zeta potential of nanostructured lipid carriers: Emphasizing some parameters for correct measurements. Colloids Surf A. 2021;620:126610-126620.
- Goyal R, Macri LK, Kaplan HM, Kohn J. Nanoparticles and nanofibers for topical drug delivery. JCR. 2016;240:77-92.
- Hassan A. Assignment of Optimum Surfactants Blend and Right Oil Phase Concentration of Oil-in-water Emulsion. Indian J Pharm Sci. 2018;80:334-341.
- Houacine C, Adams D, Singh KK. Impact of liquid lipid on development and stability of trimyristin nanostructured lipid carriers for oral delivery of resveratrol. J Mol Liq. 2020;316:113734-113752.
- Joshi DR, Adhikari N. An overview on common organic solvents and their toxicity. J Pharm Res Int. 2019;28:1-18.
- Joshi SA, Jalalpure SS, Kempwade AA, Peram MR. Fabrication and in-vivo evaluation of lipid nanocarriers based transdermal patch of colchicine. J Drug Deliv Technol. 2017;41:444-453.
- Kenechukwu FC, Isaac GT, Nnamani DO, Momoh MA, Attama AA. Enhanced circulation longevity and pharmacodynamics of metformin from surface-modified nanostructured lipid carriers based on solidified reverse micellar solutions. Heliyon. 2022;8:e09100.
- Khalil R, Hashem F, Zaki H, El-Arini S. Polymeric nanoparticles as potential carriers for topical delivery of colchicine: development and in vitro characterization. Int J Pharm Sci Res. 2014;5:1746-1756.
- Kiss E, Berkó S, Gácsi A, Kovács A, Katona G, Soós J, et al. Design and optimization of nanostructured lipid carrier containing dexamethasone for ophthalmic use. Pharmaceutics. 2019;11:679-696.
- Lademann J, Richter H, Schanzer S, Knorr F, Meinke M, Sterry W, et al. Penetration and storage of particles in human skin: Perspectives and safety aspects. Eur J Pharm Biopharm. 2011; 77; 465-468.
- Leo E, Brina B, Forni F, Vandelli MA. In vitro evaluation of PLA nanoparticles containing a lipophilic drug in water-soluble or insoluble form. Int J Pharm . 2004;278:133-141.
- Liu D, Zhang Y, Jiang G, Yu W, Xu B, Zhu J. Fabrication of dissolving microneedles with thermal-responsive coating for NIR-triggered transdermal delivery of metformin on diabetic rats. ACS Biomater Sci Eng. 2018;4:1687-1695.
- Mady OY, Al-Shoubki AA, Donia AA. An industrial procedure for pharmacodynamic improvement of metformin HCl via granulation with its paracellular pathway enhancer using factorial experimental design. Drug Des Devel Ther. 2021;4469-4487.
- Matkin J, Wang S, Li C, Huang W. Natural mixture of long-chain fatty alcohols and long-chain fatty acids, its obtension from animal and vegetable waxes and its nutraceutical uses. Google Patents. 2006.
- Mendes IT, Ruela ALM, Carvalho FC, Freitas JTJ, Bonfilio R, Pereira GR. Development and characterization of nanostructured lipid carrier-based gels for the transdermal delivery of donepezil. Colloids Surf B. 2019;177:274-281.
- Metry M, Shu Y, Abrahamsson B, Cristofoletti R, Dressman JB, Groot D, et al. Biowaiver monographs for immediate release solid oral dosage forms: metformin hydrochloride. J Pharm Sci. 2021;110:1513-1526.
- Moghadam H, Zakeri M, Samimi A. Mono-Size Distribution Index (MSDI): A new criterion for the quantitative description of size distribution. JPST. 2019;5:71-76.
- Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, et al. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J Comput Chem. 2009;30:2785-2791.
- Negi LM, Jaggi M, Talegaonkar S. Development of protocol for screening the formulation components and the assessment of common quality problems of nano-structured lipid carriers. Int J Pharm . 2014;461:403-410.
- Rizwanullah M, Amin S, Ahmad J. Improved pharmacokinetics and antihyperlipidemic efficacy of rosuvastatin-loaded nanostructured lipid carriers. J Drug Targeting. 2017;25:58-74.
- Rostamkalaei SS, Akbari J, Saeedi M, Morteza-Semnani K, Nokhodchi A. Topical gel of Metformin solid lipid nanoparticles: A hopeful promise as a dermal delivery system. Colloids Surf B . 2019;175:150-157.
- Saeed HK, Sutar Y, Patel P, Bhat R, Mallick S, Hatada AE, et al. Synthesis and characterization of lipophilic salts of metformin to improve its repurposing for cancer therapy. ACS Omega. 2021;6;2626-2637.
- Salem HF, Nafady MM, Ali AA, Khalil NM, Elsisi AA. Evaluation of metformin hydrochloride tailoring bilosomes as an effective transdermal nanocarrier. Int J Nanomed. 2022;1185-1201.
- Sharma A, Baldi A. Nanostructured lipid carriers: A review. J Dev Drugs. 2018;7:1-15.
- Sharma P, Ganta S, Denny WA, Garg S. Formulation and pharmacokinetics of lipid nanoparticles of a chemically sensitive nitrogen mustard derivative: Chlorambucil. Int J Pharm . 2009;367:187-194.
- Shete MB, Deshpande AS, Shende PK. Nanostructured lipid carrier-loaded metformin hydrochloride: Design, optimization, characterization, assessment of cytotoxicity and ROS evaluation. Chem Phys Lipids. 2023;250:105256.
- Shi L, Li Z, Yu L, Jia H, Zheng L. Effects of Surfactants and Lipids on the Preparation of Solid Lipid Nanoparticles Using Double Emulsion Method. J Disper Sci Technol. 2011;32:254-259.
- Stanescu AMA, Simionescu AA, Florea M, Diaconu CC. Is Metformin a Possible Beneficial Treatment for Psoriasis? A Scoping Review. J Pers Med. 2021;11.
- Swarnakar NK, Thanki K, Jain S. Bicontinuous cubic liquid crystalline nanoparticles for oral delivery of Doxorubicin: implications on bioavailability, therapeutic efficacy, and cardiotoxicity. Pharm Res. 2014;31:1219-1238.
- Swidan SA, Ghonaim HM, Samy AM, Ghorab MM. Efficacy and in vitro cytotoxicity of nanostructured lipid carriers for paclitaxel delivery. J Appl Pharm Sci. 2016;6:018-026.
- Tekko IA, Permana AD, Vora L, Hatahet T, McCarthy HO, Donnelly RF. Localised and sustained intradermal delivery of methotrexate using nanocrystal-loaded microneedle arrays: Potential for enhanced treatment of psoriasis. Eur J Pharm Sci . 2020;152:105469.
- Xu Q, Zhu T, Yi C, Shen Q. Characterization and evaluation of metformin-loaded solid lipid nanoparticles for celluar and mitochondrial uptake. Drug Dev Ind Pharm. 2016;42:701-706.
Publication Dates
-
Publication in this collection
20 Jan 2025 -
Date of issue
2025
History
-
Received
04 Mar 2024 -
Accepted
28 June 2024

 Metformin hydrochloride laden nanostructured lipid carriers: A promising strategy for skin diseases
Metformin hydrochloride laden nanostructured lipid carriers: A promising strategy for skin diseases