Abstract
To evaluate the neuropharmacological profile of new spiro thiazepinone and thiazolidinone compounds in CD1 mice after a computer-aided virtual screening based on a GABA-A/BZD site. From a library of 240 pyrazolo[1,4]thiazepin-3-ones and 39 pyrimidinyl thiazolidin-4-ones, two molecular prototypes of each series were selected by virtual screening using the rank consensus molecular docking approach, based on the GABA-A/BZD site. These compounds, coded as cpTP-0, cpTP-1, TAP-2 and cpTAP-2, were synthesised by multicomponent reactions and evaluated by neuropharmacological screening in CD1 mice (100 mg/kg, p.o.). The study revealed that cpTAP-2 exhibited a significant reduction of immobility time during the forced swimming test (FST), while it did not show any major effects in the rota-rod, open field, plus maze, tail suspension, pentylenetetrazole seizures, and barbiturate sleeping time tests. In a dose-response evaluation, cpTAP-2 reduced immobility time during forced swimming test in CD1 male mice at doses of 100 and 300 mg/kg with biologically relevant effect sizes. These results suggest that cpTAP-2 could elicit antidepressant effects possibly related to GABA-A/benzodiazepine site, although other mechanisms could be implicated.
Keywords:
Antidepressant; Drug design; Virtual screening; Spirothiazolidinone; GABA-A/BZD receptor
INTRODUCTION
Anxiety, depression and insomnia are some of the most prevalent psychiatric disorders and are considered to be a public health problem (Liu et al., 2015). The prevalence of these disorders has increased due to the sanitary crisis of COVID-19 (Sinanović, Muftic, Sinanović, 2020), dramatically increasing the relevance of research lines related to new treatments and strategies to assist this population.
Furthermore, the close relationship between anxiety and depression and central nervous system disorders is well known, with insomnia being the most common (Liu et al., 2015), followed by other examples such as epilepsy, which is related to the appearance of depression disorders caused by the stigmatisation that often accompanies the population suffering from this pathology (Zapata, Restrepo-Martínez, Restrepo, 2020). These relationships, along with the existence of a proportion of the population affected by these pathologies that do not respond to the existent pharmacological alternatives (Zhdanava et al., 2021), provides further relevance to the research into new treatments.
A profile of pharmacological properties related to the central nervous system (CNS) has been identified, which includes nitrogen- and sulphur-containing heterocyclic pharmacophores, such as thiazolidine derivatives, with bioactivities ranging from anxiolytic (Bhaumik et al., 2013) to antidepressant (Dhar, Bhaumik, Reddy, 2013), sedative (Faizi et al., 2017) and anticonvulsant (Faizi et al., 2017). Another example of the named heterocyclic pharmacophores is the thiazepine nucleus, which has shown a profile of anxiolytic (Yoshizawa et al., 2020), antidepressant (Pitchai et al., 2021), sedative (Vega et al., 2000) and anticonvulsant activities (Sánchez-Mateo et al., 2003).
This study describes the neuro-pharmacological profile in CD-1 mice of thiazepinone and thiazolidinone compounds designed by virtual screening, as well as the antidepressant effect of cp-TAP-2, possibly related to GABAA/BZD site. This used animal models with appropriate validity to detect acute anxiolytic (Kumar, Bhat, Kumar, 2013), antidepressant, sedative and anticonvulsant effects (Vogel, 2002).
MATERIAL AND METHODS
Animals
Male CD-1 mice aged 6-8 weeks, weighing between 35 and 40 g, and female mice aged 6-7 weeks, weighing 30-35 g, were obtained from the Pharmacy Department Animalarium, (Universidad Nacional de Colombia). They were held in plastic cages with appropriate dimensions, considering the cage population (6 mice/cage), under conditions of controlled temperature and humidity, with a photoperiod of 12 hours light/darkness (lights on at 6 am) and free access to food and water, except on the test day. The animals were subjected to a fasting period of no more than six hours in order to avoid affecting the convulsive threshold (Swinyard et al., 1989). The animals were held under standard conditions. Four animals were used per treatment in the screening stage and five animals for each gender per treatment in the dose-response evaluation. Also, at the end of the experiment, the animals were euthanised in a CO2 chamber. All procedures were conducted following the care principles for the management of laboratory animals. The Ethics Committee of the Faculty of Science at the National University of Colombia endorsed this study.
Biological tests
Irwin test
This test was used to assess the preliminary drug effect on the behavioural and physiological state of mice. The animals were observed at 15, 30, 60 and 120 min, and at 4, 6 and 24 h after administration, recording the presence or absence of: mortality, seizure, erection of the tail (Straub sign), sedation, excitation, abnormal gait, jumps, motor incoordination, abdominal torsion, piloerection, stereotypy, ticks and the increase or decrease in respiration (Roux, Sablé, Porsolt, 2003).
Open field test
This test was used to determine the basal locomotor state of each animal for the allocation. Animals, previously habituated to the test room for one hour, were placed in the centre of the maze (50x50x38 cm) one by one and the total distance travelled by each mouse was recorded by video-tracking for 5 minutes. Additionally, this procedure was used to determine the impact of each treatment on the locomotor capacity of the animals one hour after being dosed.
Rota rod
This test was used to detect potential neurotoxicity of the treatments administered. All of the animals were trained to maintain balance on an axis of three cm in diameter powered by a small engine at 12 rpm. Mice were previously trained on the rotarod for 3 cycles of one minute at a speed of 12 rpm. For testing, the animals were placed on the rotarod one hour after the administration of treatments; then, the speed was set at 12 rpm during 60s and the success or failure of the animal to maintain the balance during this time in a maximum of 10 attempts was recorded.
Elevated plus maze test (EPM)
This is a test to detect potential anxiolytic agents. Animals were divided into groups of 6 and dosed one hour before being placed one by one in the centre of the maze, elevated at 40 cm from the floor, facing a close arm. The behaviour of the animal was video tracked over 5 minutes and the percentages of time and entries in the open arms were recorded (Lister, 1990). The percentage of time in the open arms was considered the primary outcome of the test.
Forced swimming test (FST)
This is a test to detect potential antidepressant agents. Animals were divided into groups of 6 and dosed one hour before being placed one by one into a plastic cylinder (35x24 cm) containing water (20± 2°C) to a height of 13.5 cm. The total immobility time was recorded over 5 minutes (Porsolt, Bertin, Jalfre, 1997) in the screening stage. In the dose-response evaluation stage, the latency time and number of climbings were also recorded. After this time, the animal was dried carefully with paper towels to prevent hypothermia before placing it back in its cage. The total immobility time, defined as movements which are only necessary for the animal to stay afloat (Porsolt, Bertin, Jalfre, 1997), was considered as the primary outcome of the test.
Tail suspension test (TST)
This is also useful for detecting potential antidepressant agents. Animals were assigned to groups of 6 and dosed one hour before being suspended by the tail through a metal loop on the inner surface of a 20 cm high acrylic chamber. Immobility time was recorded over 5 minutes (Steru et al., 1985).
Barbiturate sleeping time
This is a useful test for detecting agents with sedative effects. Animals distributed into groups of 6 and dosed one hour before, were treated with sodic thiopental (40 mg/kg, i.p.). Latency time was recorded from the moment of the injection of sodic thiopental until the moment at which the animal completely lost its gait (Vogel, 2002); the sleeping time was recorded from the loss of the animal’s gait until the moment at which it recovered it (Lapa et al., 2002). The sleeping time was considered the primary outcome of the test and any animal that did not lose its gait after 15 min of the injection was excluded from the analysis.
Seizures induced by pentylenetetrazole (PTZ)
This is a test to detect potentially effective agents to prevent absence seizures. Groups of 6 animals dosed one hour before, were administered with PTZ (GABA antagonist, 85 mg/kg s.c.). An animal that did not show clonic seizures in its head, back or limbs for more than five seconds over 30 minutes of observation after PTZ administration was considered to be protected (Swinyard et al., 1989); after this time, the animals were euthanised in CO2 chamber.
Molecular docking and ADMET properties - in silico evaluation
Structures of compounds cpTP-0, cpTP-1, cpTAP-2 and TAP-2 were drawn in the ADMET Predictor Software (Version 8.0.4.6 Academic license) (Simulations Plus, 2016) and the SMILES codes were generated to allow Lipinski´s properties estimation. Then, the structures were optimised in Avogadro (Version 1.2.0) (Hanwell et al., 2012) using a GAFF force field with the steepest descent algorithm to convergence and were used for molecular docking assays with the crystalline structure of GABA-A receptor extracted from PDB (Code 6DW0) (Zhu et al., 2018). For molecular docking, three different software packages were used: Autodock4 (Version 4.2.6) (Morris et al., 2009), Autodock Vina (Trott, Olson, 2010; Eberhardt et al., 2021) and DOCK6 (Allen et al., 2015). Docking solutions were visualised in Discovery Studio (Version 19.1.0.20298) (Biovia, 2019) and analysed in BZD-site, scores were normalized using Equation 1 (Lanchero, 2016), and binding-energies and interactions were compared with molecular structures of clonazepam, diazepam and flunitrazepam (Handa, Saroha, 2018).
EQUATION 1 - Score normalisation equation. (Lanchero, 2016).
Compounds, reagents and solutions
Compounds cpTP-0 (Figure 1A), cpTP-1 (Figure 1B), TAP-2 (Figure 1C) and cpTAP-2 (Figure 1D) were obtained by chemical synthesis using precursors and solvents without further purification. Reactions were followed by thin layer chromatography (TLC) in Macherey-Nagel pre-coated sheets Alugram® Xtra SIL G/UV254 as a stationary phase and using a mixture of hexane/AcOEt (7:4) as the eluent. Melting points were measured in Electrothermal SMP10 apparatus and are uncorrected, FTIR spectra were measured in KBr pellets on a Nicolet-IS infrared spectrophotometer and the absorptions were reported as wavenumbers (cm-1); NMR spectra were acquired on an FT-NMR Bruker-Avance (400 MHz) spectrometer.
Compounds cpTP-0 and cpTP-1 were synthesised following the method previously reported (Becerra-Rivas, Cuervo-Prado, Orozco-Lopez, 2019), in a multicomponent reaction of aminopyrazoles A and B with cyclopentanone and mercaptoacetic acid in benzene at reflux with azeotropic distillation with Dean-Stark apparatus (Figure 2).
3’-methyl-1’-phenyl-1’,8’-dihydrospiro[cyclopentane-1,4’-pyrazolo[3,4-e][1,4]thiazepin]-7’(6’H)-one (cpTP-0): Yield: 62%, Mp: 197-199°C, IR: (cm-1): 3195 (N-H), 3075 (Ar,C-H), 2928 (C-H), 2850 (C-H), 1678 (C=O), 767 (Ar, H-C-C). 1H-NMR: (400 MHz CDCl3) δ: 1.86-1.89 (m, 2H), 1.99-2.01 (m, 2H), 2.11-2.13 (m, 2H), 2.28-2.31 (m, 2H), 2.37 (s, 3H), 3.27 (s, 2H), 7.25 (bs, 1H), 7.38-7.41 (m, 3H), 7.46-7.50 (m, 2H). 13C-NMR; (100 MHz CDCl3) δ: 15.4, 24.5, 33.0, 40.8, 54.2, 114.0, 125.4, 128.7, 129.8, 133.8, 137.2, 146.2, 171.1.
1’(4-chlorophenyl)-3´-methyl-1’,8’-dihydrospiro[cyclopentane-1,4’-pyrazolo[3,4-e][1,4]thiazepin] -7’(6’H)-one (cpTP-1): Yield: 58%, Mp: 213-215°C, IR: (cm-1): 3278 (N-H), 2999 (Ar,C-H), 2944 (C-H), 2854 (C-H), 1689 (C=O), 773 (Ar, H-C-C) 700 (C-Cl). 1H-NMR: (400 MHz CDCl3) δ: 1.84-1.93 (m, 2H), 1.97-2.05 (m, 2H), 2.11-2.17 (m, 2H), 2.23-2.26 (m, 2H), 2.38 (s, 3H), 3.27 (s, 2H), 7.39 (d, J = 8.8 Hz, 2H), 7.44 (bs, 1H), 7.47 (d, J = 8.8 Hz, 2H). 13C-NMR; (100 MHz CDCl3) δ: 15.4, 24.4, 32.9, 40.7, 54.1, 114.6, 126.4, 129.9, 133.9, 134.4, 135.9, 146.5, 171.3.
Compounds cpTAP-2 and TAP-2 were synthesised by a two-step route, first involving a reaction to obtain 4-phenyl-6-(4-ethoxyphenyl)-2-aminopyrimidine by a Biginelli-type multicomponent reaction using microwave induction and calcium chloride as a catalyst (Becerra-Rivas, Cuervo-Prado, Orozco-López, 2023) (Figure 3).
2-amino-4-(4-ethoxyphenyl)-6-phenylpyrimidine: Yield: 67 %, Mp: 91-92°C, IR: (cm-1): 3324 (N-H), 3196 (N-H), 3030 (Ar, C-H), 2929 (C-H), 2836 (C-H), 1644 (C=N), 1029 (C-O), 820 (Ar, H-C-C), 769 (Ar, H-C-C). 1H-NMR: (400 MHz CDCl ) δ: 1.48 (t, J = 6.8 Hz, 3H), 4.13 (q, J = 6.8 Hz, 2H), 5.25 (s, 2H), 7.02 (d, J = 8.0 Hz, 2H), 7.44 (s, 1H), 7.50-7.53 (m, 3H), 8.05-8.08 (m, 4H). 13C-NMR; (100 MHz CDCl ) δ: 14.8, 55.5, 103.7, 114.2, 127.2, 128.8, 128.9, 130.3, 130.5, 138.1, 161.8, 163.7, 165.8, 166.1.
2-aminopyrimidine was reacted with mercaptoacetic acid and cyclohexanone (to obtain TAP-2) or with cyclopentanone (to obtain cpTAP-2) in toluene with Dean-Stark apparatus to produce target molecules for biological assays (Figure 4) as follows:
2-amino-4-(4-ethoxyphenyl)-6-phenylpyrimidine (873 mg, 3 mmoles) was dissolved in toluene (50 mL) in a two-necked flask with stirring. The solution was heated to boiling and a previously prepared solution of mercaptoacetic acid (552 mg, 6 mmoles), cyclohexanone (882 mg, 9 mmoles) or cyclopentanone (756 mg, 9 mmoles) in 50 mL of toluene was added dropwise while the reaction was heated using Dean-Stark apparatus. Once the dropwise addition ended, the mixture was heated for 24 hours. After the reaction finished (followed by TLC: Hexane/Ethyl acetate (7:4)), it was left to stand for 1 hour and was washed with NaHCO3 (Saturated solution) (3 x 20 mL), brine (5 x 20 mL) and distilled water in a separatory funnel. The organic layer was separated and dried with anhydrous sodium sulphate, before toluene was evaporated under vacuum; the crude product was washed with hexane several times and the solid obtained was recrystallised in ethanol (70%) affording the desired product.
4-[(4-ethoxyphenyl)-6-phenylpyrimidin-2-yl]-1-thia-4-azaspiro[4.4]nonan-3-one (cpTAP-2): Yield: 57%, Mp: 91-92°C, IR: (cm-1): 3030 (Ar, C-H), 2929 (C-H), 2836 (C-H), 1644 (C=N), 1029 (C-O), 820 (Ar, H-C-C), 769 (Ar, H-C-C). 1H-NMR: (400 MHz CDCl3) δ: 1.48 (t, J = 6.8 Hz, 3H), 1,78 - 1,83 (m, 4H), 2,14 - 2,21 (m, 2H), 2,74 - 2,82 (m, 2H), 3.86 (s, 2H), 4.13 (q, J = 6.8 Hz, 2H), 7.02 (d, J = 8.0 Hz, 2H), 7.50-7.53 (m, 3H), 7.95 (s, 1H), 8.13 - 8.15 (m, 4H). 13C-NMR; (100 MHz CDCl3) δ: 14.8, 23.6, 33.4, 39.6, 63.7, 78.7, 109.5, 114.9, 127.4, 128.8, 128.9, 129.0, 131.1, 136.8, 158.0, 161.7, 166.1, 166.2, 171.9.
4-[(4-ethoxyphenyl)-6-phenylpyrimidin-2-yl]-1-thia-4-azaspiro[4.5]decan-3-one (TAP-2): Yield: 53%, Mp: 143 - 145°C. IR (cm-1): 3000 (Ar, C-H), 2927 (C-H), 2856 (C-H), 1682 (C=O), 1579 (C=N), 1045 (C-O), 828 (Ar, C-C-H). 1H-NMR: (400 MHz, CDCl3): δ: 1.08 (m, 1H), 1.46 (t, J = 7.0 Hz, 3H), 1.67 (m, 2H), 2.14 (d, J = 13.1 Hz 2H), 2.42 (dt, J = 4.0 Hz, 13.1 Hz, 2H), 3.75 (s, 2H), 4.12 (q, J = 7.0 Hz, 2H), 7.02 (d, J = 8.9 Hz 2H), 7.52 (m, 3H), 7.94 (s, 1H), 8.12 (m, 4H). 13C-NMR: (100 MHz, CDCl3): δ: 14.9, 24.0, 24.9, 32.4, 38.5, 63.8, 75.8, 109.8, 115.0, 127.5, 128.9, 129.1, 129.2, 131.2, 136.9, 158.2, 161.8, 166.3, 166.4, 172.3.
The following drugs and reagents were also used: glycerine, propylene glycol, clonazepam (Roche®) (0.2 mg/kg), imipramine (34 mg/kg), sodium thiopental (40 mg/kg) and pentylenetetrazol (85 mg/kg).
Experimental design and data analysis
The experimental pharmacological study was developed in two stages; in the first one, the screening stage, the anxiolytic, antidepressant, sedative and anticonvulsant activities of the compounds cpTP-0, cpTP-1, TAP-2 and cpTAP-2 (100 mg/kg, p.o.) were assessed in 24 male CD-1 mice allocated by minimisation, considering their weight and basal locomotor activity in the open field test to randomise the experimental units to the different treatments. The location of the cages on the shelf and the order of intervention of each cage and experimental unit were established by simple randomisation. The sample size (n=4) was calculated considering a significance threshold of 0.05, a power of 0.8 and the following effect sizes (d of Cohen) (considered biologically relevant) and variability (S) for each test: plus maze S=5.78%, d=1.5 (Rosso et al., 2022); forced swimming test S=26.4 s, d=1.56 (Kara, Stukalin, Einat, 2018); tail suspension test S=13.2 s, d=2 (Stukalin, Lan, Einat, 2020); and barbiturate sleeping S=12.6 min, d=1.98 (Vogel, 2002).
To examine the behaviour of each animal in the absence of the experimenter, a digital video camera was used for the anxiety and antidepressant tests. The results were expressed as the mean ± the standard error of the mean (SEM), except for the rotarod and seizures induced by PTZ tests, which were expressed as the number of animals that succeeded the test or were protected, respectively.
In this first stage, the animals were divided in groups of six and dosed with cpTP-0 (100 mg/kg p.o.), cpTP-1 (100 mg/kg p.o.), TAP-2 (100 mg/kg p.o.), cpTAP-2 (screening stage: 100 mg/kg p.o.), clonazepam (positive anxiolytic, sedative and anticonvulsant control, 0.2 mg/kg p.o.), imipramine (positive antidepressant control, 34 mg/ kg p.o.) or vehicle (Glycerine:Propylene glycol:Distilled water 10:10:80). For the Irwin test, the compounds were administered at doses of 10, 50 and 100 mg/kg.
In the dose-response evaluation, the aim was to confirm the antidepressant activity observed in males with cpTAP-2 (100 mg/kg) by a dose-response evaluation at doses of 30, 60, 100 and 300 mg/kg in 60 male and female CD-1 mice (30 of each gender) allocated to separate categories according to sex, which was considered to be a blocking factor in the data analysis; the allocation within blocks was performed as in the screening stage. The results were expressed as the mean ± SEM.
With regard to the data analysis, for the tests where results included continuous variables, a one-way ANOVA-Dunnet test was performed in the screening stage and a two-way ANOVA-Dunnet test in the dose-response evaluation; for those where results were categorical, a two-tailed exact Fisher test was performed, assuming a significance threshold of 0.05 in all cases.
Prior to the realisation of the ANOVA tests for each stage, normality was demonstrated by a Shapiro-Wilk test and equality of variance by a Levene test; the data analysis was performed using the statistical software GraphPad Prism 6® and EXCEL®. Finally, the effect sizes were calculated by the Cohen method.
RESULTS
Screening stage
Irwin test
According to control animals’ behaviour, no obvious neurological or autonomic changes were observed in the animals treated with cpTP-0, cpTP-1, TAP-2 and cpTAP-2 (10, 50 and 100 mg/kg p.o.). Therefore, the higher dose, 100 mg/kg, p.o., was selected for subsequent in vivo tests and dose levels of 30, 60, 100 and 300 mg/kg in the forced swimming test.
Rotarod test
None of the treatments significantly reduced the capacity of the animals to maintain balance on the rotarod (Figure 5).
Number of male CD-1 mice that successfully completed the rotarod test. Two-tailed Fisher exact test, n:4 (vehicle, clonazepam: 0.2 mg/kg, cpTP-0: 100 mg/kg, cpTP-1:100 mg/kg, TAP-2: 100 mg/kg, cpTAP-2: 100 mg/kg).
Open field test
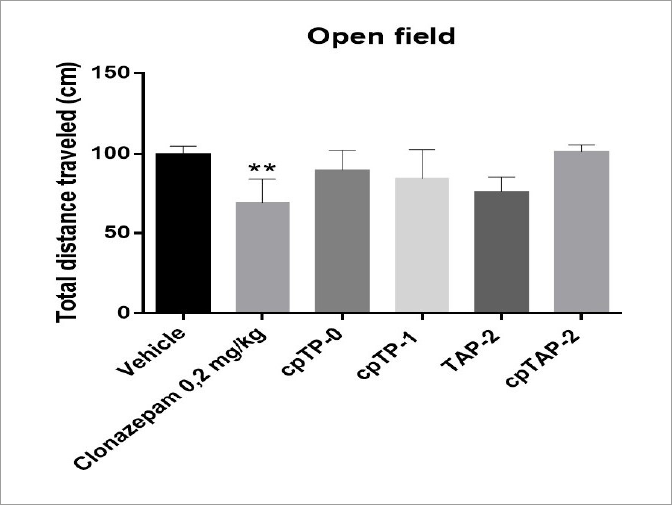
The animals treated with clonazepam showed a reduction in the total distance travelled in the open field (p<0.01). No other treatment significantly altered this parameter (Figure 6).
Total distance travelled in the open field by male CD-1 mice ± S.E.M. **p<0.01, One way ANOVA, n:4 (vehicle, clonazepam: 0.2 mg/kg, cpTP-0: 100 mg/kg, cpTP-1:100 mg/kg, TAP-2: 100 mg/kg, cpTAP-2: 100 mg/kg).
Elevated plus maze test
The animals treated with clonazepam showed an increase in the percentage of time (p<0.001, d=6.6) (Figure 7A) and entries in the open arms (p<0.01) (Figure 7B). The other compounds administered showed no change in these parameters (Figures 7A and 7B); cpTAP-2 was the compound with the biggest effect size between them, with a value of d=1.1.
Percentage of (A) time and (B) entries in the open arms of the elevated plus maze (EPM) of male CD-1 mice ± S.E.M. **p<0.01 ***p<0.001, One way ANOVA, n:4 (vehicle, clonazepam: 0.2 mg/kg, cpTP-0: 100 mg/kg, cpTP-1:100 mg/kg, TAP-2: 100 mg/kg, cpTAP-2: 100 mg/kg). (A) +d> 1.5, Cohen’s method.
Forced swimming and tail suspension tests
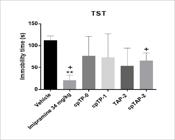
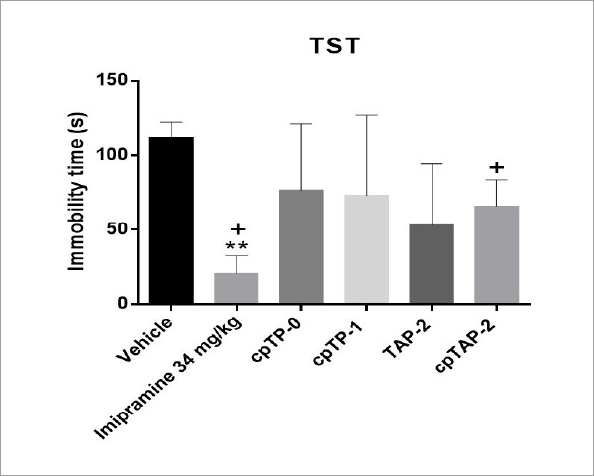
Imipramine reduced the immobility time in both forced swimming and tail suspension tests (p<0.01, d=5.06 and p<0.01, d=4.69, respectively) (Figures 8 and 9); cpTAP-2 also significantly decreased the immobility time in the FST (p<0.05, d=1.72) (Figure 8), but did not show the same effect in the TST despite having an effect size of 2.07 (Figure 9). The other treatments had the following effect sizes in the FST and TST, respectively: cpTP-0 (d=0.04 and 0.88), cpTP-1 (d=0.61 and 0.82) and TAP-2 (d=3.22 and 1.55) (Figures 8 and 9).
Immobility time in the forced swimming test (FST) of male CD-1 mice ± S.E.M. *p<0.05 **p<0.01, One way ANOVA, n:4 (vehicle, imipramine: 34 mg/kg, cpTP-0: 100 mg/kg, cpTP-1:100 mg/kg, TAP-2: 100 mg/kg, cpTAP-2: 100 mg/kg). +d > 1.56, Cohen’s method.
Immobility time in the tail suspension test (TST) of male CD-1 mice ± S.E.M. **p<0.01, One way ANOVA, n:4 (vehicle, imipramine: 34 mg/kg, cpTP-0: 100 mg/kg, cpTP-1:100 mg/kg, TAP-2: 100 mg/kg, cpTAP-2: 100 mg/kg). +d > 2.0, Cohen’s method.
Barbiturate sleeping time test
Clonazepam significantly increased the sleep duration of the mice (p<0.0001, d=10.9) (Figure 10A), but did not reduce the latency time (Figure 10B). None of the compounds reduced the latency time (Figure 10A) or increased the sleeping time, having effect sizes lower than d=1.0 (cpTP-0: d=0.28, cpTP-1: d=0.20, TAP-2: d=-0.82, cpTAP-2: d=0.79) (Figure 10B).
(A) Latency time and (B) barbiturate sleeping time of male CD-1 mice ± S.E.M. ****p<0.0001, One-way ANOVA, n:4 (vehicle, clonazepam: 0.2 mg/kg, cpTP-0: 100 mg/kg, cpTP-1:100 mg/kg, TAP-2: 100 mg/kg, cpTAP-2: 100 mg/kg). (B) +d > 1.98, Cohen’s method.
PTZ seizures
Clonazepam afforded complete protection against the seizures induced by PTZ (p<0.05) (Figure 11); cpTP-0, cpTP-1, TAP-2 and cpTAP-2 provided no protection against the seizures induced (Figure 11).
Number of male CD-1 mice protected from seizures induced by PTZ (85 mg/kg). *p<0.05, Two tails exact Fisher test, n:4 (vehicle, clonazepam: 0.2 mg/kg, cpTP-0: 100 mg/kg, cpTP-1:100 mg/kg, TAP-2: 100 mg/kg, cpTAP-2: 100 mg/kg).
Dose-response evaluation
Rotarod test
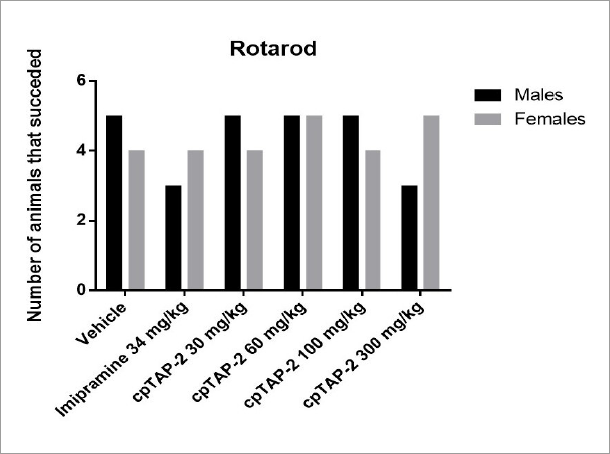
None of the treatments significantly reduced the capacity of the animals to maintain balance on the rotarod in both genders (Figure 12).
Number of male and female CD-1 mice that successfully completed the rotarod test. Two-tailed Fisher exact test. n:5 (vehicle, imipramine: 34 mg/kg, cpTAP-2: 30, 60, 100 and 300 mg/kg).
Open field test
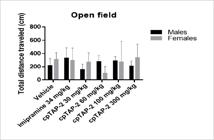
None of the treatments altered significantly the total distance travelled by the mice of both genders (Figure 13).
Total distance travelled in the open field by male and female CD-1 mice ± S.E.M. n:5 (vehicle, imipramine: 34 mg/kg, cpTAP-2: 30, 60, 100 and 300 mg/kg).
Forced swimming test
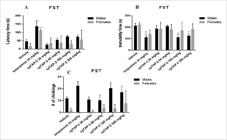
Imipramine increased the latency time in both males and females (p<0.0001 for both genders) (Figure 14A) and also reduced the immobility time in both genders (males: p<0.01, d=2.2; females: p<0.05, d=4.9) (Figure 14B), while cpTAP-2 did not increase the latency time at any dose (Figure 14a), it did reduce the immobility time in males at doses of 100 mg/kg (p<0.01, d=3.5) and 300 mg/ kg (p<0.01, d=3.0) (Figure 14B). The effect sizes obtained by the doses that showed no significant difference in the FST are as follows: 30 mg/kg (males: d=0.76, females: d=0.74), 60 mg/kg (males: d=0.62, females: d=1.0), 100 mg/kg (females: d=0.86) and 300 mg/kg (females: d=0.92) (Figure 14B). None of the treatments significantly affected the number of climbings (Figure 14C).
(A) Latency time, (B) immobility time and (C) number of climbings in the forced swimming test (FST) of male and female CD-1 mice ± S.E.M. **p<0.01 ***p<0.001 ****p<0.0001, Two-way ANOVA-Dunnet, n:5 (vehicle, imipramine: 34 mg/kg, cpTAP-2: 30, 60, 100 and 300 mg/kg). (B) +d > 1.56, Cohen’s method.
In silico ADMET-properties evaluation
Lipinski´s physicochemical descriptors of cpTP-0, cpTP-1, cpTAP-2 and TAP-2 were calculated in silico in order to determine their bioavailability as well as their agreement with the desirable parameters that are usually present in drug-like molecules. It was found that computational estimated descriptors partially agreed with Lipinski´s rule of five (Lipinski et al., 2001) and other related ADMET properties (Ghose, Viswanadhan, Wendoloski, 1999), like toxicity and relevant safety scores such as TD50, which were in a range that is comparable with other biologically active compounds (Lima, et al., 2002) (Table I).
Molecular docking of the synthesised compounds cpTP-0, cpTP-1, cpTAP-2 and TAP-2 showed average affinities with the BDZ-site of GABAA receptor, which were comparable or even higher than the values observed in benzodiazepines, used as reference (Table II). Key interactions were also analysed and compared; in this case, we found some relevant discrepancies, especially in terms of the kind and strength of the ligand-receptor interactions predicted in silico (Figures 15 to 17).
2D and 3D views of the interactions of reference compounds with the BZD-site of the GABAA receptor predicted by molecular docking: (A) Clonazepam. (B) Diazepam. (C) Flunitrazepam.
Interactions of proposed thiazepinones (cpTP-0 and cpTP-1) with the BZD-site of GABAA receptors, predicted by molecular docking.
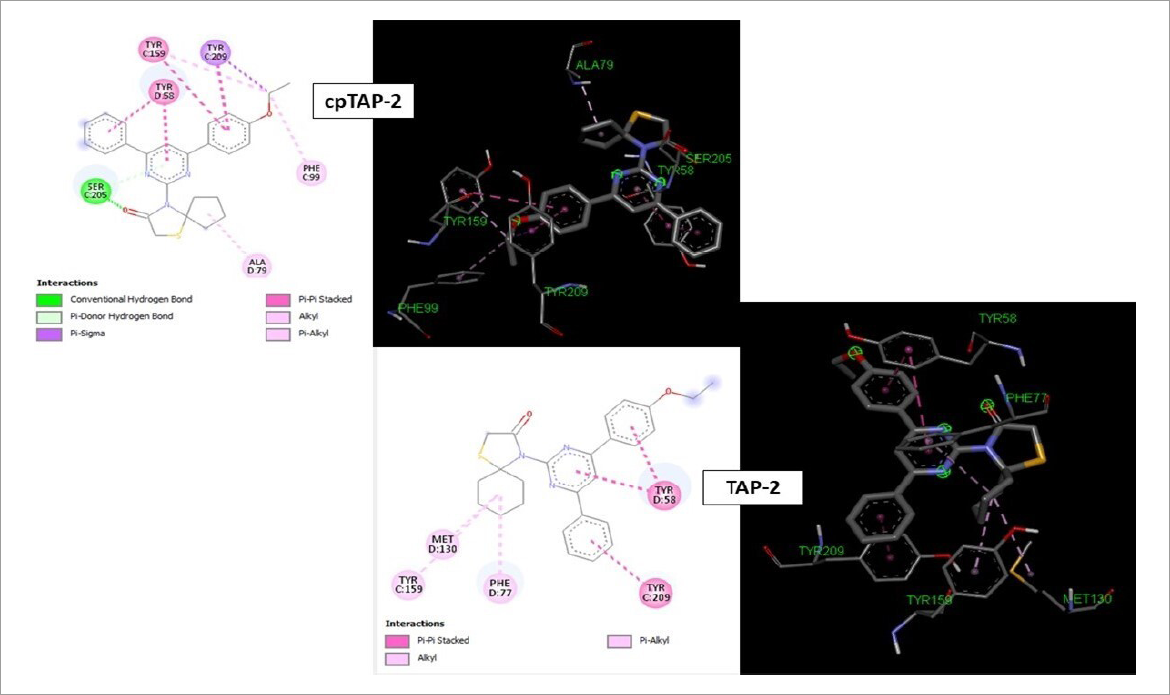
Interactions of proposed thiazolidinones (cpTAP-2 and TAP-2) with the BZD-sites of GABAA receptors predicted by molecular docking.
Predominant interactions in benzodiazepines were found as predominantly π-stacking between aromatic portions of reference molecules and residues tyrosine-209 (TYR-209), 159 (TYR-159) and 58 (TYR-58) and phenylalanine-77 (PHE-77). Interactions with those residues were observed in the evaluated molecules, especially in thiazolidine derivatives (cpTAP-2 and TAP-2) with larger conjugated systems. In the case of thiazepinone derivatives (cpTP-0 and cpTP-1), some of those residues present contact with ligands. However, instead of π-stacked bonds, some of them interacted through Van der Wals and hydrophobic cohesive forces. It was also found that some additional hydrogen bond contacts between ligands such as cpTP-0 and cpTAP-2 with serine-205 (SER-205) contributed to a higher stability of the ligand-receptor complex in calculations (Table II).
DISCUSSION
In this study, the spiro thiazolidine cpTAP-2 showed a significant antidepressant effect in male mice on the forced swimming test at doses of 100 and 300 mg/kg, without altering their locomotor activity on the open field test and their capability to maintain the balance on the rotarod test. However, none of the synthesised compounds showed a significant anxiolytic, sedative and anticonvulsant activity in the respective models, or antidepressant activity in the tail suspension test model. On the other hand, the high binding affinity of cpTAP-2 to the GABAA/BDZ site observed in the docking study suggests that its antidepressant effect could possibly be related to this site.
Previous works have suggested a profile of anxiolytic (Yoshizawa et al., 2020; Bhaumik et al., 2013), antidepressant (Pitchai et al., 2021; Dhar, Bhaumik, Reddy, 2013), sedative (Vega et al., 2000; Faizi et al., 2017) and anticonvulsant (Sánchez-Mateo et al., 2003; Faizi et al., 2017) activities for thiazepine and thiazolidine derivatives; therefore, this neuropharmacological profile was evaluated for the synthesised compounds under investigation (cpTP-0, cpTP-1, TAP-2 and cpTAP-2), by the implementation of valid animal models of anxiety (plus maze) (Kumar, Bhat, Kumar, 2013), depression (forced swimming and tail suspension tests), sedative activity (barbiturate sleeping time) and epilepsy (PTZ seizures) (Vogel, 2002). Also, models that allow the detection of alterations in locomotor activity, such as the open field test (Vogel, 2002), in addition to the Irwin test and the rotarod test which provide information about the safety of the treatments related to the central nervous system (Roux, Sablé, Porsolt, 2003; Lapa et al., 2002), were included in the previous battery.
The elevated plus maze test is an anxiety model based on the natural fear of rodents to open spaces which uses the conflict between exploration and the fear of these spaces to evaluate the anxious behaviour of the animals; an anxiolytic behaviour in this test is characterised by an increased frequency and duration in the open arms of the maze (Lister, 1990). None of the synthesised compounds increased these parameters at a dose of 100 mg/kg, suggesting the lack of an anxiolytic activity at this dose in male mice (Figures 7A and 7B), which is not unexpected according to that which is reported in the literature; in fact, although previous studies have shown anxiolytic activity of thiazepines (Yoshizawa et al., 2020) and thiazolidines (Bhaumik et al., 2013), Sánchez-Mateo et al. (2003) reported a lack of the anxiolytic activity of benzothiazepine derivatives at a dose of 100 mg/kg in this model. Also, Bhaumik et al. (2013) reported the lack of an increase in the frequency and duration in the open arms of the elevated plus maze for one of the three thiazolidine derivatives synthesised in their study.
For the evaluation of antidepressant activity, the models of FST and TST were implemented; both models are based on the induction of a state of despair in the animal due to exposure to an unescapable situation (Vogel, 2002); despite just reflecting one of the symptoms of human depression, both models are quite useful in the detection of the acute effects of a great variety of antidepressant agents, which reduce immobility time in these tests (Vogel, 2002).
In this study, cpTAP-2 reduced this immobility time in the FST at a dose of 100 mg/kg in male mice (Figure 8) but did not show the same effect in the TST (Figure 9). Furthermore, it showed a biologically relevant effect size (d=1.76) in the FST (Figure 8), suggesting an antidepressant activity of interest. It is worth highlighting that the antidepressant activity of analogue thiazolidine derivatives has been reported before (Dhar, Bhaumik, Reddy, 2013). Moreover, compounds cpTP-0, cpTP-1 and TAP-2 did not promote any reduction in immobility time for any of the models at the dose of 100 mg/kg in male mice (Figures 8 and 9). The lack of an antidepressant activity of derivatives with pyrazole nucleus similar to our compounds cpTP-0 and cpTP-1 in the TST model has been reported (Abdel-Aziz M, Abuo-Rahma, Hassan, 2009). However, there are also some reports of thiazepine derivatives such as tiazezim, which exert an important antidepressant activity (Pitchai et al., 2021).
Additionally, the open field test showed no alteration of the total distance travelled by the mice treated with the compounds synthetized (Figure 6), suggesting that the immobility time reduction in the FST caused by cpTAP-2 may not be a consequence of an increase in the locomotor activity of the animals.
The sedative activity of the named compounds was evaluated in the barbiturate sleeping model, in which it seeks to identify CNS depressors that are capable of prolonging the hypnotic-sedative activity of a barbiturate agent like sodic thiopental through the induction of a sleeping state (Vogel, 2002). In this model, none of the synthesised compounds succeeded in reducing the latency time (Figure 10A) or increasing the sleeping time of the animals (Figure 10B).
There are some reports of benzothiazepines that cause an increase in the sleeping time induced by pentobarbital (Vega et al., 2000); furthermore, there have also been reported some examples of thiazolidine-containing compounds with an important sedative activity (Faizi et al., 2017). Nevertheless, certain compounds with a thiazolidine nucleus which have been synthesized and assayed failed to exhibit sedative activity in this model (Panda, Chowdary, Rani, 2008), as seen for compounds TAP-2 and cpTAP-2.
The PTZ seizures model allows the detection of anticonvulsant agents that exert their action via the GABAA receptor, such as barbiturates and some benzodiazepines (Vogel, 2002); nevertheless, none of the compounds synthesised successfully protected the male mice from seizures at a dose of 100 mg/kg (Figure 11). Previous works have reported the anticonvulsant activity of some benzothiazepine derivatives in models of maximum electroshock (MES), while poor protection has been reported in the PTZ model for this type of compound (Sánchez-Mateo et al., 2003). The studies reporting the protection of thiazolidine derivatives in seizures induced by PTZ also report some compounds that did not succeed in the protection of the animals (Faizi et al., 2017).
In this regard, the rotarod and Irwin tests did not detect any adverse effects related to the CNS for any of the compounds synthesised at the dose of 100 mg/kg (Figure 12), suggesting the safety of them at the evaluated dose.
Due to the results obtained in the FST for cpTAP-2 in the screening stage, this compound was selected to carry out a dose-response evaluation in the same model in male and female CD-1 mice, where it displayed a reduction in the immobility time in male mice at doses of 100 and 300 mg/kg with biologically relevant effect sizes (d=3.5 and 3.0, respectively) (Figure 11B). At none of the doses evaluated did cpTAP-2 increase the number of climbings (Figure 11C), suggesting that the mechanism by which this compound exerts its antidepressant activity is not related to noradrenaline (Carr, Lucki, 2010); also, the lack of activity in the tail suspension test may suggest that a serotonin-related mechanism is not responsible of its antidepressant activity either, since this model is more sensitive to drugs that carry out their action through this pathway than the FST (Cryan, Mombereau, Vassout, 2005).
The high affinity that cpTAP-2 showed to the GABAA/BDZ binding site in the docking study suggests that this compound may act as a modulator of this site to exert its antidepressant action. Nevertheless, more studies are required to determine the exact mechanism through which cpTAP-2 manifests it.
The results obtained in the open field and rotarod test in the dose-response evaluation, showed no alteration in locomotor activity (Figure 10) or motor coordination (Figure 9) of animals of both genders at the different doses evaluated, which confirms the antidepressant activity and safety observed in the screening stage for cpTAP-2. The small sample size used during the screening stage may increase the probability of obtaining false positive results, although the results obtained in the dose-response evaluation of cpTAP-2 with a higher sample size significantly reduce this probability and increase the reliability of the antidepressant effect observed. Moreover, the limited amount of synthesised compound makes it difficult to perform further studies related to the determination of the cpTAP-2 mechanism.
In conclusion, the spirothiazolidine compound cpTAP-2 exhibited an antidepressant effect in male CD-1 mice, possibly related to the GABAA/BDZ site, and showed apparent safety related to the central nervous system.
ACKNOWLEDGEMENTS
We are grateful to Minciencias (Contract 705 of 2018) and Universidad Nacional de Colombia for the financial support (Project 110140820405 and VRI/DIB, Project 9334/6621, respectively).
REFERENCES
- Abdel-Aziz M, Abuo-Rahma GEDA, Hassan AA. Synthesis of novel pyrazole derivatives and evaluation of their antidepressant and anticonvulsant activities. Eur J Med Chem. 2009;44(9):3480-7.
- Allen WJ, Balius TE, Mukherjee S, Brozell SR, Moustakas DT, Lang PT, et al. DOCK 6: Impact of new features and current docking performance. J Comput Chem. 2015;36(15):1132-56.
- Becerra-Rivas C, Cuervo-Prado P, Orozco-Lopez F. Efficient catalyst-free tricomponent synthesis of new spiro[cyclohexane-1,4′-pyrazolo[3,4-e][1,4]thiazepin]-7′(6′H)-ones. Synth Commun. 2019;49(3):367-76.
- Becerra-Rivas CA, Cuervo-Prado PA, Orozco-Lopez F. A comparative study of microwave-assisted and conventional heating approaches for the multicomponent synthesis of 4,6-diarylpyrimidines. Univ Sci (Bogota). 2023;28(3):300-15.
- Biovia DS. Discovery Studio Visualizer. San Diego; 2019.
- Bhaumik A, Reddy AN, Chandra MA, Neelamma G, Bhaumik A. Synthesis, characterization and evaluation for anxiolytic activities of some novel 4-thiazolidinone derivatives. Schol Acad J Pharmacy. 2013;2(5):354-9.
- Carr GV, Lucki I. The role of serotonin in depression. In: Müller CP, Jacobs BL, (Eds.). Handbook of the behavioral neurobiology of serotonin. Elsevier Academic Press, 2010; p. 493-505.
- Cryan JF, Mombereau C, Vassout A. The tail suspension test as a model for assessing antidepressant activity: Review of pharmacological and genetic studies in mice. Neurosci Biobehav Rev. 2005;29(4-5):571-625.
- Dhar BV, Bhaumik A, Reddy PY. Synthesis, characterization and evaluation for antidepressant activities of some novel 4-thiazolidinone derivatives. Schol Acad J Pharmacy . 2013;2(4):289-92.
- Eberhardt J, Santos-Martins D, Tillack AF, Forli S. AutoDock Vina 1.2.0: New docking methods, expanded force field, and python bindings. J Chem Inf Model. 2021;61(8):3891-8.
- Faizi M, Jahani R, Ebadi SA, Tabatabai SA, Rezaee E, Lotfaliei M, et al. Novel 4-thiazolidinone derivatives as agonists of benzodiazepine receptors: Design, synthesis and pharmacological evaluation. EXCLI J. 2017;16:52-62.
- Ghose AK, Viswanadhan VN, Wendoloski JJ. A knowledge-based approach in designing combinatorial or medicinal chemistry libraries for drug discovery. 1. A qualitative and quantitative characterisation of known drug databases. J Comb Chem. 1999;1(1):55-68.
- Handa U, Saroha K. Research and development of diazepam solid dispersion powder using natural polymers. Int J Appl Pharmaceutics. 2018;10(5):220-5.
- Hanwell MD, Curtis DE, Lonie DC, Vandermeersch T, Zurek E, Hutchison GR. Avogadro: an advanced semantic chemical editor, visualisation, and analysis platform. J Cheminform. 2012;4(1):17.
- Lanchero IP. Estudio in silico e in vivo de compuestos inhibidores de la enzima lipasa pancreática: una contribución al reposicionamiento de fármacos antiobesidad [Tesis de Maestría]. Universidad Nacional de Colombia; 2016.
- Lapa AJ, Souccar C, Lima MT, Lima TCM. Métodos farmacológicos para el estudio de actividad sobre el sistema nervioso central. In: Métodos de evaluación de la actividad farmacológica de plantas medicinales. Santa Catarina: Florianópolis; 2002. p. 70-90.
- Lima AR, Soares-Weiser K, Bacaltchuk J, Barnes TR. Benzodiazepines for neuroleptic-induced acute akathisia. Cochrane Database Syst. Rev. 2002;1999(1): CD001950.
- Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 2001;64:4-17.
- Lister RG. Ethologically-based animal models of anxiety disorders. Pharmacol Ther. 1990;46(3):321-40.
- Liu L, Liu C, Wang Y, Wang P, Li Y, Li B. Herbal medicine for anxiety, depression and insomnia. Curr Neuropharmacol. 2015;13(4):481-93.
- Kara NZ, Stukalin Y, Einat H. Revisiting the validity of the mouse forced swim test: Systematic review and meta-analysis of the effects of prototypic antidepressants. Neurosci Biobehav Rev . 2018;84:1-11.
- Kumar V, Bhat ZA, Kumar D. Animal models of anxiety: A comprehensive review. J Pharmacol Toxicol Methods. 2013;68(2):175-83.
- Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, et al. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J Comput Chem . 2009;30(16):2785-91.
- Panda SS, Chowdary PVR, Rani S. Design and synthesis: Novel quinazolin incorporated azetidinones and thiazolidinones as sedative, antibacterial and antifungal agents. Indian drugs. 2008;45(2):84-9.
- Pitchai M, Ulaganathan S, Venkateshappa SS, Akunuri A, Rampulla R, Mathur A, et al. Concise synthesis of chiral pyrazolo[4,3-f][1,4]oxazepines and pyrazolo[4,3-f][1,4] thiazepines bearing pyrazole unit. J Heterocycl Chem. 2021;58(2):558-68.
- Porsolt RD, Bertin A, Jalfre M. Behavioural despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther. 1977;229(2):327-36.
- Rosso M, Wirz R, Loretan AV, Sutter NA, Pereira da Cunha CT, Jaric I, et al. Reliability of common mouse behavioural tests of anxiety: A systematic review and meta-analysis on the effects of anxiolytics. Neurosci Biobehav Rev . 2022;143:104928.
- Roux S, Sablé E, Porsolt RD. Primary Observation (Irwin) Test in Rodents for Assessing Acute Toxicity of a Test Agent and its Effects on Behavior and Physiological Function. In: Current Protocols in Pharmacology. John Wiley and Sons; 2003. p.10.10.01-10.10.23.
- Sánchez-Mateo C, Darias V, Albertos L, Exposito-Orta M. Psychopharmacological effects of tianeptine analogous hetero[2,1] benzothiazepine ferivatives. Arzneimittelforschung. 2003;53(01):12-20.
- Simulations Plus. ADMET PredictorTM . California: Simulations Plus Inc; 2016.
- Sinanović O, Muftic M, Sinanović S. COVID-19 pandemia: Neuropsychiatric comorbidity and consequences. Psychiatr Danub. 2020;32(2):236-44.
- Steru L, Chermat R, Thierry B, Simon P. The tail suspension test: A new method for screening antidepressants in mice. Psychopharmacology (Berl). 1985;85(3):367-70.
- Stukalin Y, Lan A, Einat H. Revisiting the validity of the mouse tail suspension test: Systematic review and meta-analysis of the effects of prototypic antidepressants. Neurosci Biobehav Rev . 2020;112:39-47.
- Swinyard E, Woodhead J, White H, Franklin M. Experimental selection, quantification, and evaluation of anticonvulsants. In: Levy R, editor. Antiepileptic Drugs. New York; 1989. p.85-102.
- Trott O, Olson AJ. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimisation, and multithreading. J Comput Chem . 2010;31(2):455-61.
- Vega S, Díaz JA, Darias V, Mateo CC. Synthesis of new thieno and pyrazolo[2,1]benzothiazepine derivatives with potential antidepressant properties. J Heterocycl Chem . 2000;37(2):389-93.
- Vogel HG. Drug Discovery and Evaluation. 2nd ed. Berlin: Springer; 2002.
- Yoshizawa K, Nakashima K, Tabuchi M, Okumura A, Nakatake Y, Yamada M, et al. Benzothiazepines, diltiazem and JTV-519, exert an anxiolytic-like effect via neurosteroid biosynthesis in mice. J Pharmacol Sci. 2020;143(3):234-7.
- Zapata Barco AM, Restrepo-Martínez M, Restrepo D. Depresión en personas con epilepsia. ¿Cuál es la conexión? Rev Colomb Psiquiatr. 2020;49(1):53-61.
- Zhdanava M, Pilon D, Ghelerter I, Chow W, Joshi K, Lefebvre P, et al. The prevalence and national burden of treatment-resistant depression and major depressive disorder in the United States. J Clin Psychiatry. 2021;82(2).
- Zhu S, Noviello CM, Teng J, Walsh RM, Kim JJ, Hibbs RE. Structure of a human synaptic GABAA receptor. Nature. 2018;559(7712):67-72.
Publication Dates
-
Publication in this collection
20 Jan 2025 -
Date of issue
2025
History
-
Received
20 Feb 2024 -
Accepted
17 July 2024

 Neuropharmacological profile of new thiazepinone and thiazolidinone compounds designed by virtual screening
Neuropharmacological profile of new thiazepinone and thiazolidinone compounds designed by virtual screening