Abstract
Hybrid structures containing multiple pharmacophore units with known activity have attracted attention due to their promising outcomes. In this study, several new hybrid structures containing benzimidazole and thiadiazole units were synthesized. The newly synthesized compounds were structurally analyzed using 1H-NMR, 13C-NMR, HRMS, and elemental analysis. The antimicrobial, in vitro anti-cancer, and antioxidant activities of all compounds were investigated. In vitro antimicrobial activities of the compounds were determined against Gram-positive (S. aureus ATCC 29213, E. faecalis ATCC 29212), Gram-negative (E. coli ATCC 25922, P. aeruginosa ATCC 27853) bacteria and fungi (C. albicans ATCC 10231) by using broth microdilution method. The compound 5g bearing 4-methoxyphenyl derivative showed the best activity with 32 μg/mL against S. aureus ATCC 29213 and P. aeruginosa ATCC 27853. The MTT test was used to determine the cytotoxicity of the produced compounds on the MCF-7 (human breast cancer) and L-929 (fibroblast) cell lines. FRAP method was used to determine the antioxidant properties of synthesized compounds. The Ferric Reducing Antioxidant Power of the compounds 5a, 5b, and 5c showed more antioxidant properties than vitamin E. The compound 5g stands out in the series in that it is not toxic on the healthy cell line and has promising antimicrobial activity.
Keywords:
Benzimidazole; Thiadiazole; Antimicrobial; Anti-cancer; Antioxidant
INTRODUCTION
Cancer is a major public health issue and is the leading cause of death worldwide. In 2020, 19.3 million new cases of cancer and 10 million cancer-related deaths were reported (Anastassova et al., 2022). According to the World Cancer Report of the WHO, the number of deaths due to cancer is estimated to more than double in the next few years (Parkin, 2001). Chemotherapy is one of the most efficient ways to treat cancer since it slows the growth and metastasis of tumors, and a variety of chemical compounds with various structures have been designed to treat different forms of cancer. The use of cytotoxic drugs is a tried-and-true treatment strategy to slow down the growth of tumors (Ismael et al., 2008). However, one of the drawbacks of anti-cancer treatment and the biggest challenge in cancer therapy is that anti-cancer medications are also harmful to normal cells, causing numerous side effects (Aydemir et al., 2003). This non-targeted drug delivery constrains the therapeutic efficacy of the current anti-cancer treatment. Thus, the hunt for medicines that are effective with fewer adverse effects is the main aim of researchers in the development of anti-cancer therapies. Reactive oxygen species (ROS) promote the growth of cancer cells. They interact with tumor initiation, propagation, tumor spread, tumor microenvironment, and therapeutic resistance to facilitate cancer development (Xing et al., 2022). Antioxidants guard organisms against these reactive oxygen species. These are chemicals that neutralize free radicals, which can damage an organism’s tissues and cells, and inhibit oxidation processes that occur during chemical reactions in metabolic pathways (Sies, 1997). Thus, research into the antioxidant characteristics of synthesized compounds is extensively explored in medicinal chemistry. The search for active compounds that might stop or minimize the effects of oxidative stress on cells has become an important area of research nowadays. Benzimidazole-thiadiazole complexes have shown promising antioxidant activity against reactive oxygen species.
One of the major public health issues of the twenty-first century is antimicrobial resistance (AMR), which poses a threat to the effective prevention and treatment of an expanding number of microbial infections that are no longer susceptible to the conventional medications used to treat them (Prestinaci, Pezzotti, Pantosti, 2015). Microbial resistance is one of the most urgent issues in clinical practice, and a primary objective of current biomedical research is to discover new, powerful drugs that can combat multi-resistant bacteria. Antibiotic abuse and pharmaceutical corporations’ lack of interest in investing in antibiotic development have made the discovery of new antibiotic classes inevitable. Benzimidazole is known as a fortunate structure in pharmaceutical chemistry, having a variety of biological functions. Benzimidazole has a benzene ring system in which the benzene ring is attached to a five-member imidazole ring having nitrogen atoms at positions 1 and 3 and thus is known as a heterocyclic aromatic compound (Khokra, Choudhary, 2011). Additionally, benzimidazole is a tremendous scaffold of therapeutic importance with promising pharmacological properties. The safety and efficacy profiles of benzimidazole medications in clinical use are well-established.
The term “benzimidazole” refers to the ring structure in which the benzene ring is fused to the 4,5-positions of the imidazole ring (Debus, 1858). It is one of the earliest nitrogen heterocycles initially created by Hobrecker in 1872. Benzimidazole has become an extensively employed heterocyclic moiety in modern times (Wright, 1951). Because of its active pharmacophore, it has applications as antitubercular (Mohapatra, Ganguly, 2024; Ranjith et al., 2013), anti-inflammatory (Jahan, et al., 2024; Vasantha et al., 2015), antitrichinellosis (Keri et al., 2015), anti-cancer (Acar Çevik et al., 2024; Yadav, Narasimhan, 2016), antioxidant (Işık et al., 2022; Arora et al., 2014), antihistaminic (Sridevi et al., 2010), and antimicrobial agent (Celik et al., 2022; Ansari, Lal, 2009). The purine base and the benzimidazole core’s structural resemblance make it easy for benzimidazole to interact with living systems. A wide range of pharmacological activities of this compound draws attention to the inclusion of this beneficial structure in the design and synthesis of this molecule with other structures to make complex compounds.
1,3,4-Thiadiazoles is a poly-heteroatomic system containing a five-membered heterocyclic ring associated with conjugated p electrons and distinct regions of positive and negative charges (Karki et al., 2011). Due to its dense and highly polarizable structure and net neutral electron charge, it can traverse cellular membranes and interact with biological targets. The 1,3,4-thiadiazole ring’s inductive action and the comparatively high aromaticity make this compound behave as a modestly weak base. The (S-C=N) toxophoric units that are present in substituted thiadiazols give rise to a wide spectrum of pharmacological actions, including anti-cancer, antibacterial, anti-inflammatory, antifungal, anticonvulsant, antiviral, and antiparasitic properties (Khadri et al., 2020).
Since cancer drugs suppress the immune system of patients, they become more susceptible to microbial infections. When microbial infections occur in cancer patients, they have to use multiple drugs. The development of antimicrobial and anti-cancer drugs is very important to protect cancer patients from microbial infections. Considering the effect of oxidative stress on cancer, it is very important to synthesize compounds with both anti-cancer, antimicrobial, and antioxidant effects. The main objectives of this study include the synthesis, characterization, and in vitro testing of seven distinct derivatives with benzimidazole and thiadiazole rings, which are believed to have antioxidant, antibacterial, antifungal, and anti-cancer properties.
MATERIAL AND METHODS
Chemistry
All compounds were purchased from Sigma-Aldrich (Sigma-Aldrich Corp., St. Louis, MO, USA) or Merck (Merck KGaA, Darmstadt, Germany). The synthesized compounds’ melting points (ºC, uncorrected) were determined using a Mettler-Toledo MP 90 melting point instrument (Columbus, Ohio, OH, USA). With Bruker 75 MHz and 300 MHz digital FT-NMR spectrometers (Bruker Bioscience, Billerica, MA, USA), 13C-NMR and 1H-NMR, spectra were captured in DMSO-d6 solvent with TMS acting as an internal standard. The units of coupling constants (J) are Hertz. Shimadzu LC/MS ITTOF equipment was used to determine M+1 peaks (Shimadzu, Tokyo, Japan). On Silica Gel 60 F254 TLC plates, thin-layer chromatography (TLC) experiments were conducted (Merck KGaA, Darmstadt, Germany).
Synthesis of Benzaldehyde derivative (1) sodium metabisulfite salt: Ethanol was used to dissolve of methyl 4-formyl benzoate (5g, 0.03 mol). Drop by drop, ethanol-dissolved sodium metabisulfite (6.84 g, 0.036 mol) was added to the benzaldehyde solution. The reaction’s components were mixed for an hour at room temperature once the dripping process was finished. It was filtered to remove the precipitated product.
Synthesis of methyl 4-(5-chloro-1H-benzo[d]imidazole-2-yl) benzoate (2): 4-Chlorobenzene-1,2-diamine (0.022 mol) was dissolved in DMF, and sodium metabisulfite salt of benzaldehyde derivative (7.09 g, 0.026 mol) was added. By adding the reaction’s contents to iced-water at the end, the result was precipitated. From the ethanol, the precipitated product was removed and crystallized.
Synthesis of 4-(5-chloro-1H-benzo[d]imidazole-2-yl) benzohydrazide derivatives (3): Compound 2 (0.018 mol) and excess of hydrazine hydrate (5 mL) were placed in the same vial and ethanol (15 mL) was added. The mixture was refluxed for 12h. When the reaction was completed, the mixture was poured into iced-water, the product was filtered.
N-substituted-2-(4-(5-chloro-1H-benzo[d]imidazole-2-yl)benzoyl)hydrazine-1-carbothioamide (4): The appropriate quantity of isothiocyanate (1.1 mmol) was added after the hydrazide derivative compound 3 (1 mmol) had been dissolved in 10 ml of ethanol, and the mixture was then refluxed for three hours. It was filtered to remove the precipitated product.
N-substituted-5-(4-(5-chloro-1H-benzo[d]imidazole-2-yl) phenyl) 1,3,4-thiadiazole-2-amine (5a-5g): The appropriate thiosemicarbazide derivative was stirred in 10 ml of H2SO4 in an ice bath. Then it was stirred for another 10 minutes at room temperature, at the end of the time it was poured slowly on ice, adjusted to pH=8 with aqueous ammonia and filtered. It is washed with water and crystallized from ethanol.
N-ethyl-5-(4-(5-chloro-1H-benzo[d]imidazole-2-yl)phenyl)-1,3,4-thiadiazole-2-amine (5a): Yield: 72 %. M.p. 311.5 oC. 1H-NMR (300 MHz, DMSO-d6): δ=1.21 (3H, t, J=7.17 Hz, -CH3), 5.33-5.35 (2H, m, -CH2), 7.24 (1H, dd, J1=3.03 Hz, J2=9.21 Hz, Benzimidazole CH), 7.64-7.67 (2H, m, Benzimidazole CH), 7.93 (2H, d, J=8.52 Hz, 1,4-disubstituted benzene), 8.25 (2H, d, J=8.43 Hz, 1,4-disubstituted benzene). 13C-NMR (75 MHz, DMSO-d6): δ(ppm): 14.65, 35.53, 114.47, 117.31, 119.64, 123.59, 126.19, 126.65, 128.36, 128.77, 129.04, 130.79, 134.80, 135.98, 155.41. HRMS (m/z): [M+H]+ calcd for C17H14N5SCl: 356.0731; found: 356.0732. Anal. calcd. For C17H14N5SCl, C, 57.38; H, 3.97; N, 19.68. Found: C, 57.46; H, 3.96; N, 19.72.
N-(2-chloroethyl)-5-(4-(5-chloro-1H-benzo[d]imidazole-2-yl)phenyl)-1,3,4-thiadiazole-2-amine (5b): Yield: 65 %. M.p. 197.3 oC. 1H-NMR (300 MHz, DMSO-d6): δ=3.47-3.60 (4H, m, -CH2), 7.24-7.27 (1H, m, Aromatic C-H), 7.62-7.68 (2H, m, Aromatic C-H), 7.97-7.99 (1H, m, Aromatic C-H), 8.09-8.12 (1H, m, Aromatic C-H), 8.23-8.27 (2H, m, Aromatic C-H). 13C-NMR (75 MHz, DMSO-d6): δ(ppm): 25.46, 45.61, 112.63, 117.51, 121.57, 121.98, 123.85, 125.92, 127.28, 128.53, 129.31, 130.71, 138.29, 152.22, 154.82. HRMS (m/z): [M+H]+ calcd for C17H13N5SCl2: 391.0329; found: 391.0334. Anal. calcd. For C17H13N5SCl2, C, 52.32; H, 3.36; N, 17.94. Found: C, 52.48; H, 3.35; N, 17.98.
N-phenyl-5-(4-(5-chloro-1H-benzo[d]imidazole-2-yl)phenyl)-1,3,4-thiadiazole-2-amine (5c): Yield: 62 %. M.p. 310.3 oC. 1H-NMR (300 MHz, DMSO-d6): δ=7.52-7.55 (3H, m, Aromatic CH), 7.62-7.65 (2H, m, Aromatic CH), 7.93-7.96 (2H, m, Aromatic CH), 8.21-8.28 (3H, m, Aromatic CH), 8.35-8.40 (2H, m, Aromatic CH).13C-NMR (75 MHz, DMSO-d6): δ(ppm): 105.67, 109.51, 112.94, 114.71, 119.18, 121.67, 124.53, 126.82, 128.13, 129.10, 129.33, 130.15, 132.24, 134.77, 135.07, 148.68, 151.18. HRMS (m/z): [M+H]+ calcd for C21H14N5SCl: 404.0731; found: 404.0721. Anal. calcd. For C21H14N5SCl, C, 62.45; H, 3.49; N, 17.34. Found: C, 62.64; H, 3.48; N, 17.39.
N-(2-chlorophenyl)-5-(4-(5-chloro-1H-benzo[d]imidazole-2-yl)phenyl)-1,3,4-thiadiazole-2-amine (5d): Yield: 89 %. M.p. 307.4 oC. 1H-NMR (300 MHz, DMSO-d6): δ=7.68 (2H, d, J=8.46 Hz, Aromatic C-H), 7.73 (2H, s, Aromatic C-H), 8.06 (3H, d, J=8.76 Hz, Aromatic C-H), 8.28-8.31 (4H, s, Aromatic C-H). 13C-NMR (75 MHz, DMSO-d6): δ(ppm): 115.75, 122.79, 123.67, 124.50, 125.17, 126.84, 127.25, 127.64, 127.79, 127.98, 128.47, 128.86, 129.37, 130.28, 130.60, 131.32, 132.40, 148.83, 151.89. Anal. calcd. For C21H13N5SCl2, C, 57.54; H, 2.99; N, 15.98. Found: C, 57.72; H, 2.98; N, 15.93.
N-cyclohexyl-5-(4-(5-chloro-1H-benzo[d]imidazole-2-yl)phenyl)-1,3,4-thiadiazole-2-amine (5e): Yield: 91 %. M.p. 272.2 oC. 1H-NMR (300 MHz, DMSO-d6): δ=1.18-1.32 (6H, m, cyclohexyl CH), 1.61-1.74 (3H, m, cyclohexyl CH), 1.97-1.99 (2H, m, cyclohexyl CH), 7.33 (1H, dd, J1=1.68 Hz, J2=8.64 Hz, Benzimidazole CH), 7.69 (1H, d, J=8.55 Hz, Benzimidazole C-H), 7.73-7.74 (1H, m, Benzimidazole C-H), 7.97 (2H, d, J=8.52 Hz, 1,4-disubstituted benzene), 8.25 (2H, d, J=8.73 Hz, 1,4-disubstituted benzene). 13C-NMR (75 MHz, DMSO-d6): δ(ppm): 21.09, 23.59, 25.35, 30.03, 32.42, 57.56, 113.14, 114.29, 121.19, 122.91, 124.68, 125.17, 126.28, 127.01, 127.74, 128.22, 129.88, 136.63, 155.05. Anal. calcd. For C21H20N5SCl, C, 61.53; H, 4.92; N, 17.08. Found: C, 61.71; H, 4.93; N, 17.11.
N-isopropyl-5-(4-(5-chloro-1H-benzo[d]imidazole-2-yl)phenyl)-1,3,4-thiadiazole-2-amine (5f): Yield: 92 %. M.p. 294.4 oC. 1H-NMR (300 MHz, DMSO-d6): δ=1.22-1.25 (6H, m, -CH3), 3.86-3.91 (1H, m, -CH), 7.25 (1H, dd, J1=2.10, Hz, J2=8.52 Hz, Benzimidazole CH), 7.61-7.67 (1H, m, Aromatic CH), 7.92-7.95 (2H, m, Aromatic C-H), 8.01-8.03 (1H, m, Aromatic C-H), 8.23-8.27 (2H, m, Aromatic C-H). 13C-NMR (75 MHz, DMSO-d6): δ(ppm): 22.55, 47.59, 116.58, 118.66, 119.90, 123.96, 127.26, 127.69, 130.61, 133.83, 140.68, 142.24, 144.22, 152.33, 155.44. HRMS (m/z): [M+H]+ calcd for C18H16N5SCl: 370.0888; found: 370.0882. Anal. calcd. For C18H16N5SCl, C, 58.45; H, 4.36; N, 18.93. Found: C, 58.63; H, 4.37; N, 18.97.
N-(4-methoxyphenyl)-5-(4-(5-chloro-1H-benzo[d]imidazole-2-yl)phenyl)-1,3,4-thiadiazole-2-amine (5g): Yield: 89 %. M.p. Semi-solid. 1H-NMR (300 MHz, DMSO-d6): δ=3.76 (3H, s, -OCH3), 7.28-7.31 (3H, m, Aromatic C-H), 7.60 (2H, d, J=9.03 Hz, Aromatic C-H), 7.66-7.69 (2H, m, Aromatic C-H), 8.04 (2H, d, J=8.55 Hz, 1,4-disubstituted benzene), 8.30 (2H, d, J=8.55 Hz, 1,4-disubstituted benzene). 13C-NMR (75 MHz, DMSO-d6): δ(ppm): 55.69, 113.14, 114.82, 115.60, 120.00, 123.31, 123.41, 127.08, 127.64, 127.81, 128.58, 130.67, 132.29, 134.39, 150.43, 155.25, 156.54, 165.60. Anal. calcd. For C22H16N5OSCl, C, 60.90; H, 3.72; N, 16.14. Found: C, 61.08; H, 3.73; N, 16.18.
Antimicrobial Activity
In vitro antimicrobial activities of the compounds were evaluated against Gram-positive (Staphylococcus aureus ATCC 29213, Enterococcus faecalis ATCC 29212), Gram-negative (Escherichia coli ATCC 25922, Pseudomonas aeruginosa ATCC 27853) bacteria and fungi (Candida albicans ATCC 10231). For this purpose, minimum inhibitory concentrations (MIC) of the compounds along with reference antimicrobials were determined against these microorganisms. The study was conducted according to the Clinical Laboratory Standards Institute (CLSI) M100-S28 protocol for bacteria (Wayne, 2017) and CLSI M27-A3 protocol for fungi (Wayne, 2008) Mueller Hinton Broth (MHB) for bacteria and RPMI-1640 for fungi were used in this research.
The serial dilutions of each compound at the range of 2-512 µg/mL were prepared in 96-well microplates after all compounds dissolved in DMSO. The McFarland 0.5 standard was used to prepare suspensions of each microbe, and as a result, densities of 105 cfu/ml were achieved. Bacteria and fungus were placed in microplates and incubated for 24 and 48 hours, respectively, at 37 and 35 degrees Celsius. These microorganisms were evaluated against the reference antimicrobials. Growth control of microorganisms and sterilization control of the mediums were tested at the same time. Besides, DMSO which is used as a solvent in this test was also tested for antimicrobial activity. The wells with the lowest concentration without microbial growth were determined as MICs of each compound, after the incubation period. The detection was made by macroscopic evaluation using dye MTT (Shi et al., 2007). The experiment was repeated three times.
Anti-cancer Activity
Cell Culture
MCF-7 and L929 cell lines were purchased from ATCC (American Type Culture Collection) and studied. Cells were mixed with 88% DMEM (Dulbecco’s modified Eagle’s medium; Gibco, Thermo Fisher Scientific), 10% FBS (Fetal Bovine Serum; Sigma Aldrich), 1% glutamine (Sigma Aldrich), and 1% penicillin (Sigma-Aldrich) solutions. The cells to which the medium was added were allowed to grow by incubating at 37°C in an environment containing 95% humidity and 5% CO2. All syntheses were dissolved with DMSO solution and it was ensured that their final concentration did not exceed 0.5% at the time of introduction into the cell.
Cell Viability Assay
The cytotoxic effects of all compounds synthesized by MTT analysis on L929 and MCF7 cell lines were investigated. 96-well plates were used for seeding cells. Approximately 10000 cells were seeded in each well. Cells were allowed to adhere for 24 hours and then 20 mM solution was prepared (with DMSO) for each synthesis. The syntheses were given to each well in triplicate, ensuring that the DMSO ratio in the final well concentration did not exceed 0.5% and the final concentration was 100µM. The wells were then incubated for 48 hours. All wells without synthesis were used as positive controls. After the incubation, the wells were treated with MTT solution to determine metabolically active cells and incubated at 37°C for 3 hours. After the MTT interaction, the wells were emptied and DMSO solution was placed in them. The formazan crystals formed were dissolved with this solution and the number of viable cells in each well was determined by color change. The absorbance values were read at 540 nm with the help of a microplate and the values found were represented as mean±standard deviation (±SD). The experiment was repeated two times.
Antioxidant Activity
This method, in which the reducing capacity of iron (III) is determined by antioxidants, was developed by Benzie and Strain in 1996. Fe(III)-TPTZ complex is formed as a result of the reaction of Fe(III) with tripyridyltriazine (TPTZ), and this complex is reduced to Fe(II)-TPTZ complex with the antioxidant in the environment. The color of this complex is dark blue and gives maximum absorbance at 593 nm (Benzie, Strain, 1996). Incubation is performed for up to 30 minutes to complete the reaction and read the correct absorbance values. The results obtained can be given as Trolox equivalent or as IC50 values. The experiment was repeated two times.
RESULTS AND DISCUSSION
Chemistry
The synthesis phase of the new structures was carried out in five stages, as shown in Figure 1. The aldehyde portion of the methyl 4-formyl benzoate compound was treated with sodium metabisulfite in ethanol. Thus, the sodium metabisulfite addition product of the aldehyde was obtained. In the second step, as a result of the condensation reaction of benzaldehyde sodium metabisulfite product and 4-chlorobenzene-1,2-diamine under reflux and methyl 4-(5-chloro-1H-benzo[d]imidazole-2-yl)benzoate (2) was obtained. Compound 3 was synthesized by treating compound 2 with hydrazine hydrate in ethanol in the following procedure (3). The suitable isothiocyanate derivatives and the hydrazide derivative were refluxed in ethanol, and the precipitated product was filtered out. The next step involved cyclizing the thiosemicarbazide molecule in the presence of strong sulfuric acid to produce the thiadiazole derivatives (5a-g).
Synthesis pathway of 1,3,4-thiazole derivative target benzimidazole compounds 5a-5g. Reagent and conditions: (i) Na2S2O5/EtOH, (ii) DMF/120°C, (iii) NH2NH2/EtOH, (iv) RNCS/EtOH and (v) H2SO4.
Antimicrobial Activity
By employing the broth microdilution method, the compounds were examined for their in vitro antimicrobial activities against Gram-positive (S. aureus ATCC 29213, E. faecalis ATCC 29212), Gram-negative (E. coli ATCC 25922, P. aeruginosa ATCC 27853) bacteria and fungi (C. albicans ATCC 10231). As reference antimicrobial medications, this study used ampicillin, gentamycin, and vancomycin for their antibacterial activity and fluconazole for their antifungal activity. The MIC values determined for each substance and reference antimicrobial agents because of the experiment were presented in Table I.
According to result of the antimicrobial study, MICs ranged from 32 to 256 μg/mL against Gram-positive bacteria. In terms of antibacterial activity against Gram-positive bacteria, compound 5g showed the best activity with 32 μg/mL on S. aureus ATCC 29213. Although this MIC value did not reach those of the reference antibacterial agents, it stands out as a promising value.
MICs ranged from 32 to 256 μg/mL against Gram-negative bacteria. Compound 5g has the lowest MIC value on Gram-negative bacteria as well as Gram-positive bacteria. This MIC value on P. aeruginosa ATCC 27853 is 32 μg/mL which is higher than the MIC of gentamicin (1 μg/mL) on this microorganism. However, it can still be considered promising in terms of antibacterial activity. The MIC values of the compounds in the study on C. albicans ATCC 10231 were determined as 256 μg/mL, except for compound 5g which had MIC at 128 μg/mL. The findings indicate that the compounds in this study showed generally modest levels of antibacterial activity and fell short of the MIC values of the reference antimicrobials. However, compound 5g outperformed all the other compounds in the series in terms of antibacterial activity.
In the study by Işık et al. (2024) compounds bearing benzimidazole-thiadiazole structure were investigated and their antimicrobial effects were evaluated. Promising results were obtained for the compounds. In this study, a nonsubstituted benzimidazole derivative was used. In general, it was concluded that the antibacterial effect was enhanced by substituting the 5th position of the thiadiazole ring with an alkyl group in the compounds. The presence of a methoxy group on the phenyl ring attached to the thiadiazole ring was found to increase the antimicrobial effect (Işık et al., 2024). Celik et al. (2022), synthesized 5,6-dimethyl-1H-benzimidazole derivatives containing thiadiazole ring and evaluated their antimicrobial effects. In this study, high antimicrobial effect was obtained by 4-methoxyphenyl substitution of thiadiazole ring. The electron-withdrawing -Cl group at the 2-position of the ethyl group was found to enhance both antibacterial and antifungal activity (Celik et al., 2022).
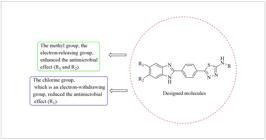
Çevik et al. (2022), synthesized 5-methyl-1H-benzo[d]imidazole derivative compounds bearing thiadiazole structure and investigated their antimicrobial effects. Many of the compounds were found to have promising antibacterial and antifungal effects. In this study, compound 5g bearing 4-methoxyphenyl ring showed promising antibacterial effect. However, when compared with other studies, it was observed that nonsubstituted, 5-methyl or 5,6-dimethyl substitution of the benzimidazole ring had a positive effect on the antimicrobial effect, while the antimicrobial effect decreased with the introduction of the -Cl substituent, which is an electron withdrawing group on the benzimidazole ring (Figure 2).
Anti-cancer Activity
L929 and MCF-7 cell lines were used to test the cytotoxic effects of compounds 5a-5g. For the first screening, the MTT test was used to assess the in vitro cytotoxic bioactivity of produced compounds against the L929 and MCF-7 cell lines. Both cell lines were exposed to the target substances at a constant concentration of 100 µM to assess their cytotoxic potential. After the cells had been treated for 48 hours, cell viability percentages were calculated. Preliminary Cytotoxic Effect results of compounds 5a-5g against L929 and MCF-7 cell lines are presented in Table II and Figure 3. The data obtained at the end of the study showed that all compounds between 5a-5g do not have an anti-cancer effect. As a result of the maximum dose applied, almost all compounds showed IC50 values which is higher than 100 µM except compound 5e for the L929 cell line (which is 97,52 µM). Apart from this, L929 fibroblast cells showed lower cell viability than MCF-7 breast cancer cells with the maximum dose applied.
IC50 values (µM) and percent of vitality of compounds 5a-5g and reference drug cisplatin at 100 µM concentration for MCF-7 and L929 cell lines
Antioxidant Activity
Like some reducing agents, antioxidants also cause the Fe+3 ferricyanide complex to be reduced to Fe+2. In this method, the color of the test solution changes from yellow to green, depending on the reducing power of the sample tested. This green color gives maximum absorbance at 700 nm and increasing absorbance indicates increasing reduction strength. According to this method, trolox was used as the standard antioxidant compound, and measurements were made by the procedure determined by Benzie and Strain in 1996. The Ferric Reducing Antioxidant Power values greater than or equal to 1.0 mmol Trolox Equiv./L are considered as high and desired levels. The Ferric Reducing Antioxidant Power of the compounds 5a, 5b, and 5c showed more antioxidant properties than vitamin E for iron reduction which is shown in Table III.
CONCLUSION
In this study, we designed and synthesized for the first time a series of novel benzimidazole-thiadiazole hybrids as antimicrobial, anti-cancer agents, and antioxidants using a molecular hybridization protocol. The antibacterial activity was carried out against four different bacterial strains (S. aureus ATCC 29213, E. faecalis ATCC 29212, E. coli ATCC 25922, P. aeruginosa ATCC 27853) and one fungi strain (C. albicans) by using broth microdilution method. The synthesized compound was analyzed for its in vitro anti-cancer activity on the MCF7 cell line by using an MTT assay. According to the results, the compound 5g showed moderate antibacterial activity against S. aureus ATCC 29213 and P. aeruginosa ATCC 27853. The compound was also analyzed for its antioxidant capacity using the FRAP method. Compounds were found to be ineffective against cancer cells, while compounds 5a, 5b and 5c were found to have promising antioxidant effects.
REFERENCES
- Acar Çevik U, Kaya B, Celik I, Rudrapal M, Rakshit G, Karayel A, et al. New benzimidazole-triazole derivatives as topoisomerase I Inhibitors: Design, synthesis, anti-cancer screening, and molecular modeling studies. ACS omega. 2024; 9(11): 13359-13372.
- Anastassova N, Georgieva I, Milanova V, Tzoneva R, Radev K, Yancheva D, et al. Synthesis of new triazole and thiadiazole derivatives of the n, n’-disubstituted benzimidazole-2-thione and evaluation of their antitumor potential. J Chem Technol Metall. 2022; 57(4): 709-717.
- Ansari KF, Lal C. Synthesis, physicochemical properties and antimicrobial activity of some new benzimidazole derivatives. Eur J Med Chem. 2009; 44(10): 4028-4033.
- Arora ROK, Kaur N, Bansal Y, Bansal G. Novel coumarin-benzimidazole derivatives as antioxidants and safer anti-inflammatory agents. Acta Pharm Sin B. 2014; 4(5): 368-375.
- Aydemir N, Bilaloğlu R. Genotoxicity of two anti-cancer drugs, gemcitabine and topotecan, in mousebone marrow in vivo. Mutat Res Genet Toxicol Environ Mutagen. 2003; 537(1): 43-51.
- Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996; 239(1): 70-76.
- Celik I, Çevik UA, Karayel A, Işık A, Kayış U, Gül UD, et al. Synthesis, Molecular Docking, Dynamics, Quantum-Chemical Computation, and Antimicrobial Activity Studies of Some New Benzimidazole-Thiadiazole Hybrids. ACS omega. 2022; 7(50): 47015-47030.
- Çevik UA, Işık A, Evren A E, Kapusız Ö, Gül ÜD, Özkay Y, et al. Synthesis of new benzimidazole derivatives containing 1, 3, 4-thiadiazole: their in vitro antimicrobial, in silico molecular docking and molecular dynamic simulations studies. SAR QSAR Environ Res. 2022; 33(11): 899-914.
- Debus H. Ueber die einwirkung des ammoniaks auf glyoxal. Justus Liebigs Ann Chem. 1858; 107(2): 199-208.
- Hobrecker F. Reduction-products of nitracetamide compounds. Deut Chem Ges Ber. 1872; 5: 920-924.
- Ismael GF, Rosa DD, Mano MS, Awada A. Novel cytotoxic drugs: old challenges, new solutions. Cancer Treat Rev. 2008; 34(1): 81-91.
- Işık A, Çevik UA, Çelik I, Bostancı HE, Karayel A, Gündoğdu G, et al. Benzimidazole-hydrazone derivatives: Synthesis, in vitro anti-cancer, antimicrobial, antioxidant activities, in silico DFT and ADMET studies. J Mol Struct. 2022; 1270: 133946.
- Işık A, Acar Çevik U, Karayel A, Ahmad I, Patel H, Çelik İ, Gul UD, Bayazıt G, Bostancı HE, Kocak A, Ozkay Y, Kaplancıklı ZA. Synthesis, DFT Calculations, In Silico Studies, and Antimicrobial Evaluation of Benzimidazole-Thiadiazole Derivatives. ACS Omega. 2024; 9(16); 18469-18479
- Jahan J, Barik S, Chagaleti BK, Kathiravan MK, Deivasigamani P. The synthetic approach of benzimidazole derivatives as anti-inflammatory agents. Jmpas. 2023; 12(5): 6049-6058.
- Karki SS, Panjamurthy K, Kumar S, Nambiar M, Ramareddy SA, Chiruvella KK, et al. Synthesis and biological evaluation of novel 2-aralkyl-5-substituted-6-(4’-fluorophenyl)-imidazo [2, 1-b][1, 3, 4] thiadiazole derivatives as potent anticancer agents. Eur J Med Chem. 2011; 46(6): 2109-2116.
- Keri RS, Hiremathad A, Budagumpi S, Nagaraja BM. Comprehensive review in current developments of benzimidazole-based medicinal chemistry. Chem Biol Drug Des. 2015; 86(1): 19-65.
- Khadri MN, Begum AB, Sunil MK, Khanum SA. Synthesis, docking and biological evaluation of thiadiazole and oxadiazole derivatives as antimicrobial and antioxidant agents. Res Chem. 2020; 2: 100045.
- Khokra SL, Choudhary D. Benzimidazole an important scaffold in drug discovery. Asian J Biochem Pharm Res. 2011; 3(1): 476-486.
- Mohapatra TR, Ganguly S. Design, Synthesis, Antibacterial and Antitubercular Studies of Some New Benzimidazoles. A Combined Experimental and Computational Approach. ChemistrySelect. 2024; 9(12): e202304791.
- Parkin DM. Global cancer statistics in the year 2000. Lancet Oncol. 2001; 2(9): 533-543.
- Prestinaci F, Pezzotti P, Pantosti A. Antimicrobial resistance: a global multifaceted phenomenon. Pathog Glob Health. 2015; 109(7): 309-318.
- Ranjith PK, Rajeesh P, Haridas KR, Susanta NK, Row TNG, Rishikesan R, et al. Design and synthesis of positional isomers of 5 and 6-bromo-1-[(phenyl) sulfonyl]-2-[(4-nitrophenoxy) methyl]-1H-benzimidazoles as possible antimicrobial and antitubercular agents. Bioorg Med Chem Lett. 2013; 23(18): 5228-5234.
- Shi L, Ge HM, Tan SH, Li HQ, Song YC, Zhu HL, et al. Synthesis and antimicrobial activities of Schiff bases derived from 5-chloro-salicylaldehyde. Eur J Med Chem. 2007; 42(4): 558-564.
- Sies H. Oxidative stress: oxidants and antioxidants. Exp Physiol. 1997; 82(2): 291-295.
- Sridevi CH, Balaji K, Naidu A, Sudhakaran R. Synthesis of some phenylpyrazolo benzimidazolo quinoxaline derivatives as potent antihistaminic agents. J Chem. 2010; 7: 234-238.
- Vasantha K, Basavarajaswamy G, Rai MV, Boja P, Pai VR, Shruthi N, et al. Rapid ‘one-pot’synthesis of a novel benzimidazole-5-carboxylate and its hydrazone derivatives as potential anti-inflammatory and antimicrobial agents. Bioorg. Med. Chem. Lett. 2015; 25(7): 1420-1426.
- Yadav S, Narasimhan B. Perspectives of benzimidazole derivatives as anticancer agents in the new era. Anti-Cancer Agents Med Chem. 2016; 16(11): 1403-1425.
- Wayne, P. Clinical and L.S. Institute, Performance standards for antimicrobial susceptibility testing. Clinical and Laboratory Standards Institute. 2017.
- Wayne P. Clinical and Laboratory Standards Institute: Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard. CLSI document M27-A3 and Supplement S. 2008; 3: 6-12.
- Wright JB. The chemistry of the benzimidazoles. Chem Rev. 1951; 48(3): 397-541.
- Xing F, Hu Q, Qin Y, Xu J, Zhang B, Yu X, et al. The relationship of redox with hallmarks of cancer: the importance of homeostasis and context. Front Oncol. 2022; 12: 862743.
Publication Dates
-
Publication in this collection
20 Jan 2025 -
Date of issue
2025
History
-
Received
19 Dec 2023 -
Accepted
12 June 2024

 Synthesis, characterization, antimicrobial, antioxidant, and anti-cancer activity of new hybrid structures based on benzimidazole and thiadiazole
Synthesis, characterization, antimicrobial, antioxidant, and anti-cancer activity of new hybrid structures based on benzimidazole and thiadiazole