Abstract
MON810 is a genetically-modified (GM) maize (Zea mays) event commonly employed in insect-resistant GM maize hybrids. GM events obtained by biolistic transformation methods, such as MON810, are generated by insertion of a recombinant gene expression cassette in a random locus of the plant genome, and this process may cause emergent properties besides the intended modification. Here, we compared morphophysiological parameters of MON810 GM maize hybrid AG-5011YG and its non-GM near-isogenic hybrid (NIH) AG-5011, using in vitro cultures as an interactive model. NIH callogenesis frequency, callus friability, and de novo morphogenesis were compared using two explant types and different 2,4-dichlorophenoxyacetic acid (2,4-D) levels. 2,4-D modulated the morphophysiological responses of both NIHs, but when using root segments as explants, the GM showed significantly different trends for callus induction and friability, with lower responses at higher 2,4-D concentrations, indicating an emergent property related to altered cell response to 2,4-D.
Keywords:
Genetically modified; Zea mays; tissue culture; model; emergent properties
INTRODUCTION
MON810 (Monsanto Company, YieldGard® maize, genetically-modified (GM) event MON810, unique identifier MON-ØØ81Ø-6 (CERA 2016CERA - Center for Environmental Risk Assessment/ILSI Research Foundation. GM crop database, MON-ØØ81Ø-6(MON810) (2016) Available at: <Available at: http://cera-gmc.org/GmCropDatabaseEvent/MON810/short
>. Accessed on Feb 20, 2016.
http://cera-gmc.org/GmCropDatabaseEvent/...
) is an insect-resistant GM maize (Zea mays) used for the production of GM maize hybrids. This GM event was generated by biolistic insertion of a recombinant-gene-expression cassette (GEC) in the genome of maize hybrid Hi-II (CERA 2016CERA - Center for Environmental Risk Assessment/ILSI Research Foundation. GM crop database, MON-ØØ81Ø-6(MON810) (2016) Available at: <Available at: http://cera-gmc.org/GmCropDatabaseEvent/MON810/short
>. Accessed on Feb 20, 2016.
http://cera-gmc.org/GmCropDatabaseEvent/...
), and has the third-highest number of approvals for use in food, feed, and processing around the world (James 2016James C (2016) Executive summary of global status of commercialized biotech/gm crops: 2016. In ISAAA Brief 52. Ithaca, New York, 135p.).
Several genetic transformation techniques have been developed to genetically engineer plants that can tolerate environmental stress, and improve productivity and quality of plant species, although the efficiency of current gene transfer techniques is still low (Kumari et al. 2017Kumari A, Baskaran P and Van Staden J (2017) Gene transfer utilizing pollen-tubes of Albuca nelsonii and Tulbaghia violacea. Crop Breeding and Applied Biotechnlogy 17: 222-228.). Before approval for cultivation and commercialization, usually a new GM event must undergo a risk assessment, where not only the recombinant construct's integration, stability, and expression must be demonstrated, but also evaluated for possible unintended genetic and phenotypic abnormalities compared to the most similar non-GM counterpart available (Bartsch et al. 2010Bartsch D, Devos Y, Hails R, Kiss J, Krogh PH, Mestdagh S, Nuti M, Sessitsch A, Sweet J and Gathmann A (2010) Environmental impact of genetically modified maize expressing Cry1 proteins. In Kempken F and Jung C (eds) Genetic modification of plants: agriculture, horticulture and forestry. Springer, Berlin, 675p.). Depending on plant species, the appropriate comparison may be made between transformed and non-transformed material from the same genotype in the case of predominantly vegetative-propagated species or near-isogenic lines/hybrids (NILs/NIHs, respectively) in the case of predominately-sexually reproduced species (Cellini et al. 2004Cellini F, Chesson A, Colquhoun I, Constable A, Davies HV, Engel KH, Gatehouse AM, Kärenlampi S, Kok EJ, Leguay JJ, Lehesranta S, Noteborn HP, Pedersen J and Smith M (2004) Unintended effects and their detection in genetically modified crops. Food and Chemical Toxicology 42: 1089-1125.), such as maize.
GM NILs are obtained by introgressing a target locus on conventional inbred lines, performing an initial cross with the target locus-donor plant, and consecutive backcrossings with the parental inbred line. After 6-8 backcrossings, the genotype of produced seeds will be more than 99% similar to the backcrossed parental line, and, additionally, contain the target locus. Based on this, NILs are defined as two or more lines that share the same genetic makeup except for one selected locus, and possibly a few additional loci genetically-linked to the target locus (Zeven and Waninge 1986Zeven AC and Waninge J (1986) The degree of phenotypic resemblance of the near-isogenic lines of the wheat cultivar Thatcher with their recurrent parent. Euphytica 35: 665-676.).
Plant growth regulators such as the synthetic auxin 2,4-D are widely-employed in plant tissue culture (Gaspar et al. 1996Gaspar T, Kevers C, Penel C, Greppin H, Reid DM and Thorpe TA (1996) Plant hormones and plant growth regulators in plant tissue culture. In Vitro Cellular & Developmental Biology - Plant 32: 272-289.), and differences in the in vitro regenerative capacity of maize genotypes are known to be affected by growth regulator concentration in the culture medium (Vasil et al. 1985Vasil V, Lu C and Vasil IK (1985) Histology of somatic embryogenesis in cultured immature embryos of maize (Zea mays L.). Protoplasma 127: 1-8, Zhonget al. 1992Zhong H, Srinivasan C and Stricklen MB (1992) In-vitro morphogenesis of corn (Zea mays L.). I. Differentiation of multiple shoot clumps and somatic embryos from shoot tips. Planta 187: 483-489.). Here, we report an interactive in vitro model for the study of transgenic plants based on the rationale that in vitro conditions provide a highly-controllable environment, which minimizes random environmental effects and increases reproducibility, scalability, and resolution of effects.
The objective of this study was to compare induction rate, frequency of friable calli, and morphogenesis responses of a GM maize hybrid (AG5011YG) and its non-GM NIH AG-5011, using seedling root segments and seedling apical shoot meristem (SAM) thin cell layers (TCLs) as explants cultured in medium with a range of 2,4-D concentrations (0, 4 and 17 µM for SAM TCLs, and 0, 13, 17 and 21 µM for root segments). The main hypothesis tested was: near-isogenic hybrid, explant type, and 2,4-D concentration affect callus induction, friability and morphogenic potential in an interactive fashion.
MATERIAL AND METHODS
Plant material
Seeds of GM maize hybrid AG5011YG (MON810) and non-GM NIH AG5011 were purchased at a seed market in Erval do Oeste, Santa Catarina, south Brazil. Seeds were analyzed to confirm their GM or non-GM status using a lateral flow strip kit for detection of Cry1Ab protein (Envirologix Quickstix AS-003-CRLS kit) and qualitative PCR for detection of the CAMV-35S promoter and the recombinant cry1Ab gene, using zein as endogenous control (Data not shown). Seeds of the GM and non-GM NIHs (n=30) were thoroughly-washed under tap water for 5 min; immersed in distilled water with Tween 20 (40 μL 100 mL-1) and shaken for 5 min; transferred to an aseptic laminar flow hood;surface sterilized with 70% alcohol for 3 min; treated with NaClO 1% plus Tween 20 (40 μL 100 mL-1) for 30 min; washed five times with sterile distilled water; and immersed in sterile distilled water in a glass flask for 72 h to soften and facilitate subsequent embryo excision. All subsequent manipulations of seeds, embryos, seedlings and cultures took place under aseptic conditions in a laminar flow hood, using aseptic technique and sterilized tools and materials. Mature zygotic embryos were excised from softened seeds placed on paper plates, using forceps and scalpels under a stereomicroscope. Excised embryos were placed in glass flasks with sterile distilled water to prevent dehydration, and then were again surface-sterilized with 70% ethanol for 1 min, NaClO 1% for 10 min, washed five times with sterile distilled water, and inoculated in test tubes containing 15 ml germination medium (MS salts (Murashige and Skoog 1962Murashige T and Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiologia Plantarum 15: 473-497.)) supplemented with Morel vitamins (Morel and Wetmore 1951Morel G and Wetmore RH (1951) Fern callus tissue culture. American Journal of Botany 38: 141-143.), and 3% sucrose. Germinating embryos were kept at 25 ºC in the dark for 3 weeks, and plantlets were used as source of different explant types for callus induction.
Callus induction
The basal nutrient medium used for induction of maize in vitro cultures consisted of MS salts supplemented with Morel vitamins, 3% sucrose, enzymatic casein hydrolysate (200 gL-1) and adenine (40 mg L-1); this basal medium was supplemented with a range of 2,4-D concentrations for the induction experiment. The pH of all media was adjusted to 5.8; 0.2% of Phytagel was then added; and the medium was then autoclaved at 121 ºC and 1.5 atm for 20 min.
Thin cell layers (TCLs, ~0.75 mm) (4 TCLs around the first node per plantlet) and root segments (~2 cm) (10-16 segments per seedling) were harvested from contaminant-free and well-developed GM and non-GM maize seedlings (n = 24 for GM and 28 for non-GM) for explants used in the callus induction experiment. Explants were excised from plantlets placed on paper plates, using scalpels and forceps, and immediately inoculated in plastic Petri dishes containing 25 ml basal nutrient medium supplemented with a gradient of 2,4-D concentrations (0, 4 and 17 μM for TCLs; 0, 13, 17 and 21 μM for root segments). The ranges were determined on the basis of previous experiments with the GM maize (data not shown). A total of 229 TCLs and 576 root segments were inoculated. Cultures were kept at 25 ºC in the dark.
Callogenesis, and callus friability were evaluated 4 weeks and morphogenesis 8 weeks after induction. Here, callus is defined as an unorganized cell mass (Ikeuchi et al. 2013Ikeuchi M, Sugimoto K and Iwase A (2013) Plant callus: mechanisms of induction and repression. Plant Cell 25: 3159-3173.), friable callus is defined as a friable (easily fragmented into small pieces), yellowish callus capable of producing globular structures that develop only roots (Type III callus - Emons et al. 1993Emons AMC, Samallo-Droppers A and Van der Toom C (1993) The influence of sucrose, mannitol, L-proline, abscisic acid and gibberellic acid on the maturation of somatic embryos of Zea mays L. from suspension cultures. Plant Physiology 142: 597-604.,Emons and Kieft 1995), and non-friable callus is defined as soft, spongy, translucent to brown callus that did not produce globular structures but was also capable of producing roots. All endpoints were recorded by visual inspection in a stereomicroscope. Callus samples were photographed inside Petri dishes in an Olympus stereomicroscope attached to an Olympus DP-71 image capture system.
Statistical Analysis
Data were analyzed in a generalized linear mixed model framework (Bolker et al. 2009Bolker BM, Brooks ME, Clark CJ, Geange SW, Poulsen JR, Stevens MHH and White JSS (2009) Generalized linear mixed models: A practical guide for ecology and evolution. Trends in Ecology and Evolution 24: 127-135.). Data on callus induction, callus friability and morphogenesis (only rhizogenesis was recorded) were evaluated as binary variables (yes = 1; no = 0) and analyzed with logistic regression. For every analysis, the initial statistical models included all explanatory variables, interactions, and quadratic terms for quantitative variables (e.g. 2,4-D concentration). Model selection was carried out by means of stepwise elimination of variables followed by inspection of the small-sample-corrected Akaike Information Criterion (cAIC) (Hurvich and Tsai 1989Hurvich CM and Tsai C-L (1989) Regression and time series model selection in small samples. Biometrika 76: 297-307., Burnham and Anderson 2004Burnham KP and Anderson DR (2004) Multimodel inference: understanding AIC and BIC inmodel selection. Sociological Methods & Research 33: 261-304.). Model assumptions about residual variance and distribution were evaluated graphically for all models. Effects were considered significant when P < 0.05. Significant trend- and point-wise differences were detected by comparison of 95% confidence intervals/envelopes (Cumming et al. 2007Cumming G, Fidler F and Vaux DL (2007) Error bars in experimental biology. Journal of Cell Biology 177: 7-11. ). Analyses were carried out in the R statistical language (R Core Team 2019R Core Team (2019) R: A language and environment for statistical computing, reference index version 3.5.3. R Foundation for Statistical Computing, Vienna, 3642p.).
RESULTS AND DISCUSSION
TCLs (~0.75 mm) from the first-node (Figure 1) and root segments (~2 cm) (Figure 2) of in vitro germinated plantlets from both maize NIHs (GM and non-GM) readily produced large amounts of callus. These cultures strongly favored rhizogenesis, so that this protocol will require further adjustments to promote the growth of other types of tissue, such as shoot organogenesis or somatic embryogenesis. Typically, plant regeneration is obtained in Zea mays using immature zygotic embryos as explants (Green and Phillips 1975Green CE and Philips RL (1975) Plant regeneration from tissue culture of maize. Crop Science 15: 417-421., Vasil et al. 1985Vasil V, Lu C and Vasil IK (1985) Histology of somatic embryogenesis in cultured immature embryos of maize (Zea mays L.). Protoplasma 127: 1-8, Emons and Kieft 1995Emons AMC and Kieft H (1995) Somatic embryogenesis in maize (Zea mays L.). In Bajaj YPS (ed) Biotechnology in agriculture and forestry 31. Springer, Berlin , p. 24-39.), but other organs/tissues were successfully employed, such as immature tassels (Rhodes et al. 1986Rhodes CR, Green CE and Phillips RL (1986) Factors affecting tissue culture initiation from maize tassels. Plant Science 46: 225-232.), leaf segments (Conger et al. 1987Conger BV, Novak FJ, Afza R and Erdelsky KE (1987) Somatic embryogenesis from cultured leaf segments of Zea mays. Plant Cell Reports 6: 345-347.), and shoot apical meristems (Zhong et al. 1992Zhong H, Srinivasan C and Stricklen MB (1992) In-vitro morphogenesis of corn (Zea mays L.). I. Differentiation of multiple shoot clumps and somatic embryos from shoot tips. Planta 187: 483-489.). Nonetheless, the results of this study validate the use of seedling SAM TCLs and, especially, seedling root segments, as viable options for maize in vitro culture initiation, while establishing an initial in vitro model for the study of GM plants.
Calli of non-GM (a-c) and GM (MON810) (d-f) near-isogenic maize hybrids induced on thin cell layers (TCLs) (1 mm) of shoot apical meristem (SAM) of in vitro germinated plantlets, on media supplemented with 2,4-D at 0 μM (a and d), 4 μM (b and e) and 17 μM (c and f).
Calli of non-GM (a-d) and GM (MON810) (e-h) near-isogenic maize hybrids induced on root segments (2 mm) of in vitro germinated plantlets, on media supplemented with 2,4-D at 0 μM (a and e), 13 μM (b and f), 17 μM (c and g) and 21 μM (d and h).
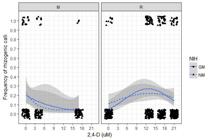
Generally, as 2,4-D concentration increased, so did the rate of callus induction for both NIHs and types of explant (Figure 3) in a clear curvilinear trend (P<0.001). However, significant interactions between NIHs and explant types (P < 0.05), between explant type and 2,4-D concentration (P < 0.001), and between NIH and 2,4-D concentration (P < 0.001) affected in vitro callogenesis. The interaction between NIH and explant type indicated that SAM TCLs and root segments responded differently depending on the NIH: SAM TCLs showed similar induction trends for both NIHs throughout the tested 2,4-D gradients, while root segments exhibited significantly different trends for GM and non-GM, with the non-GM NIH presenting higher induction frequency at 2,4-D concentrations above 15 µM. The interaction between explant type and 2,4-D indicated that TCLs and root segments presented significantly different induction trends across the 2,4-D gradient: quadratic trends were exhibited by both TCLs and root segments, but root segments required higher 2,4-D to attain maximum induction compared to TCLs, and showed higher maximum induction rates.
Frequency of callus induction on thin cell layers (TCLs) (0.75 mm) of shoot apical meristem (SAM) (M) and root segments (2 cm) (R) of in vitro germinated plantlets of GM (GM) and non-GM (NM) near-isogenic maize hybrids, along a gradient of 2,4-D concentrations in basal culture media. Black dots and triangles represent observations (GM and non-GM, respectively - all 0 and 1 on the y axis, slightly dislocated vertically and horizontally to improve visualization). Shaded bands indicate 95% confidence envelopes.
NIH type and 2,4-D concentration, but not explant type, significantly affected callus friability. Increasing 2,4-D concentration in the culture medium increased the frequency of friable calli, with the highest frequencies achieved in the highest tested 2,4-D concentrations for GM and non-GM NIHs, as well as for TCLs and root segments (Figure 4). The non-GM NIH showed higher frequency of friable calli across the tested 2,4-D gradient for calli derived from root segments, but not from TCLs.
Frequency of friable calli of GM (GM - solid line) and non-GM (NM - dashed line) near-isogenic maize hybrids, 4 weeks after induction, using thin cell layers (TCLs) (0.75 mm) of shoot apical meristem (M) or root segments (2 cm) (R) of in vitro germinated plantlets as explants, along gradients of 2,4-D concentrations in basal culture media. Black dots and triangles represent observations (GM and non-GM, respectively - all 0 and 1 on the y axis, slightly dislocated vertically and horizontally to improve visualization). Shaded bands indicate 95% confidence envelopes.
Eight weeks after callus induction, the majority of morphogenic responses observed in calli were either a lack of organization or a tendency to rhizogenesis, depending on experimental conditions and especially on 2,4-D concentration (Figure 5). 2,4-D induced curvilinear responses (p<0.001) with opposite signs (opposite curvatures) depending on explant type, denoting a significant interaction between explant type and 2,4-D concentration (p<0.001). There was evidence of a significant interaction between NIH and a quadratic trend for 2,4-D concentration (p=0.008). The interaction between 2,4-D and NIH suggested that, by averaging both explant types, 2,4-D had an almost constant effect in the non-GM NIH, but the curvilinear effect for the GM NIH was more pronounced. However, callus rhizogenesis for the GM and non-GM NIHs depended on explant type: overall, averaging between both NIHs, root-derived calli demonstrated more pronounced rhizogenesis, with a curvilinear trend and higher frequency of rhizogenic calli induced in mid-level 2,4-D concentrations, whereas increased 2,4-D concentrations in the culture medium continuously inhibited rhizogenesis in TCL-derived calli.
Frequency of rhizogenic calli of GM (GM - solid line) and non-GM (NM - dashed line) near-isogenic maize hybrids, 8 weeks after induction, using thin cell layers (TCLs) (0.75 mm) of shoot apical meristem (M) or root segments (2 cm) (R) of in vitro germinated plantlets as explants, along gradients of 2,4-D concentrations in basal culture media. Black dots and triangles represent observations (GM and non-GM, respectively - all 0 and 1 on the y axis, slightly dislocated vertically and horizontally to improve visualization). Shaded bands indicate 95% confidence envelopes.
Calli were effectively induced by 2,4-D on both explant types, but root segments required higher 2,4-D concentration than SAM TCLs to reach maximum induction frequency. Since roots represent a more differentiated tissue than SAMs, the requirement of higher 2,4-D levels to induce cell dedifferentiation in root tissues is consistent with the plant tissue culture literature. Interestingly, induction frequencies on SAM TCLs and root segments was not only dependent on 2,4-D, but also on maize NIH: induction trends for SAM TCLs were similar for GM and non-GM maize, but for root segments higher induction frequencies were observed in non-GM compared to the GM, indicating a different cell response to auxin between the tested NIHs, which was only detectable when using explants that require higher 2,4-D concentration for dedifferentiation.
Maize callus friability is an indicator of a low degree of differentiation, and usually friability is associated with high regeneration capacity through somatic embryogenesis (Emons and Kieft 1995Emons AMC and Kieft H (1995) Somatic embryogenesis in maize (Zea mays L.). In Bajaj YPS (ed) Biotechnology in agriculture and forestry 31. Springer, Berlin , p. 24-39.). However, some friable maize calli are also known to be highly determined to rhizogenesis, with the main difference between maize embryogenic and rhizogenic callus being that meristems in embryogenic calli are formed exogenously from a protodermal cell layer on the outside of the callus tissue, while in rhizogenic calli they are formed endogenously from a parenchymatous cell layer within the tissue (Emons et al. 1993Emons AMC, Samallo-Droppers A and Van der Toom C (1993) The influence of sucrose, mannitol, L-proline, abscisic acid and gibberellic acid on the maturation of somatic embryos of Zea mays L. from suspension cultures. Plant Physiology 142: 597-604.).
Callus friability was significantly affected by 2,4-D and NIH, but not by explant type. Increasing 2,4-D concentration continuously enhanced friable callus frequency in both NIHs, but the non-GM NIH showed a stronger response to 2,4-D, producing significantly more friable calli. Combining the results of callus induction and callus friability, we observed that the 2,4-D concentration which maximizes callus induction did not correspond to the concentration that maximizes callus friability, since models estimated a decrease in induction towards the higher end of the tested 2,4-D gradient, while the frequency of friable calli maintained an increasing trend throughout the whole gradient.
After induction, calli were left in the same culture medium for 8 weeks, in order to detect possible late induction responses from inoculated explants, and allow calli to enter into a morphogenic pathway (Emons and Kieft 1995Emons AMC and Kieft H (1995) Somatic embryogenesis in maize (Zea mays L.). In Bajaj YPS (ed) Biotechnology in agriculture and forestry 31. Springer, Berlin , p. 24-39.). Several calli became rhizogenic, as previously reported in other studies (Emons and Kieft 1995), and no development of shoots nor somatic embryos was observed, characterizing these as Type III calli (Emons and Kieft 1995Emons AMC and Kieft H (1995) Somatic embryogenesis in maize (Zea mays L.). In Bajaj YPS (ed) Biotechnology in agriculture and forestry 31. Springer, Berlin , p. 24-39.).
CONCLUSIONS
This is the first study to establish in vitro cultures of a commercial GM maize hybrid and its conventional, non-GM NIH, allowing for the evaluation of off-target effects of genetic transformation by employing in vitro cultures as an interactive model. The synthetic auxin 2,4-D effectively modulated the morphophysiological responses of the GM and non-GM NIHs, using either SAM TCL's or root segments as explant. By averaging between both explant types, or considering root segments alone, the highest value for callogenesis and callus friability across the tested 2,4-D gradients was significantly different between the NIHs, indicating altered cell response to 2,4-D. Of the tested explants, root segments gave rise to the highest rates of callus induction and callus friability in both NIHs, although it required higher 2,4-D doses (~20 µM) than SAM TCLs for maximum induction. Moreover, seedling roots were often plentiful in vitro, making it an easily-available explant source. The high rates of rhizogenesis offer a challenge and an opportunity for comparison between GM and non-GM hybrids in understanding maize in vitro morphogenesis. Further research efforts are required to investigate the environmental parameters of control in the maintenance/proliferation of induced cultures, as well as their possible re-determination to organogenic or embryogenic states. The use of in vitro cultures is a novel approach to perform comparisons between GM maize and their non-GM counterparts, providing a sensitive model for detection of emergent properties or off-target effects of genetic modification, as reflected in altered phenotypic responses of GM maize cells to environmental stimuli in vitro.
ACKNOWLEDGEMENTS
The authors thank CAPES for fellowship to DH, and CNPq for fellowship to RON and MPG. Financial support was provided by The Norwegian Agency for Development Cooperation (Ministry of Foreign Affairs, Norway) under the GenØk South-America Research Hub grant FAPEU 077/2012. This was a joint project between UFSC and GenØk-Centre for Biosafety.
REFERENCES
- Bartsch D, Devos Y, Hails R, Kiss J, Krogh PH, Mestdagh S, Nuti M, Sessitsch A, Sweet J and Gathmann A (2010) Environmental impact of genetically modified maize expressing Cry1 proteins. In Kempken F and Jung C (eds) Genetic modification of plants: agriculture, horticulture and forestry. Springer, Berlin, 675p.
- Bolker BM, Brooks ME, Clark CJ, Geange SW, Poulsen JR, Stevens MHH and White JSS (2009) Generalized linear mixed models: A practical guide for ecology and evolution. Trends in Ecology and Evolution 24: 127-135.
- Burnham KP and Anderson DR (2004) Multimodel inference: understanding AIC and BIC inmodel selection. Sociological Methods & Research 33: 261-304.
- Cellini F, Chesson A, Colquhoun I, Constable A, Davies HV, Engel KH, Gatehouse AM, Kärenlampi S, Kok EJ, Leguay JJ, Lehesranta S, Noteborn HP, Pedersen J and Smith M (2004) Unintended effects and their detection in genetically modified crops. Food and Chemical Toxicology 42: 1089-1125.
- CERA - Center for Environmental Risk Assessment/ILSI Research Foundation. GM crop database, MON-ØØ81Ø-6(MON810) (2016) Available at: <Available at: http://cera-gmc.org/GmCropDatabaseEvent/MON810/short >. Accessed on Feb 20, 2016.
» http://cera-gmc.org/GmCropDatabaseEvent/MON810/short - Conger BV, Novak FJ, Afza R and Erdelsky KE (1987) Somatic embryogenesis from cultured leaf segments of Zea mays. Plant Cell Reports 6: 345-347.
- Cumming G, Fidler F and Vaux DL (2007) Error bars in experimental biology. Journal of Cell Biology 177: 7-11.
- Emons AMC and Kieft H (1995) Somatic embryogenesis in maize (Zea mays L.). In Bajaj YPS (ed) Biotechnology in agriculture and forestry 31. Springer, Berlin , p. 24-39.
- Emons AMC, Samallo-Droppers A and Van der Toom C (1993) The influence of sucrose, mannitol, L-proline, abscisic acid and gibberellic acid on the maturation of somatic embryos of Zea mays L. from suspension cultures. Plant Physiology 142: 597-604.
- Gaspar T, Kevers C, Penel C, Greppin H, Reid DM and Thorpe TA (1996) Plant hormones and plant growth regulators in plant tissue culture. In Vitro Cellular & Developmental Biology - Plant 32: 272-289.
- Green CE and Philips RL (1975) Plant regeneration from tissue culture of maize. Crop Science 15: 417-421.
- Hurvich CM and Tsai C-L (1989) Regression and time series model selection in small samples. Biometrika 76: 297-307.
- Ikeuchi M, Sugimoto K and Iwase A (2013) Plant callus: mechanisms of induction and repression. Plant Cell 25: 3159-3173.
- James C (2016) Executive summary of global status of commercialized biotech/gm crops: 2016. In ISAAA Brief 52. Ithaca, New York, 135p.
- Kumari A, Baskaran P and Van Staden J (2017) Gene transfer utilizing pollen-tubes of Albuca nelsonii and Tulbaghia violacea Crop Breeding and Applied Biotechnlogy 17: 222-228.
- Morel G and Wetmore RH (1951) Fern callus tissue culture. American Journal of Botany 38: 141-143.
- Murashige T and Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiologia Plantarum 15: 473-497.
- R Core Team (2019) R: A language and environment for statistical computing, reference index version 3.5.3. R Foundation for Statistical Computing, Vienna, 3642p.
- Rhodes CR, Green CE and Phillips RL (1986) Factors affecting tissue culture initiation from maize tassels. Plant Science 46: 225-232.
- Vasil V, Lu C and Vasil IK (1985) Histology of somatic embryogenesis in cultured immature embryos of maize (Zea mays L.). Protoplasma 127: 1-8
- Zeven AC and Waninge J (1986) The degree of phenotypic resemblance of the near-isogenic lines of the wheat cultivar Thatcher with their recurrent parent. Euphytica 35: 665-676.
- Zhong H, Srinivasan C and Stricklen MB (1992) In-vitro morphogenesis of corn (Zea mays L.). I. Differentiation of multiple shoot clumps and somatic embryos from shoot tips. Planta 187: 483-489.
Publication Dates
-
Publication in this collection
01 Aug 2019 -
Date of issue
Apr-Jul 2019
History
-
Received
22 Feb 2018 -
Accepted
12 Dec 2018