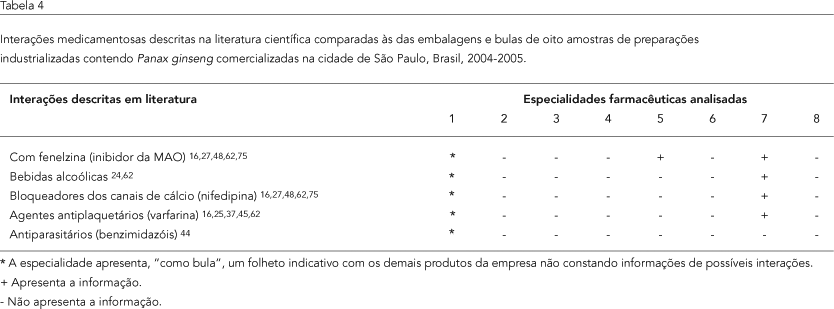
The information provided on package inserts and inner wrapping of eight products containing Panax ginseng from different manufacturers was compared internally and checked against data from the scientific literature. The inserts included extensive text, containing abundant information on indications for use, but no scientific evidence in humans. All the inserts lacked information on potential adverse effects and drug interaction. There was no standardization as to dose regimens, particularly in relation to the dried extract and ginsenoside concentration. The eight inserts thus showed no concern over standardization, indication for usage, or possible side effects and drug interactions.
Panax ginseng; Medicine Package; Drug Utilization