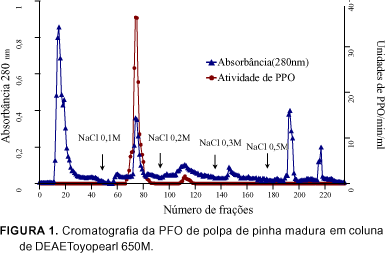
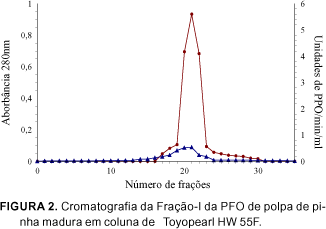
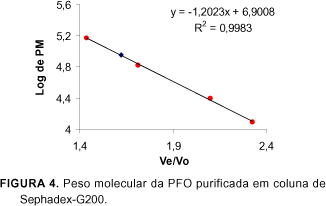
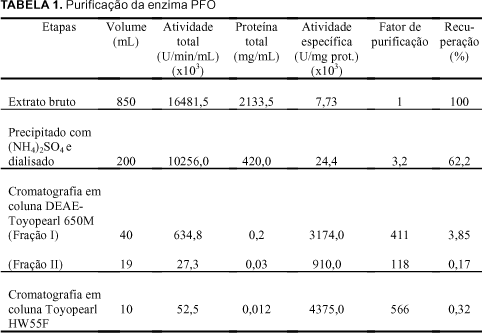
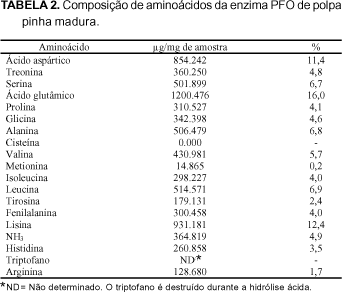
The PPO (EC 1.10.3.2) extract of ripe custard apple (Annona squamosa L.) pulps, was partially purified by ammonium sulphate fractionation and purified 411 (Fraction I) and 118 (Fraction II) fold in an ion exchange column of DEAE-Toyopearl 650M, and 566 fold in a gel column of Toyopearl HW 55F. The enzyme of the most active fraction was characterized biochemically. The partially purified and purified enzyme used the o-diphenols as substrates and no activity towards monophenols was detected. With respect to the kinetic parameters, the purified enzyme presented values for Km and Vmax of 7.14 mM and 302.0 units/min/ml for catechol and 25.0 mM and 180.2 units/min/ml for L-dopa respectively, substrates which show greater specificity. The molecular weight was estimated as 90.700 daltons using gel filtration on Sephadex G-200. In the analysis of copper, the purified enzyme gave a value of 11ppm by weight of the liofilized sample. The amino acid composition of the custard apple fruit PPO, presented greater amounts of aspartic acid, glutamic acid and lysine and smaller amounts of methionine, arginine and tyrosine, with an absence of cysteine.
purification; polyphenoloxidase; custard apple; Annona squamosa L