Abstract
Ulcerative colitis (UC) is a chronic and non-specific inflammatory bowel disease (IBD). Its pathogenesis remains unclear, but its morbidity shows an increasing trend year by year. The pathogenesis of UC may be related to the genetic susceptibility, immune factor and intestinal microflora. In the last few years, an increasing number of studies have examined the relationship between the expression levels of peripheral CircRNA and UC. We aimed to evaluate the efficacy of CircRNA MFHAS1 in colitis and its possible mechanism. The expression of CircRNA MFHAS1 was reduced in colitis, and miR-486-5p expression was also increased in citrobacter rodentium-induced murine colitis. In vitro model, over-expression of CircRNA MFHAS1 reduced inflammatory responses, induced SIRT1 protein expression, and suppressed NF-κB protein expression. However, miR-486-5p CircRNA MFHAS1 suppressed SIRT1 protein expression, and induced NF-κB protein expression in vitro model. The inactivation of SIRT1 reduced the anti-inflammation effects of CircRNA MFHAS1 on inflammatory responses in vitro model. Over-expression of miR-486-5p also reduced the anti-inflammation effects of CircRNA MFHAS1 in vitro model. Our results demonstrated that CircRNA MFHAS1 reduces inflammatory responses in Colitis via SIRT1/NF-κB by miR-486-5p.
Keywords:
CircRNA MFHAS1; Colitis; NF-κB; SIRT1; miR-486-5p
1 Background
Ulcerative colitis (UC) is a chronic, non-specific, inflammatory disease in which lesions are mainly located in the colonic mucosa (Meade et al., 2008Meade, M. O., Cook, D. J., Griffith, L. E., Hand, L. E., Lapinsky, S. E., Stewart, T. E., Killian, K. J., Slutsky, A. S., & Guyatt, G. H. (2008). A study of the physiologic responses to a lung recruitment maneuver in acute lung injury and acute respiratory distress syndrome. Respiratory Care, 53(11), 1441-1449. PMid:18957146.). The precise etiology and pathogenesis of UC remain unclear. UC is associated with chronic disease and easy and repeated recurrence. Therefore, it is difficult to diagnose and treat in clinical practice. The World Health Organization (WHO) classifies UC as a modern refractory disease. In recent years, UC morbidity rates have been increasing (Dhamija et al., 2014Dhamija, P., Hota, D., Kochhar, R., Sachdev, A., & Chakrabarti, A. (2014). Randomized clinical trial: Atorvastatin versus placebo in patients with acute exacerbation of mild to moderate ulcerative colitis. Indian Journal of Gastroenterology, 33(2), 151-156. http://dx.doi.org/10.1007/s12664-013-0420-4. PMid:24222372.
http://dx.doi.org/10.1007/s12664-013-042...
) due to the influence of factors such as dietary habit changes and aggravated mental stress. Currently, no specific targeted treatment is available for UC in Western medicine (Meade et al., 2008Meade, M. O., Cook, D. J., Griffith, L. E., Hand, L. E., Lapinsky, S. E., Stewart, T. E., Killian, K. J., Slutsky, A. S., & Guyatt, G. H. (2008). A study of the physiologic responses to a lung recruitment maneuver in acute lung injury and acute respiratory distress syndrome. Respiratory Care, 53(11), 1441-1449. PMid:18957146.); comparatively speaking, traditional Chinese medicine (TCM) and its preparations are more effective. TCM UC treatment can not only relieve the clinical symptoms of UC, but can also effectively prevent recurrence (Sandborn et al., 2016Sandborn, W. J., Bhandari, B. R., Fogel, R., Onken, J., Yen, E., Zhao, X., Jiang, Z., Ge, D., Xin, Y., Ye, Z., French, D., Silverman, J. A., Kanwar, B., Subramanian, G. M., McHutchison, J. G., Lee, S. D., Shackelton, L. M., Pai, R. K., Levesque, B. G., & Feagan, B. G. (2016). Randomised clinical trial: A phase 1, dose-ranging study of the anti-matrix metalloproteinase-9 monoclonal antibody GS-5745 versus placebo for ulcerative colitis. Alimentary Pharmacology & Therapeutics, 44(2), 157-169. http://dx.doi.org/10.1111/apt.13653. PMid:27218676.
http://dx.doi.org/10.1111/apt.13653...
).
NF-kB mainly exists in the inactive form under normal physiological conditions. However, it can initiate transcription of multiple genes through various signal transduction pathways in the case of any abnormality (Bing et al., 2017Bing, X., Xuelei, L., Wanwei, D., Linlang, L., & Keyan, C. (2017). EGCG Maintains Th1/Th2 balance and mitigates ulcerative colitis induced by dextran sulfate sodium through TLR4/MyD88/NF-kappaB signaling pathway in rats. Canadian Journal of Gastroenterology & Hepatology, 2017, 3057268. http://dx.doi.org/10.1155/2017/3057268. PMid:29404307.
http://dx.doi.org/10.1155/2017/3057268...
). It plays a vital role in multiple physiopathological activities, particularly in inflammation and immune response (Eissa et al., 2017Eissa, N., Hussein, H., Kermarrec, L., Elgazzar, O., Metz-Boutigue, M. H., Bernstein, C. N., & Ghia, J. E. (2017). Chromofungin (CHR: CHGA47-66) is downregulated in persons with active ulcerative colitis and suppresses pro-inflammatory macrophage function through the inhibition of NF-kappaB signaling. Biochemical Pharmacology, 145, 102-113. http://dx.doi.org/10.1016/j.bcp.2017.08.013. PMid:28827109.
http://dx.doi.org/10.1016/j.bcp.2017.08....
). It can regulate the expression of immunity- and inflammation-related factors and inflammatory mediators to exert its effects. Excessive NF-kB expression also plays an important role in the genesis and development of various diseases (Bing et al., 2017Bing, X., Xuelei, L., Wanwei, D., Linlang, L., & Keyan, C. (2017). EGCG Maintains Th1/Th2 balance and mitigates ulcerative colitis induced by dextran sulfate sodium through TLR4/MyD88/NF-kappaB signaling pathway in rats. Canadian Journal of Gastroenterology & Hepatology, 2017, 3057268. http://dx.doi.org/10.1155/2017/3057268. PMid:29404307.
http://dx.doi.org/10.1155/2017/3057268...
), including some inflammatory diseases, cardiovascular diseases, and tumors (Gu et al., 2017Gu, P., Zhu, L., Liu, Y., Zhang, L., Liu, J., & Shen, H. (2017). Protective effects of paeoniflorin on TNBS-induced ulcerative colitis through inhibiting NF-kappaB pathway and apoptosis in mice. International Immunopharmacology, 50, 152-160. http://dx.doi.org/10.1016/j.intimp.2017.06.022. PMid:28666238.
http://dx.doi.org/10.1016/j.intimp.2017....
). Interaction area of NF-kB and profilin IkB, and the nuclear localization sequence are responsible for the binding of NF-kB with DNA, dimerization, and interaction with IkB, respectively. Thus, it allows the activated NF-kB to enter the nucleus to exert its function (Bing et al., 2017Bing, X., Xuelei, L., Wanwei, D., Linlang, L., & Keyan, C. (2017). EGCG Maintains Th1/Th2 balance and mitigates ulcerative colitis induced by dextran sulfate sodium through TLR4/MyD88/NF-kappaB signaling pathway in rats. Canadian Journal of Gastroenterology & Hepatology, 2017, 3057268. http://dx.doi.org/10.1155/2017/3057268. PMid:29404307.
http://dx.doi.org/10.1155/2017/3057268...
).
Silent information regulator 2 (Sir2)-related enzyme is a highly conserved NAD+-dependent histone deacetylase. There are 7 Sir2 homologous genes in humans, referred to as SIRT1–7. Of these, SIRT1 shows the highest homology and has become an important research focus in recent years (Lv et al., 2018Lv, Q., Wang, K., Qiao, S., Yang, L., Xin, Y., Dai, Y., & Wei, Z. (2018). Norisoboldine, a natural AhR agonist, promotes Treg differentiation and attenuates colitis via targeting glycolysis and subsequent NAD(+)/SIRT1/SUV39H1/H3K9me3 signaling pathway. Cell Death & Disease, 9(3), 258. http://dx.doi.org/10.1038/s41419-018-0297-3. PMid:29449535.
http://dx.doi.org/10.1038/s41419-018-029...
). It was reported that SIRT1 protein has anti-inflammatory and anti-oxidative stress effects, and can reduce cell injury (Akimova et al., 2014Akimova, T., Xiao, H., Liu, Y., Bhatti, T. R., Jiao, J., Eruslanov, E., Singhal, S., Wang, L., Han, R., Zacharia, K., Hancock, W. W., & Beier, U. H. (2014). Targeting sirtuin-1 alleviates experimental autoimmune colitis by induction of Foxp3+ T-regulatory cells. Mucosal Immunology, 7(5), 1209-1220. http://dx.doi.org/10.1038/mi.2014.10. PMid:24549276.
http://dx.doi.org/10.1038/mi.2014.10...
).
miRNA is a class of small-molecule nucleotides that can regulate gene expression. It is involved in the UC-related gene or protein expression. The single-nucleotide polymorphism (SNP) of miRNA precursor can partly affect the sensitivity and pathogenetic process in UC patients (Bian et al., 2011Bian, Z., Li, L., Cui, J., Zhang, H., Liu, Y., Zhang, C. Y., & Zen, K. (2011). Role of miR-150-targeting c-Myb in colonic epithelial disruption during dextran sulphate sodium-induced murine experimental colitis and human ulcerative colitis. The Journal of Pathology, 225(4), 544-553. http://dx.doi.org/10.1002/path.2907. PMid:21590770.
http://dx.doi.org/10.1002/path.2907...
), and it is of vital importance to the study of UC pathogenesis, as well as diagnosis and prognosis for UC. According to reports, miRNA is expressed in human serum. Moreover, serum miRNA can bind with protein and lipoprotein, thus avoiding being degraded by RNA hydrolase (Ghobadi et al., 2017Ghobadi, F., Vaisi-Raygani, A., Bahrehmand, F., Tanhapour, M., Kiani, A., Rahimi, Z., & Pourmotabbed, T. (2017). Genetic variants of Pre-microRNAs A-499G(rs3746444) and T-196a2C(rs11614913) with Ulcerative Colitis (UC) and investigated with Thiopurine-S-Methyltransferase (TPMT) Activity. Clinical Laboratory, 63(10), 1683-1690. http://dx.doi.org/10.7754/Clin.Lab.2017.170502. PMid:29035443.
http://dx.doi.org/10.7754/Clin.Lab.2017....
). Serum miRNA derives from the pathways for cell apoptosis, cell necrosis, and cell secretion, as well as circulating cell lysis. Mature intracellular miRNA can be wrapped by lipoprotein or lipid to form the exosome, which is thereby transported out of the cell into the blood. Exosomes that enter the blood can enter the receptor cell again through endocytosis (Netz et al., 2017Netz, U., Carter, J., Eichenberger, M. R., Feagins, K., Galbraith, N. J., Dryden, G. W., Pan, J., Rai, S. N., & Galandiuk, S. (2017). Plasma microRNA Profile Differentiates Crohn’s Colitis From Ulcerative Colitis. Inflammatory Bowel Diseases, 24(1), 159-165. http://dx.doi.org/10.1093/ibd/izx009. PMid:29272478.
http://dx.doi.org/10.1093/ibd/izx009...
). Its coating can be removed in the receptor cell to release miRNA, thus exerting its biological function. The present study assessed the efficacy of downregulation of CircRNA MFHAS1 in preventing inflammatory responses in a model of Citrobacter rodentium-induced murine colitis.
2 Material and Methods
2.1 Animals
Fourteen C57BL/6 mice (age 5-6 weeks, weight 20-21 g, male) were housed at 22-23 °C, 55-60% humidity, 12/12 h light/dark cycle, and had free access to water and standard chow. All mice were allocated into 2 groups: sham (n = 6), colitis (n = 8). Mice in the colitis group were administered 5×109 CFU/day of Citrobacter rodentium for 7 days. The animal protocol was approved by the Institutional Animal Care and Use Committee of the Chongming branch of Xinhua Hospital.
2.2 Histopathology assay
Tissue samples were washed with PBS and fixed with 4% paraformaldehyde for 24 h. Tissue samples was deparaffinized, hydrated, and stained using hematoxylin and eosin for 5 min. Tissue samples were examined using an Olympus BX51 polarizing microscope (Olympus Corporation, Tokyo, Japan).
2.3 Enzyme-linked immunosorbent assay (ELISA) KIT
Tissue samples and cells were lysed with RAPA assay containing PMSF at 4 °C for 15 min. We used 10 μg protein to measure the IL-1β, IL-6, TNF-α, and IL-18 levels using ELISA.
2.4 Western blot analysis
Cells were lysed with RAPA containing PMSF at 4 °C for 15 min and the were centrifuged at 12 000 g for 10 min at 4 °C. Protein concentrations were estimated by bicinchoninic acid (BCA) method (Thermo Scientific, Rockford, IL). We used SDS-PAGE to resolve 50 μg of total protein, which was then transferred to PVDF membranes. Membranes were blocked with 5% non-fat milk in TBST for 1 h and immunoblotted overnight at 4 °C with p65NF-κB, SIRT1, and GAPDH (Santa Cruz). Membranes were incubated with horseradish peroxidase-conjugated secondary antibodies (1:5000; Santa Cruz) for 1 h at 37 °C. The membranes were washed with TBST for 15 min and detected by a chemiluminescent reagent. Western blot bands were quantified using Image-ProPlus 6.0 software (Media Cybernetics, Inc., Rockville, MD, USA).
2.5 Cell culture and in vitro model
THP-1 cells were cultured in Marker-5A medium (Sigma-Aldrich, St. Louis, MO, USA) containing 10% FBS (Invitrogen) at 37 °C in a 95% humidity incubator at 37 °C and 5% CO2. THP-1 cells were transfected with Lipofectamine 2000 (Invitrogen) with CircRNA MFHAS1, CircRNA MFHAS1 inhibitor, and negative mimics for 48 h and then treated with 1×108 CFU/day of Citrobacter rodentium for 6 h to induce an in vitro colitis model. The cells were further divided into CircRNA MFHAS1 overexpression group, CircRNA MFHAS1 inhibited group and negative group according to different treatment.
2.6 Immunofluorescence and microscopy
Cells were washed with PBS and fixed with 4% paraformaldehyde for 20 min. Then, cells were blocked with 5% BSA containing 0.1% Triton X-100 at 25 °C for 1 h and incubated overnight with SIRT1 (1:100, Abcam; USA) at 4 °C. Cells were then washed with PBS for 15 min and incubated with Alexa Fluor 488 conjugated with anti-rabbit immunoglobulin G (IgG) (1:500 dilution) for 1 h. Cells were then washed with PBS for 15 min and stained with DAPI for 15 min at the dark. Images of cells were captured using an Eclipse TE200 fluorescent microscope (Nikon, Melville, NY).
2.7 Statistical analysis
Data were analyzed using SPSS 17.0 and are expressed as mean ± standard deviation (SD). One-way analysis of variance (ANOVA) and Tukey's post hoc test were used for comparisons of individual data. p<0.05 was considered to be statistically significant.
3 Results
3.1 The expression of CircRNA MFHAS1 in Citrobacter rodentium-induced murine colitis
The explored the mechanism and function of CircRNA MFHAS1 in Citrobacter rodentium-induced murine colitis. HE staining showed ulcer in Citrobacter rodentium-induced murine colitis, but it did not occur in the sham control group (Figure 1A). We found that body weight was decreased, and there were higher levels of TNF-α, IL-1β, IL-6, and IL-18 in the Citrobacter rodentium-induced murine colitis group compared with the sham control group (Figure 11E). CircRNA MFHAS1 expression was decreased in the Citrobacter rodentium-induced murine colitis group compared with the control group (Figure 11H). These results suggest that CircRNA MFHAS1 is involved in the occurrence and development of Citrobacter rodentium-induced murine colitis.
The expression of CircRNA MFHAS1 in Citrobacter rodentium-induced murine colitis. HE staining (A), body weight (B), TNF-α (C), IL-1β (D), IL-6 (E), and IL-18 (F) levels, CircRNA MFHAS1 expression (G and H) using gene chip and QPCR. **p<0.01 compared with sham control group. Sham, sham control group; Colitis, Citrobacter rodentium-induced murine colitis group.
3.2 CircRNA MFHAS1 regulates inflammation in vitro
We investigated the mechanism underlying the action of CircRNA MFHAS1 in colitis inflammation and assessed whether CircRNA MFHAS1 or CircRNA MFHAS1 inhibitor mimics increased or reduced the expression of CircRNA MFHAS1in the in vitro model compared with the negative group (Figure 22B). Overexpression of circRNA MFHAS1 reduced the levels of TNF-α, IL-1β, IL-6, and IL-18 in the in vitro model compared with the negative group (Figure 22F), and downregulation of circRNA MFHAS1 increased the levels of TNF-α, IL-1β, IL-6, and IL-18 in the in vitro model compared with the negative group (Figure 22J).
CircRNA MFHAS1 regulates inflammation in vitro. CircRNA MFHAS1 expression (A and B) using overexpression of MFHAS1 or downregulation of MFHAS1; TNF-α (C), IL-1β (D), IL-6 (E) and IL-18 (F) levels by overexpression of MFHAS1; TNF-α (G), IL-1β (H), IL-6 (I) and IL-18 (J) levels by downregulation of MFHAS1. Negative, negative control group; MFHAS1, overexpression of CircRNA MFHAS1 group; MFHAS1 inhibitor, downregulation of CircRNA MFHAS1 group. **p<0.01 compared with negative control group.
3.3 CircRNA MFHAS1 regulates inflammation in the in vitro model via miR-486-5p.
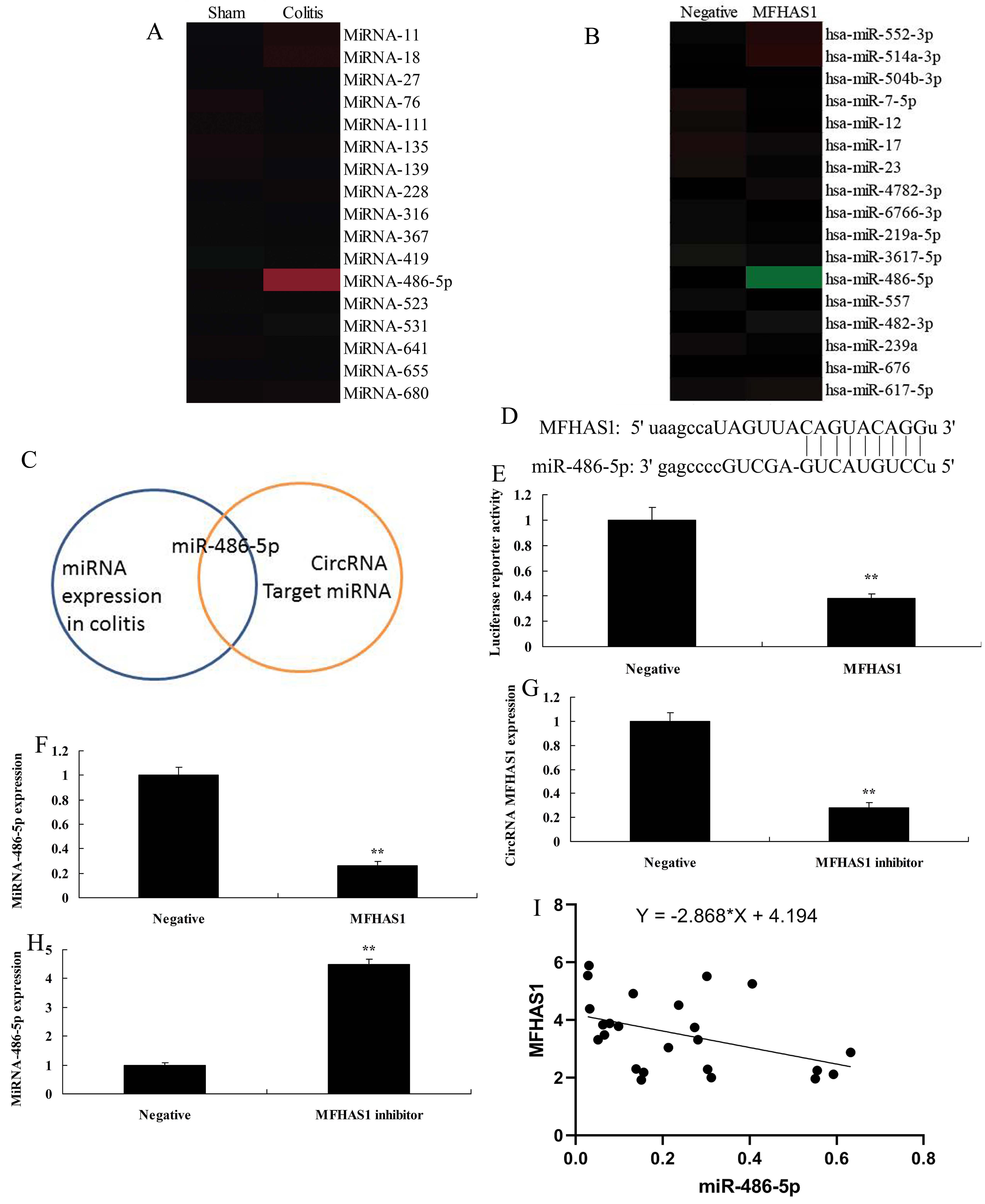
As shown in Figure 3A, there was increased expression of miR-486-5p in the colitis group compared with the negative group. Overexpression of CircRNA MFHAS1 reduced miR-486-5p expression in vitro compared with the negative group (Figure 3B). Data in Figure 3C show that CircRNA MFHAS1 regulates miR-486-5p to adjust inflammation in vitro. Figures 33E show that CircRNA MFHAS1 regulates the expression of miR-486-5p by directly targeting its mRNA 3'-UTR, and luciferase assay activity levels were reduced in the overexpression of CircRNA MFHAS1 group compared with the negative group. Overexpression of CircRNA MFHAS1 reduced miR-486-5p expression in vitro compared with the negative group (Figure 3F). CircRNA MFHAS1 inhibitor mimics reduced CircRNA MFHAS1 expression and increased miR-486-5p expression in vitro compared with the negative group (Figure 33H). We found a negative correlation between CircRNA MFHAS1 and miR-486-5p (Figure 3I).
CircRNA MFHAS1 regulates inflammation in vitro model by miR-486-5p. Gene chip results for miR-486-5p expression (A and B) in vivo and in vitro of overexpression of CircRNA MFHAS1; Analysis chart (C), CircRNA MFHAS1 regulates the expression of miR-486-5p by directly targeting its mRNA 3'-UTR (D), luciferase assay activity levels (E), miR-486-5p expression by overexpression of CircRNA MFHAS1 (F); CircRNA MFHAS1 expression (G) and miR-486-5p expression (H) by downregulation of CircRNA MFHAS1 group; negative correlation between CircRNA MFHAS1 and miR-486-5p (I). Negative, negative control group; MFHAS1, overexpression of CircRNA MFHAS1 group; MFHAS1 inhibitor, downregulation of CircRNA MFHAS1 group. **p<0.01 compared with negative control group.
3.4 miR-486-5p regulates the SIRT1/NF-κB signaling pathway
We used gene chip technology to analyze the inflammation signal pathway in the in vitro model.. MiR-486-5p mimics increased the expression of miR-486-5p in vitro compared with the negative group (Figure 4A). We found that SIRT1 expression was reduced and NF-κBp65 expression was increased in the in vitro model by overexpression of miR-486-5p compared with the negative group (Figure 4B). Our results show that the SIRT1/NF-κB signaling pathway may be regulated by miR-486-5p to further affect the inflammation in colitis (Figure 4C). We also found that miR-486-5p regulates the expression of SIRT1 by directly targeting its mRNA 3'-UTR (Figure 4D). Luciferase assay activity levels were reduced in miR-486-5p over-expression group compared with the negative group (Figure 4E). Overexpression of miR-486-5p suppressed SIRT1 protein expression and increased NF-κB protein expression in vitro compared with the negative group (Figure 44H). It showed that overexpression of CircRNA MFHAS1 suppressed SIRT1 protein expression in vitro compared with the negative group (Figure 4I). Overexpression of CircRNA MFHAS1 suppressed NF-κB protein expression and increased SIRT1 protein expression in vitro compared with the negative group (Figure 44L). Therefore, miR-486-5p regulates the NF-κB signaling pathway by SIRT1 to promote inflammatory responses in colitis.
miR-486-5p regulates the SIRT1/NF-κB signaling pathway. miR-486-5p expression (A), Gene chip results for SIRT1/NF-κB signaling pathway (B), Analysis chart (C), miR-486-5p regulates the expression of SIRT1 by directly targeting its mRNA 3'-UTR (D), luciferase assay activity levels (E), SIRT1 and NF-κB protein expression (F and G) and Western blot assays of SIRT1 and NF-κB (H) following overexpression of miR-486-5p; SIRT1 protein expression by IF (I) and SIRT1 and NF-κB protein expression (J and K) and Western blot assays of SIRT1 and NF-κB (L) following overexpression of CircRNA MFHAS1. Negative, negative control group; MFHAS1, overexpression of CircRNA MFHAS1 group; miR-486-5p, overexpression of miR-486-5p group. **p<0.01 compared with negative control group.
3.5 miR-486-5p reduced the pro-inflammation effects of CircRNA MFHAS1 downregulation in vitro
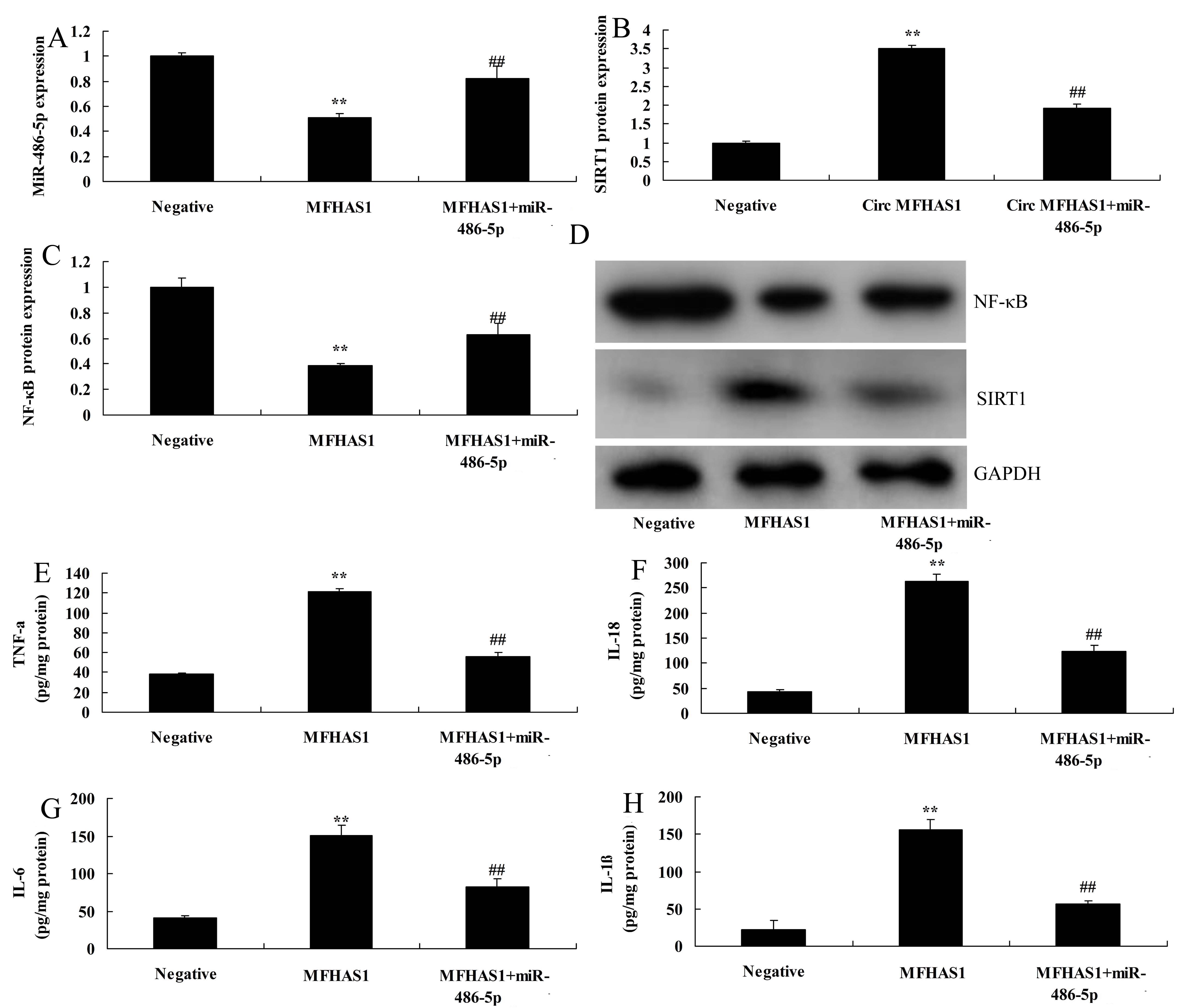
Next, we used the in vitro model to analyze the mechanism of CircRNA MFHAS1 on inflammation in Citrobacter rodentium-induced murine colitis. Overexpression of miR-486-5p increased miR-486-5p expression, suppressed SIRT1 protein expression, and increased NF-κB protein expression in vitro following overexpression of CircRNA MFHAS1 compared with CircRNA MFHAS1 overexpression group (Figure 55D). miR-486-5p reduced the anti-inflammation effects of CircRNA MFHAS1 overexpression on inhibition of TNF-α, IL-1β, IL-6, and IL-18 levels in vitro compared with the CircRNA MFHAS1 overexpression group (Figure 55H).
miR-486-5p reduced the pro-inflammation effects of CircRNA MFHAS1 downregulation in vitro. miR-486-5p expression (A), SIRT1 and NF-κB protein expression (B and C) and Western blot assays of SIRT1 and NF-κB (D), TNF-α (C), IL-1β (D), IL-6 (E), and IL-18 (F) levels. Negative, negative control group; MFHAS1, overexpression of CircRNA MFHAS1 group; miR-486-5p, overexpression of miR-486-5p group. **p<0.01 compared with negative control group, ##p<0.01 compared with overexpression of CircRNA MFHAS1group.
3.6 The inactivation of SIRT1 reduced the anti-inflammation effects of CircRNA MFHAS1 on inflammatory responses in vitro
The study investigated the mechanism of anti-inflammation effects of CircRNA MFHAS1 in a Citrobacter rodentium-induced murine colitis model. Si-SIRT1 suppressed SIRT1 protein expression, and induced NF-κB protein expression in vitro by overexpression of CircRNA MFHAS1 compared with the CircRNA MFHAS1 overexpression group (Figure 66C). The inactivation of SIRT1 reduced TNF-α, IL-1β, IL-6, and IL-18 levels in vitro by overexpression of CircRNA MFHAS1 compared with the CircRNA MFHAS1 overexpression group (Figure 66G). These results show that CircRNA MFHAS1 regulates the SIRT1/NF-κB signaling pathway to affect inflammation in colitis through miR-486-5p.
The inactivation of SIRT1 reduced the anti-inflammation effects of CircRNA MFHAS1 on inflammatory responses in vitro. SIRT1 and NF-κB protein expression (A and B) and Western blot assays of SIRT1 and NF-κB (C), TNF-α (D), IL-1β (E), IL-6 (F), and IL-18 (G) levels. Negative, negative control group; MFHAS1, overexpression of CircRNA MFHAS1 group; miR-486-5p, overexpression of miR-486-5p group. **p<0.01 compared with negative control group, ##p<0.01 compared with overexpression of CircRNA MFHAS1group.
4 Discussion
UC is most common in Western countries. Recent data indicate that the morbidity of UC in the USA is 200/100 000. Annually, about 0.1-0.15 billion US dollars is spent on UC treatment. In China, the economy has been rapidly developed in the past 10 years (Oliva et al., 2012Oliva, S., Di Nardo, G., Ferrari, F., Mallardo, S., Rossi, P., Patrizi, G., Cucchiara, S., & Stronati, L. (2012). Randomised clinical trial: The effectiveness of Lactobacillus reuteri ATCC 55730 rectal enema in children with active distal ulcerative colitis. Alimentary Pharmacology & Therapeutics, 35(3), 327-334. http://dx.doi.org/10.1111/j.1365-2036.2011.04939.x. PMid:22150569.
http://dx.doi.org/10.1111/j.1365-2036.20...
) and dietary habits have greatly changed (Meade et al., 2008Meade, M. O., Cook, D. J., Griffith, L. E., Hand, L. E., Lapinsky, S. E., Stewart, T. E., Killian, K. J., Slutsky, A. S., & Guyatt, G. H. (2008). A study of the physiologic responses to a lung recruitment maneuver in acute lung injury and acute respiratory distress syndrome. Respiratory Care, 53(11), 1441-1449. PMid:18957146.), with lower intake of vegetables and higher intake of processed Western-style foods (Hua et al., 2015Hua, F., Ribbing, J., Reinisch, W., Cataldi, F., & Martin, S. (2015). A pharmacokinetic comparison of anrukinzumab, an anti- IL-13 monoclonal antibody, among healthy volunteers, asthma and ulcerative colitis patients. British Journal of Clinical Pharmacology, 80(1), 101-109. http://dx.doi.org/10.1111/bcp.12589. PMid:25614144.
http://dx.doi.org/10.1111/bcp.12589...
). A recent investigation suggests that UC morbidity is negatively correlated with vegetable intake (Sandborn et al., 2012Sandborn, W.J., van Assche, G., Reinisch, W., Colombel, J.F., D'Haens, G., Wolf, D.C., Kron, M., Tighe, M.B., Lazar, A., Thakkar, R.B. (2012). Adalimumab induces and maintains clinical remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology, 142(2), 257-265.). In addition, ω-6 fatty acid, total fat, and meat intake are positively correlated with UC morbidity (Sandborn et al., 2012Sandborn, W.J., van Assche, G., Reinisch, W., Colombel, J.F., D'Haens, G., Wolf, D.C., Kron, M., Tighe, M.B., Lazar, A., Thakkar, R.B. (2012). Adalimumab induces and maintains clinical remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology, 142(2), 257-265.). From 2000 to 2012, the annual number of diagnosed cases of UC increased by 250%, with the recurrence rate reaching 72% worldwide. Moreover, USis affecting a younger population (Hua et al., 2015Hua, F., Ribbing, J., Reinisch, W., Cataldi, F., & Martin, S. (2015). A pharmacokinetic comparison of anrukinzumab, an anti- IL-13 monoclonal antibody, among healthy volunteers, asthma and ulcerative colitis patients. British Journal of Clinical Pharmacology, 80(1), 101-109. http://dx.doi.org/10.1111/bcp.12589. PMid:25614144.
http://dx.doi.org/10.1111/bcp.12589...
). In this study, CircRNA MFHAS1 expression was found to be decreased in the Citrobacter rodentium-induced murine colitis model compared with the sham control group. Shi et al showed that MFHAS1 suppresses inflammation in Thr239 via inhibition of the TLR4 signaling pathway (Shi et al., 2017Shi, Q., Xiong, B., Zhong, J., Wang, H., Ma, D., & Miao, C. (2017). MFHAS1 suppresses TLR4 signaling pathway via induction of PP2A C subunit cytoplasm translocation and inhibition of c-Jun dephosphorylation at Thr239. Molecular Immunology, 88, 79-88. http://dx.doi.org/10.1016/j.molimm.2017.06.017. PMid:28609714.
http://dx.doi.org/10.1016/j.molimm.2017....
).
The etiology and pathogenesis of UC remain unclear. With the development of cytokine research, it was found that cytokines are closely correlated with the pathogenesis of inflammatory bowel disease (Scott & Lichtenstein, 2018Scott, F. I., & Lichtenstein, G. R. (2018). Biosimilars in the treatment of inflammatory bowel disease: Supporting Evidence in 2017. Current Treatment Options in Gastroenterology, 16(1), 147-164. http://dx.doi.org/10.1007/s11938-018-0177-z. PMid:29492747.
http://dx.doi.org/10.1007/s11938-018-017...
). Cytokines can be classified into 2 types-pro-inflammatory cytokine and anti-inflammatory-based on their effects on inflammatory response. The mechanism underlying the association between the difference two types has attracted growing attention (Rosario et al., 2017Rosario, M., French, J. L., Dirks, N. L., Sankoh, S., Parikh, A., Yang, H., Danese, S., Colombel, J. F., Smyth, M., Sandborn, W. J., Feagan, B. G., Reinisch, W., Sands, B. E., Sans, M., & Fox, I. (2017). Exposure-efficacy Relationships for Vedolizumab Induction Therapy in Patients with Ulcerative Colitis or Crohn’s Disease. Journal of Crohn’s and Colitis, 11(8), 921-929. http://dx.doi.org/10.1093/ecco-jcc/jjx021. PMid:28333288.
http://dx.doi.org/10.1093/ecco-jcc/jjx02...
). The imbalance of the two types of cytokines is considered to be the main cause of the injury of intestinal mucosa (Allamneni et al., 2018Allamneni, C., Venkata, K., Yun, H., Xie, F., DeLoach, L., & Malik, T. A. (2018). Comparative effectiveness of vedolizumab vs. infliximab induction therapy in ulcerative colitis: experience of a real-world cohort at a tertiary inflammatory bowel disease center. Gastroenterology Research, 11(1), 41-45. http://dx.doi.org/10.14740/gr934w. PMid:29511405.
http://dx.doi.org/10.14740/gr934w...
). Cytokines are proteins or small-molecule polypeptides (Cho et al., 2016Cho, B. O., Yin, H. H., Park, S. H., Byun, E. B., Ha, H. Y., & Jang, S. I. (2016). Anti-inflammatory activity of myricetin from Diospyros lotus through suppression of NF-kappaB and STAT1 activation and Nrf2-mediated HO-1 induction in lipopolysaccharide-stimulated RAW264.7 macrophages. Bioscience, Biotechnology, and Biochemistry, 80(8), 1520-1530. http://dx.doi.org/10.1080/09168451.2016.1171697. PMid:27068250.
http://dx.doi.org/10.1080/09168451.2016....
) synthesized and secreted by immune cells (eg, T cells, B cells, macrophages, and monocytes) and non-immune cells (eg, vascular endothelial cells, epidermal cells, and fibroblasts). Cytokines can transfer information between cells and have immunological functions (Rosario et al., 2017Rosario, M., French, J. L., Dirks, N. L., Sankoh, S., Parikh, A., Yang, H., Danese, S., Colombel, J. F., Smyth, M., Sandborn, W. J., Feagan, B. G., Reinisch, W., Sands, B. E., Sans, M., & Fox, I. (2017). Exposure-efficacy Relationships for Vedolizumab Induction Therapy in Patients with Ulcerative Colitis or Crohn’s Disease. Journal of Crohn’s and Colitis, 11(8), 921-929. http://dx.doi.org/10.1093/ecco-jcc/jjx021. PMid:28333288.
http://dx.doi.org/10.1093/ecco-jcc/jjx02...
). Cytokines can extensively regulate immune response and hematopoietic function, and they participate in pathological processes such as inflammation and injury (Allamneni et al., 2018Allamneni, C., Venkata, K., Yun, H., Xie, F., DeLoach, L., & Malik, T. A. (2018). Comparative effectiveness of vedolizumab vs. infliximab induction therapy in ulcerative colitis: experience of a real-world cohort at a tertiary inflammatory bowel disease center. Gastroenterology Research, 11(1), 41-45. http://dx.doi.org/10.14740/gr934w. PMid:29511405.
http://dx.doi.org/10.14740/gr934w...
). Our in vitro results demonstrate that overexpression of CircRNA MFHAS1 reduces levels of TNF-α, IL-1β, IL-6, and IL-18. Zhong et al. (2015)Zhong, J., Shi, Q. Q., Zhu, M. M., Shen, J., Wang, H. H., Ma, D., & Miao, C. H. (2015). MFHAS1 Is associated with sepsis and stimulates TLR2/NF-kappaB signaling pathway following negative regulation. PLoS One, 10(11), e0143662. http://dx.doi.org/10.1371/journal.pone.0143662. PMid:26599367.
http://dx.doi.org/10.1371/journal.pone.0...
reported that MFHAS1 reduced inflammation in sepsis via the TLR2/NF-κB signaling pathway.
In the absence of external stimulation, NF-κB exists in the cytoplasm in the form of NF-κB-IkB complex. IkBs are degraded in the presence of external stimulation (Galvez-Llompart et al., 2011Galvez-Llompart, M., Recio, M. C., & Garcia-Domenech, R. (2011). Topological virtual screening: a way to find new compounds active in ulcerative colitis by inhibiting NF-kappaB. Molecular Diversity, 15(4), 917-926. http://dx.doi.org/10.1007/s11030-011-9323-4. PMid:21717125.
http://dx.doi.org/10.1007/s11030-011-932...
; Yu et al., 2011Yu, Z. H., Huang, F., Xu, N., Zhao, D. M., Hu, F. A., Liu, J., & Liu, H. F. (2011). Expression of Toll-like receptor 4, CD14, and NF-kappaB in Chinese patients with ulcerative colitis. Journal of Immunoassay & Immunochemistry, 32(1), 47-56. http://dx.doi.org/10.1080/15321819.2010.538108. PMid:21253969.
http://dx.doi.org/10.1080/15321819.2010....
). They include cytokine, oxidant, protein kinase C activator, virus, immunological stimulant, ultraviolet rays, and lipopolysaccharide. As a result, the free NF-κB dimer is released (Yu et al., 2011Yu, Z. H., Huang, F., Xu, N., Zhao, D. M., Hu, F. A., Liu, J., & Liu, H. F. (2011). Expression of Toll-like receptor 4, CD14, and NF-kappaB in Chinese patients with ulcerative colitis. Journal of Immunoassay & Immunochemistry, 32(1), 47-56. http://dx.doi.org/10.1080/15321819.2010.538108. PMid:21253969.
http://dx.doi.org/10.1080/15321819.2010....
). At this time, NF-κB is translocated from cytoplasm to cell nucleus and binds with the intranuclear target gene kB sequence (Galvez-Llompart et al., 2011Galvez-Llompart, M., Recio, M. C., & Garcia-Domenech, R. (2011). Topological virtual screening: a way to find new compounds active in ulcerative colitis by inhibiting NF-kappaB. Molecular Diversity, 15(4), 917-926. http://dx.doi.org/10.1007/s11030-011-9323-4. PMid:21717125.
http://dx.doi.org/10.1007/s11030-011-932...
). Thus, it affects the gene transcription of multiple adhesion molecules, cytokines, immune receptors, acute-phase proteins, and stress response protein. Research indicates that NF-κB is the central regulator of stress and inflammatory response (Yu et al., 2011Yu, Z. H., Huang, F., Xu, N., Zhao, D. M., Hu, F. A., Liu, J., & Liu, H. F. (2011). Expression of Toll-like receptor 4, CD14, and NF-kappaB in Chinese patients with ulcerative colitis. Journal of Immunoassay & Immunochemistry, 32(1), 47-56. http://dx.doi.org/10.1080/15321819.2010.538108. PMid:21253969.
http://dx.doi.org/10.1080/15321819.2010....
). It plays an immunoregulation role (Gu et al., 2017Gu, P., Zhu, L., Liu, Y., Zhang, L., Liu, J., & Shen, H. (2017). Protective effects of paeoniflorin on TNBS-induced ulcerative colitis through inhibiting NF-kappaB pathway and apoptosis in mice. International Immunopharmacology, 50, 152-160. http://dx.doi.org/10.1016/j.intimp.2017.06.022. PMid:28666238.
http://dx.doi.org/10.1016/j.intimp.2017....
) and its signaling pathway is extensively involved in cell survival, differentiation, proliferation, and apoptosis. It plays a key role in the genesis, development, and outcome of multiple diseases. We demonstrated that CircRNA MFHAS1 reduced inflammation in an in vitro model through reducing the expression of miR-486-5p.
SIRT1 not only reduces the release of inflammatory factors, but can also suppress the interactions between inflammatory factors and the corresponding cell receptors. Thus, it can restrain the inflammatory factor-mediated cytotoxic reaction (Sands et al., 2016Sands, B. E., Joshi, S., Haddad, J., Freudenberg, J. M., Oommen, D. E., Hoffmann, E., McCallum, S. W., & Jacobson, E. (2016). Assessing colonic exposure, safety, and clinical activity of SRT2104, a Novel Oral SIRT1 activator, in patients with mild to moderate Ulcerative Colitis. Inflammatory Bowel Diseases, 22(3), 607-614. http://dx.doi.org/10.1097/MIB.0000000000000597. PMid:26595549.
http://dx.doi.org/10.1097/MIB.0000000000...
). SIRT1 exerts its cell-protection effect through multiple mechanisms. For example, it can silence some apoptosis-inducing proteins through histone modification. In addition, it can act on non-histones (such as NF-κB) through deacetylation, and reduce the expression of its downstream genes like iNOS. In addition, it was reported that in NF-κB-treated intestinal epithelial cells, the SIRT1 expression level decreases with increased NF-κB concentration (Wang et al., 2017Wang, K., Li, Y. F., Lv, Q., Li, X. M., Dai, Y., & Wei, Z. F. (2017). Bergenin, acting as an agonist of PPARgamma, Ameliorates experimental colitis in mice through improving expression of SIRT1, and therefore inhibiting NF-kappaB-Mediated macrophage activation. Frontiers in Pharmacology, 8, 981. http://dx.doi.org/10.3389/fphar.2017.00981. PMid:29375382.
http://dx.doi.org/10.3389/fphar.2017.009...
). The above results suggest that SIRT1 is involved in NF-κB-induced intestinal epithelial cell injury. Moreover, it plays a role in protecting intestinal epithelial cells in this process. CircRNA MFHAS1 reduced inflammation in an in vitro model of SCI through the SIRT1/NF-κB signaling pathway and reducing the expression of miR-486-5p. Kong et al revealed that miR-486-5p and miR-320b expression were increased in knee osteoarthritis (Kong et al., 2017Kong, R., Gao, J., Si, Y., & Zhao, D. (2017). Combination of circulating miR-19b-3p, miR-122-5p and miR-486-5p expressions correlates with risk and disease severity of knee osteoarthritis. American Journal of Translational Research, 9(6), 2852-2864. PMid:28670374.).
5 Conclusions
In this study, we demonstrated that CircRNA MFHAS1 reduced inflammatory responses in colitis by inhibiting the SIRT1/NF-κB signaling pathway and reducing the expression of miR-486-5p. These results show that CircRNA MFHAS1 has an anti-inflammation effect in colitis and has potential as a new drug or target for treatment of colitis.
-
Practical Application: Reduction of inflammatory responses by CircRNA MFHAS1 in colitis.
References
- Akimova, T., Xiao, H., Liu, Y., Bhatti, T. R., Jiao, J., Eruslanov, E., Singhal, S., Wang, L., Han, R., Zacharia, K., Hancock, W. W., & Beier, U. H. (2014). Targeting sirtuin-1 alleviates experimental autoimmune colitis by induction of Foxp3+ T-regulatory cells. Mucosal Immunology, 7(5), 1209-1220. http://dx.doi.org/10.1038/mi.2014.10 PMid:24549276.
» http://dx.doi.org/10.1038/mi.2014.10 - Allamneni, C., Venkata, K., Yun, H., Xie, F., DeLoach, L., & Malik, T. A. (2018). Comparative effectiveness of vedolizumab vs. infliximab induction therapy in ulcerative colitis: experience of a real-world cohort at a tertiary inflammatory bowel disease center. Gastroenterology Research, 11(1), 41-45. http://dx.doi.org/10.14740/gr934w PMid:29511405.
» http://dx.doi.org/10.14740/gr934w - Bian, Z., Li, L., Cui, J., Zhang, H., Liu, Y., Zhang, C. Y., & Zen, K. (2011). Role of miR-150-targeting c-Myb in colonic epithelial disruption during dextran sulphate sodium-induced murine experimental colitis and human ulcerative colitis. The Journal of Pathology, 225(4), 544-553. http://dx.doi.org/10.1002/path.2907 PMid:21590770.
» http://dx.doi.org/10.1002/path.2907 - Bing, X., Xuelei, L., Wanwei, D., Linlang, L., & Keyan, C. (2017). EGCG Maintains Th1/Th2 balance and mitigates ulcerative colitis induced by dextran sulfate sodium through TLR4/MyD88/NF-kappaB signaling pathway in rats. Canadian Journal of Gastroenterology & Hepatology, 2017, 3057268. http://dx.doi.org/10.1155/2017/3057268 PMid:29404307.
» http://dx.doi.org/10.1155/2017/3057268 - Cho, B. O., Yin, H. H., Park, S. H., Byun, E. B., Ha, H. Y., & Jang, S. I. (2016). Anti-inflammatory activity of myricetin from Diospyros lotus through suppression of NF-kappaB and STAT1 activation and Nrf2-mediated HO-1 induction in lipopolysaccharide-stimulated RAW264.7 macrophages. Bioscience, Biotechnology, and Biochemistry, 80(8), 1520-1530. http://dx.doi.org/10.1080/09168451.2016.1171697 PMid:27068250.
» http://dx.doi.org/10.1080/09168451.2016.1171697 - Dhamija, P., Hota, D., Kochhar, R., Sachdev, A., & Chakrabarti, A. (2014). Randomized clinical trial: Atorvastatin versus placebo in patients with acute exacerbation of mild to moderate ulcerative colitis. Indian Journal of Gastroenterology, 33(2), 151-156. http://dx.doi.org/10.1007/s12664-013-0420-4 PMid:24222372.
» http://dx.doi.org/10.1007/s12664-013-0420-4 - Eissa, N., Hussein, H., Kermarrec, L., Elgazzar, O., Metz-Boutigue, M. H., Bernstein, C. N., & Ghia, J. E. (2017). Chromofungin (CHR: CHGA47-66) is downregulated in persons with active ulcerative colitis and suppresses pro-inflammatory macrophage function through the inhibition of NF-kappaB signaling. Biochemical Pharmacology, 145, 102-113. http://dx.doi.org/10.1016/j.bcp.2017.08.013 PMid:28827109.
» http://dx.doi.org/10.1016/j.bcp.2017.08.013 - Galvez-Llompart, M., Recio, M. C., & Garcia-Domenech, R. (2011). Topological virtual screening: a way to find new compounds active in ulcerative colitis by inhibiting NF-kappaB. Molecular Diversity, 15(4), 917-926. http://dx.doi.org/10.1007/s11030-011-9323-4 PMid:21717125.
» http://dx.doi.org/10.1007/s11030-011-9323-4 - Ghobadi, F., Vaisi-Raygani, A., Bahrehmand, F., Tanhapour, M., Kiani, A., Rahimi, Z., & Pourmotabbed, T. (2017). Genetic variants of Pre-microRNAs A-499G(rs3746444) and T-196a2C(rs11614913) with Ulcerative Colitis (UC) and investigated with Thiopurine-S-Methyltransferase (TPMT) Activity. Clinical Laboratory, 63(10), 1683-1690. http://dx.doi.org/10.7754/Clin.Lab.2017.170502 PMid:29035443.
» http://dx.doi.org/10.7754/Clin.Lab.2017.170502 - Gu, P., Zhu, L., Liu, Y., Zhang, L., Liu, J., & Shen, H. (2017). Protective effects of paeoniflorin on TNBS-induced ulcerative colitis through inhibiting NF-kappaB pathway and apoptosis in mice. International Immunopharmacology, 50, 152-160. http://dx.doi.org/10.1016/j.intimp.2017.06.022 PMid:28666238.
» http://dx.doi.org/10.1016/j.intimp.2017.06.022 - Hua, F., Ribbing, J., Reinisch, W., Cataldi, F., & Martin, S. (2015). A pharmacokinetic comparison of anrukinzumab, an anti- IL-13 monoclonal antibody, among healthy volunteers, asthma and ulcerative colitis patients. British Journal of Clinical Pharmacology, 80(1), 101-109. http://dx.doi.org/10.1111/bcp.12589 PMid:25614144.
» http://dx.doi.org/10.1111/bcp.12589 - Kong, R., Gao, J., Si, Y., & Zhao, D. (2017). Combination of circulating miR-19b-3p, miR-122-5p and miR-486-5p expressions correlates with risk and disease severity of knee osteoarthritis. American Journal of Translational Research, 9(6), 2852-2864. PMid:28670374.
- Lv, Q., Wang, K., Qiao, S., Yang, L., Xin, Y., Dai, Y., & Wei, Z. (2018). Norisoboldine, a natural AhR agonist, promotes Treg differentiation and attenuates colitis via targeting glycolysis and subsequent NAD(+)/SIRT1/SUV39H1/H3K9me3 signaling pathway. Cell Death & Disease, 9(3), 258. http://dx.doi.org/10.1038/s41419-018-0297-3 PMid:29449535.
» http://dx.doi.org/10.1038/s41419-018-0297-3 - Meade, M. O., Cook, D. J., Griffith, L. E., Hand, L. E., Lapinsky, S. E., Stewart, T. E., Killian, K. J., Slutsky, A. S., & Guyatt, G. H. (2008). A study of the physiologic responses to a lung recruitment maneuver in acute lung injury and acute respiratory distress syndrome. Respiratory Care, 53(11), 1441-1449. PMid:18957146.
- Netz, U., Carter, J., Eichenberger, M. R., Feagins, K., Galbraith, N. J., Dryden, G. W., Pan, J., Rai, S. N., & Galandiuk, S. (2017). Plasma microRNA Profile Differentiates Crohn’s Colitis From Ulcerative Colitis. Inflammatory Bowel Diseases, 24(1), 159-165. http://dx.doi.org/10.1093/ibd/izx009 PMid:29272478.
» http://dx.doi.org/10.1093/ibd/izx009 - Oliva, S., Di Nardo, G., Ferrari, F., Mallardo, S., Rossi, P., Patrizi, G., Cucchiara, S., & Stronati, L. (2012). Randomised clinical trial: The effectiveness of Lactobacillus reuteri ATCC 55730 rectal enema in children with active distal ulcerative colitis. Alimentary Pharmacology & Therapeutics, 35(3), 327-334. http://dx.doi.org/10.1111/j.1365-2036.2011.04939.x PMid:22150569.
» http://dx.doi.org/10.1111/j.1365-2036.2011.04939.x - Rosario, M., French, J. L., Dirks, N. L., Sankoh, S., Parikh, A., Yang, H., Danese, S., Colombel, J. F., Smyth, M., Sandborn, W. J., Feagan, B. G., Reinisch, W., Sands, B. E., Sans, M., & Fox, I. (2017). Exposure-efficacy Relationships for Vedolizumab Induction Therapy in Patients with Ulcerative Colitis or Crohn’s Disease. Journal of Crohn’s and Colitis, 11(8), 921-929. http://dx.doi.org/10.1093/ecco-jcc/jjx021 PMid:28333288.
» http://dx.doi.org/10.1093/ecco-jcc/jjx021 - Sandborn, W. J., Bhandari, B. R., Fogel, R., Onken, J., Yen, E., Zhao, X., Jiang, Z., Ge, D., Xin, Y., Ye, Z., French, D., Silverman, J. A., Kanwar, B., Subramanian, G. M., McHutchison, J. G., Lee, S. D., Shackelton, L. M., Pai, R. K., Levesque, B. G., & Feagan, B. G. (2016). Randomised clinical trial: A phase 1, dose-ranging study of the anti-matrix metalloproteinase-9 monoclonal antibody GS-5745 versus placebo for ulcerative colitis. Alimentary Pharmacology & Therapeutics, 44(2), 157-169. http://dx.doi.org/10.1111/apt.13653 PMid:27218676.
» http://dx.doi.org/10.1111/apt.13653 - Sandborn, W.J., van Assche, G., Reinisch, W., Colombel, J.F., D'Haens, G., Wolf, D.C., Kron, M., Tighe, M.B., Lazar, A., Thakkar, R.B. (2012). Adalimumab induces and maintains clinical remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology, 142(2), 257-265.
- Sands, B. E., Joshi, S., Haddad, J., Freudenberg, J. M., Oommen, D. E., Hoffmann, E., McCallum, S. W., & Jacobson, E. (2016). Assessing colonic exposure, safety, and clinical activity of SRT2104, a Novel Oral SIRT1 activator, in patients with mild to moderate Ulcerative Colitis. Inflammatory Bowel Diseases, 22(3), 607-614. http://dx.doi.org/10.1097/MIB.0000000000000597 PMid:26595549.
» http://dx.doi.org/10.1097/MIB.0000000000000597 - Scott, F. I., & Lichtenstein, G. R. (2018). Biosimilars in the treatment of inflammatory bowel disease: Supporting Evidence in 2017. Current Treatment Options in Gastroenterology, 16(1), 147-164. http://dx.doi.org/10.1007/s11938-018-0177-z PMid:29492747.
» http://dx.doi.org/10.1007/s11938-018-0177-z - Shi, Q., Xiong, B., Zhong, J., Wang, H., Ma, D., & Miao, C. (2017). MFHAS1 suppresses TLR4 signaling pathway via induction of PP2A C subunit cytoplasm translocation and inhibition of c-Jun dephosphorylation at Thr239. Molecular Immunology, 88, 79-88. http://dx.doi.org/10.1016/j.molimm.2017.06.017 PMid:28609714.
» http://dx.doi.org/10.1016/j.molimm.2017.06.017 - Wang, K., Li, Y. F., Lv, Q., Li, X. M., Dai, Y., & Wei, Z. F. (2017). Bergenin, acting as an agonist of PPARgamma, Ameliorates experimental colitis in mice through improving expression of SIRT1, and therefore inhibiting NF-kappaB-Mediated macrophage activation. Frontiers in Pharmacology, 8, 981. http://dx.doi.org/10.3389/fphar.2017.00981 PMid:29375382.
» http://dx.doi.org/10.3389/fphar.2017.00981 - Yu, Z. H., Huang, F., Xu, N., Zhao, D. M., Hu, F. A., Liu, J., & Liu, H. F. (2011). Expression of Toll-like receptor 4, CD14, and NF-kappaB in Chinese patients with ulcerative colitis. Journal of Immunoassay & Immunochemistry, 32(1), 47-56. http://dx.doi.org/10.1080/15321819.2010.538108 PMid:21253969.
» http://dx.doi.org/10.1080/15321819.2010.538108 - Zhong, J., Shi, Q. Q., Zhu, M. M., Shen, J., Wang, H. H., Ma, D., & Miao, C. H. (2015). MFHAS1 Is associated with sepsis and stimulates TLR2/NF-kappaB signaling pathway following negative regulation. PLoS One, 10(11), e0143662. http://dx.doi.org/10.1371/journal.pone.0143662 PMid:26599367.
» http://dx.doi.org/10.1371/journal.pone.0143662
Publication Dates
-
Publication in this collection
30 Oct 2020 -
Date of issue
2021
History
-
Received
02 July 2020 -
Accepted
08 Aug 2020