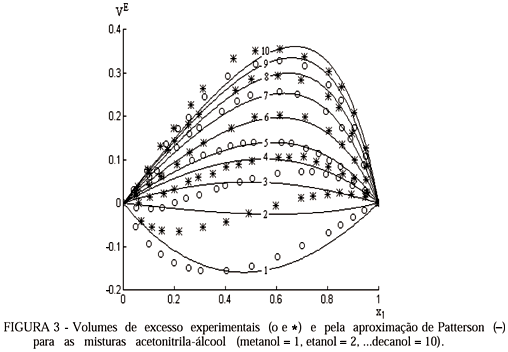
For mixtures in which exist interactions between differents molecules, in the relationship of the variables appears an energetic adjustable parameter of interaction. The thermodynamic properties of excess give qualitative information of the possible types of interaction. Two important properties in this sense are the excess volume and excess enthalpy. In this paper different methods are studied, regression with least squares and by minimizing absolute and relative residues, to determine their possibilities of application to evaluate molecular interaction parameters in thermodynamic models of solutions, using experimental data of excess molar volume for the whole composition ranges of 10 binary systems of molar fractions formed by acetonitrile + primary alcohol (from metanol to n-decanol). The calculated values and the experimental data of the properties are compared, and the comparative results are analyzed.
Regression; Least Squares; Interaction molecular; Binary Sistems