Abstract
Comparison of G-bands from 2n = 10 and 2n = 16 karyotypes of Akodon revealed tandem fusions, pericentric inversions and Robertsonian rearrangement in autosomes and addition/deletion of constitutive heterochromatin in sex chromosomes. Cytochrome-b sequences indicate that the 2n = 10 karyotype is a new species and show it to be a sister taxon of the 2n = 14, 2n = 15 and 2n = 16 karyotypes. Indeed, this group shows a particular evolutionary situation in which a unique taxonomic unit based on morphological data can be detected, but, karyologically, it can be separated into two groups (2n = 14-15-16 and 2n = 10). Cytochrome-b sequences show a finer resolution, indicating that these four karyotypes represent three molecular entities (2n = 14-15, 2n = 16 and 2n = 10) that may be derived from a common ancestor with a 2n = 16 karyotype.
Akodon; cytochrome-b; G-bands; karyotypical evolution; phylogeny
ANIMAL GENETICS
RESEARCH ARTICLE
Phylogenetic relationships and karyotype evolution in the sigmodontine rodent Akodon (2n = 10 and 2n = 16) from Brazil
Maria José de J. SilvaI, II; James L. PattonIII; Yatiyo Yonenaga-YassudaI
IDepartamento de Biologia, Instituto de Biociências, Universidade de São Paulo, São Paulo, SP, Brazil
IILaboratório de Genética, Instituto Butantan, São Paulo, SP, Brazil
IIIMuseum of Vertebrate Zoology, University of California, Berkeley, CA, USA
Send correspondence to Send correspondence to Maria José de J. Silva Laboratório de Genética Instituto Butantan Av. Vital Brasil 1500 05503-900 São Paulo, SP, Brazil E-mail: mariajo@usp.br.
ABSTRACT
Comparison of G-bands from 2n = 10 and 2n = 16 karyotypes of Akodon revealed tandem fusions, pericentric inversions and Robertsonian rearrangement in autosomes and addition/deletion of constitutive heterochromatin in sex chromosomes. Cytochrome-b sequences indicate that the 2n = 10 karyotype is a new species and show it to be a sister taxon of the 2n = 14, 2n = 15 and 2n = 16 karyotypes. Indeed, this group shows a particular evolutionary situation in which a unique taxonomic unit based on morphological data can be detected, but, karyologically, it can be separated into two groups (2n = 14-15-16 and 2n = 10). Cytochrome-b sequences show a finer resolution, indicating that these four karyotypes represent three molecular entities (2n = 14-15, 2n = 16 and 2n = 10) that may be derived from a common ancestor with a 2n = 16 karyotype.
Key words:Akodon, cytochrome-b, G-bands, karyotypical evolution, phylogeny.
INTRODUCTION
The Akodontini tribe encompasses about 35% of the total diversity of the extant species among 10 tribes of the subfamily Sigmodontinae of South American rodents (Smith and Patton 1993), although relationships at genera and species levels within this tribe remain uncertain.
Karyotypes have been useful in generating species classification and clarifying some systematic problems, especially those related to cryptic species within the genus Akodon. In Akodon the diploid chromosome number ranges from 2n = 9 or 10 to 2n = 52, with the majority of the members of this genus being characterized by relatively low chromosome numbers (Spotorno and Fernandez, 1976; Yonenaga et al., 1976; Kasahara and Yonenaga-Yassuda, 1984; Liascovich and Reig, 1989; Svartman and Almeida, 1994; Geise et al., 1998; Fagundes and Yonenaga-Yassuda, 1998; Fagundes et al., 1998; Silva and Yonenaga-Yassuda, 1998). Brazilian Akodon species with known karyotypes are as follows: Akodon sp. n. (2n = 9, 10); A. cursor (sensu Christoff, 1997) with 2n = 14, 15 and 16 (the latter diploid number has been cited as belonging to Akodon aff. cursor by Rieger et al., 1995, and Geise et al., 2001); A. montensis (2n = 23-26); A. azarae (2n = 38); A. reinhardti (2n = 37, 38); A. lindberghi (2n = 42); A. serrensis (2n = 46); A. toba (2n = 42 or 43) and A. paranaensis (2n = 44) (Yonenaga, 1972; Yonenaga, 1975; Yonenaga-Yassuda, 1979; Yonenaga-Yassuda et al., 1987; Castro, 1989; Sbalqueiro, 1989; Geise et al., 1996, 1998; Sbalqueiro and Nascimento, 1996; Fagundes et al., 1998; Silva and Yonenaga-Yassuda, 1998; Christoff et al., 2000).
Silva and Yonenaga-Yassuda (1998) suggested that the new 2n = 9, 10 karyotype (Akodon sp. n.) from Mato Grosso state in central Brazil ranks as a new Akodon species. This karyotype has an autosomal polymorphism in chromosome 3, which can be either an acrocentric or a submetacentric due to a pericentric inversion, and the odd diploid number (2n = 9) being caused by monosomy of the X chromosome.
The 2n = 14, 15 and 16 karyotypes have been assigned to the same taxonomic unit, A. cursor (Christoff, 1997; Fagundes et al., 1998), but based on cytochrome-b mitochondrial gene sequences Geise et al. (2001) argued that 2n = 16 specimens of this species from the Brazilian states of Paraná, Bahia and Minas Gerais should be recognized as being different from A. cursor and should be referred to as Akodon aff. cursor.
As the traditional classification of a large number of South American sigmodontine taxa remain poorly understood (Smith and Patton, 1999), chromosomal data and, more recently, mitochondrial DNA sequences (which allow better identification of species and phylogeographic patterns) have provided relevant information to establish relationships among this and other groups (Smith and Patton, 1993, 1999; Myers et al. 1995; Da Silva and Patton 1998; Garcia 1999; Geise et al. 2001; Bonvicino and Moreira 2001).
In this paper we compare G-band patterns between the 2n = 10 and 16 Akodon karyotypes, and discuss the phylogenetic implications based on cytochrome-b sequences. Comparison of G-banded chromosomes, molecular systematics and biogeographic information provide additional data to aid our understanding of the evolutionary process as well as the present status and phylogeny of Akodon.
Material and Methods
Cytogenetic procedures
We used Seabright's method (1971) to obtain somatic cell G-banding patterns from 28 Akodon specimens with a 2n = 10 karyotype and compared the G-banded chromosomes of 2n = 10 specimens from Gaúcha do Norte in the Brazilian state of Mato Grosso and 2n = 16 (referred to as A. cursor by Fagundes et al., 1998) from the Brazilian state of São Paulo.
Sequencing procedures
Total genomic DNA from seven Akodon specimens with 2n = 10 (specimens MZUSP-29658, 29671, 29673, 29678, 29684, 29685 and APC-270) was extracted from muscle or liver using the Chelex (BioRad) method (Walsh et al., 1991). Tissues had previously been frozen in liquid nitrogen and transferred to 95% ethanol. The cytochrome-b gene was amplified by the polymerase chain reaction (PCR) with the MVZ05 and MVZ16 primer set as described by Smith and Patton (1993) and amplification confirmed by 2% agarose gel electrophoresis. The PCR products were sequenced using an ABI 377 automated sequencer using a d-Rhodamine cycle sequencing kit and the MVZ05 primer. The sequences were edited using the Sequence Navigator software (Applied Biosystems, Inc. 1994). Phylogenetic analyses under parsimony criterion were performed using the PAUP*4.0b6 program with 100 bootstrap replicates and Kimura-2-parameter distances (Kimura 1980) were also calculated.
The first 750 bp mitochondrial cytochrome-b sequences were obtained from seven Akodon specimens, four from Gaúcha do Norte and three from Vila Rica (both in Mato Grosso state), all specimens having been previously karyotyped. Since data indicated that these specimens share similar haplotypes we only use one specimen from each locality: the specimen MZUSP-29671 (GenBank accession number DQ631966) from Gaúcha do Norte and MZUSP-29685 (DQ631965) from Vila Rica in order to access the phylogenetic reconstruction. Other Akodon sequences and Bolomys (U03528) (Necromys after Smith and Patton, 1999) were obtained from GenBank, and the latter being used as the outgroup taxon. Voucher specimens with 2n = 9, 10 were deposited at the Museu de Zoologia da Universidade de São Paulo (MZUSP), São Paulo state, Brazil.
Results and Discussion
Conventionally stained and banding patterns karyotypes of Akodon with 2n = 14, 15 and 15 have been reported by various authors (Yonenaga, 1972, 1975; Yonenaga-Yassuda, 1979); Sbalqueiro and Nascimento, 1996; Fagundes et al., 1998) and samples with a 2n = 9, 10 karyotype have been described by Silva and Yonenaga-Yassuda (1998).
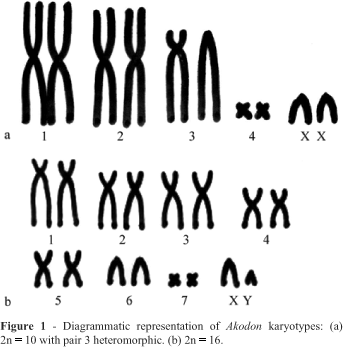
We compared G-bands of the 2n = 10 and 2n = 16 karyotypes, as fewer steps were involved in the differentiation between these karyotypes than between 2n = 10 and 2n = 14. This analysis suggests that a similar karyotype or a diploid number higher than 2n = 16 gave rise to the lowest chromosome number of Akodon sp. n. (2n = 10). Diagrams of the 2n = 16 and 2n = 10 karyotypes are being shown in Figure 1.
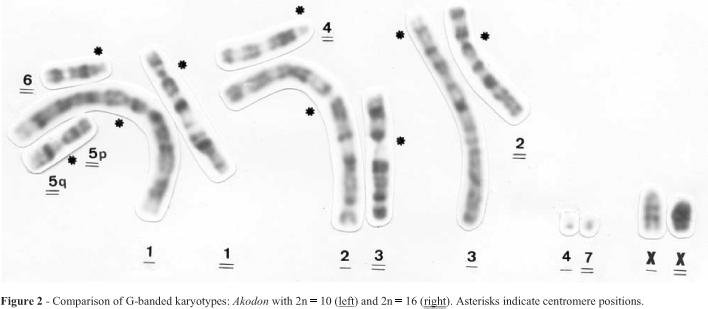
Comparison of 2n = 10 and 16 G-banded metaphases (Figure 2) revealed the following complex chromosomal rearrangements: chromosome 1 of 2n = 10 could have derived from multiple tandem fusions of chromosomes 1 (after a pericentric inversion), 6 and 5q of a 2n = 16-like karyotype; chromosome 2 of 2n = 10 could have originated by a Robertsonian rearrangement of chromosomes 3 (after a pericentric inversion) and 4 of 2n = 16; chromosome 3 could have resulted from a tandem fusion involving chromosome 2 and probably 5p of 2n = 16; pair 4 of 2n = 10 and pair 7 of 2n = 16 were the smallest chromosomes of each complement and seemed to be totally homologous in both karyotypes. The X chromosome was larger in the 2n = 10 than in the 2n = 16 karyotype due to the presence of a block of constitutive heterochromatin in the proximal region of the long arm and the Y chromosome was a minute subtelocentric whereas in 2n = 16 karyotype it was acrocentric.
Although we did not identify any homologies between the distal region of chromosome 3 of the 2n = 10 karyotype and the other components of the 2n = 16 karyotype it is possible that this sequence was lost from the ancestral 2n = 16 karyotype to give rise to the 2n = 16 karyotype and was then maintained in the 2n = 10 karyotype, although the distal region of chromosome 3 could have arisen as a result of amplification of a segment in 2n = 10 karyotype. Up to now we are not able to mention the origin, nature or function of this genetic material or if it implies difference in the fitness of the individuals. We can just mention that morphologically 2n = 10 and 2n = 16 seem to be indistinguishable (Christoff, in prep.)
Previous cytogenetic studies by Silva and Yonenaga-Yassuda (1998) revealed interstitial silver-stained nucleolar organizer regions (Ag-NORs) located at 1p and 1q, on the telomeric region of the 2p chromosome and the short arm of one pair 4 homologue of the 2n = 10 karyotype, the 2n = 16 karyotype reported by Fagundes et al. (1997) exhibited Ag-NORs on the telomeres of chromosome pairs 4 and 5. As far as we could detect, there was correspondence of Ag-NOR positions on the telomeric regions of both the 2p chromosome for the 2n = 10 karyotype and the 4p chromosome for the 2n = 16 karyotype. Absence of Ag-NORs on the 1p telomeres and their presence on one 2n = 10 pair 4 homologue could be due to either loss of NORs or relocation of ribosomal genes.
According to Fagundes et al. (1997) fluorescence in situ hybridization analysis (FISH) with a telomeric (TTAGGG)n probe resulted in only telomeric signals on the 2n = 16 karyotype chromosomes. Interestingly, Silva and Yonenaga-Yassuda (1998) reported conspicuous interstitial telomeric sites (ITS) on the pericentromeric region and the short arm of pair 1 and on the long arm of pair 3 of the 2n = 10 karyotype, in addition to the regular telomeric signals. However these signals detected on the 2n = 10 karyotype 1p and 3q chromosomes did not seem to be coincident with the specific positions involved in the chromosomal rearrangements. In addition, the pericentromeric signal of chromosome 1 was co-localized with pericentromeric constitutive heterochromatin, which is a highly repetitive DNA sequence. All this evidence led us to believe that much more complex rearrangements than we observe after the comparison of G-banded chromosomes had occurred during the evolutionary process which drove the differentiation of the 2n = 10 and 2n = 16 karyotypes.
Phylogenetic analyses and parsimony of cytochrome-b sequences support our previous chromosome results suggesting that the 2n = 10 Akodon karyotype is a new species. These animals from Gaúcha do Norte and Vila Rica (localities geographically separated by nearly 450 km) share quite similar haplotypes (Kimura-2-parameter equal to 0.002) which are grouped in a strongly supported monophyletic group (bootstrap value of 100%), reinforcing the requirement of a new taxonomic status for this species (Figure 3). Furthermore, this species is recovered as the sister group (with bootstrap support of 100%) of Akodon specimens with 2n = 14, 15 and 16 karyotypes, where the 2n = 16 karyotype is the sister group of 2n = 14 and 15 karyotypes. All of these three karyotypes show a common ancestor relative to other species of the cursor-complex, such as A. montensis and A. mistax (Figure 3).
Geise et al. (2001) reported a bootstrap value of 94% supporting Akodon with a karyotype of 2n = 14, 15 as a sister of the 2n = 16 karyotype, and referred to them as A. cursor and Akodon aff. cursor, respectively. After including our data (2n = 10 cytochrome-b sequences), the support for the relationships of these two taxa decreased to 72%, while the bootstrap values to support the 2n = 14, 15 and 2n = 16 karyotypes as monophyletic groups increased respectively to 88 and 90%.
Kimura-2-parameter (K2p) distances within and among taxa were as follow: geographic samples of the 2n = 10 karyotype differed by 0.002; 2n = 14-15 by 0.007-0.023; 2n = 16 by 0.032-0.052; and 2n = 10 differed from 2n = 14-15 by 0.053-0.062 and from 2n = 16 by 0.057-0.071; 2n = 14, 15 differed from 2n = 16 by 0.040-0.067. Akodon montensis differed from them by 0.090-0.113 and Necromys lasiurus also differed from them by 0.155-0.190 K2p.
Maia and Langguth (1981) described an Akodon population from the Brazilian state of Pernambuco in which all specimens had a karyotype of 2n = 16. More recently, Akodon specimens with a 2n = 16 karyotype have also been found in the Brazilian states of Paraíba, Minas Gerais, São Paulo, Bahia and Paraná (Figure 4) (Rieger et al., 1995; Sbalqueiro and Nascimento 1996; Fagundes et al., 1998; Geise et al., 2001). Regardless of the karyotype diversity in Akodon specimens with 2n = 14, 15 and 16 karyotypes (a total of 28 karyotypes), Christoff (1997) and Fagundes et al. (1998), considered these specimens as a single species (Akodon cursor) and explained the variation as being due to pericentric inversions in three different chromosome pairs, a complex rearrangement involving centric fusion with previous pericentric inversions of two pairs, trisomy of pair 7 and monosomy of the X chromosome. But Christoff (1997) noted that Akodon specimens with 2n = 14, 15 and 16 karyotypes were not distinctive taxonomically by external or cranio-dental morphology, and grouped as one unique species. However, the phylogenetic analyses of mitochondrial sequence data revealed, with relatively high support (72%), a node in which the Akodon specimens from São Paulo with a 2n = 14 and 15 karyotype is the sister group of the Akodon specimens from Paraíba, Bahia and Minas Gerais with a karyotype of 2n = 16. Moreover, according to Geise et al. (2001), cytochrome-b sequences of Akodon specimens from Una (Bahia state) with a 2n = 14-15 karyotype share more similarities to specimens with the same karyotype which were 1500 km away than to 2n = 16 karyotype specimens collected in the same locality. Therefore if the 2n = 14 and 16 karyotypes were panmictic (randomly interbreeding) there should be no reason for their mitochondrial DNA to appear so different as the results show.
Rieger et al. (1995) used 22 structural loci encoding 14 proteins to check genetic variability in 22 Akodon specimens from Paraná with 2n = 16 karyotypes, 41 specimens from Espírito Santo with 2n = 14 and 15 karyotypes and 206 specimens from the Brazilian states of Santa Catarina and three localities in the Brazilian state of Rio Grande do Sul with 2n = 24, 25 and 26 karyotypes. On the basis of phenetic and phylogenetic data, Rieger et al. (1995) suggested that members of the cursor-complex have undergone recent differentiation and that chromosomal divergence has proceeded without eletromorphic divergence. We can add here that mitochondrial sequences can identify and separate these three units plus the new species with the 2n = 10 karyotype.
It is also interesting to point out that preliminary morphometric studies (Christoff et al., in prep.) showed no differences between 2n = 10 karyotype Akodon and A. cursor (sensu Christoff, 1997), indicating the possibility that they are cryptic species as already observed in A. cursor and A. montensis (Christoff, 1997).
It therefore seems that a special evolutionary situation exists in which a group is recognized as a unique species on the basis of morphological traits but karyologically is clearly separated into 2n = 14, 15 and 16 forms and 2n = 10, with cytochrome-b sequences indicating that the 2n = 10 karyotypes represents a well supported full species and 2n = 24 is another strongly bootstrapped species while Akodon with 16 chromosomes is probably a different entity distinct from Akodon with 2n = 14-15 chromosomes as previously hypothesized by Geise et al. (2001).
Acknowledgments
We thank Dr. Yuri Leite for the valuable contributions during the lab-work, data analysis, and elaboration of the manuscript. We are also grateful to Dr. Valéria Fagundes for the concession of 2n = 16 G-bands, Dr. Katia Pellegrino and Sheda Morshed for suggestions in the manuscript; MSc. Ana Paula Carmignotto and Dr. Alexandre U. Christoff who collected specimens, and to Dr. Miguel T. Rodrigues, Dr. Vinícius Xavier da Silva, Dr. Gabriel Skuk and MSc. Dante Pavan for helping in fieldwork; to Miriam Romeo, Tais Machado and Yukie Sato for technical assistance. We also thank Dr. Yuri Leite, Margareth Smith and Lena Geise for providing sequences which are also available in the GenBank, and the MVZ for the accessibility and infra-structure. Grants from Fundação de Amparo à Pesquisa do Estado de São Paulo (including 00/06591-0 to Dr. M.J.J.S. and BIOTA 98/5075 to Dr. Mario de Vivo); Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Conselho Nacional de Desenvolvimento Científico e Tecnológico and National Science Fundation (grants to Dr. James Patton) are also acknowledged.
Received: July 28, 2005; Accepted: December 29, 2005.
Associate Editor: João S. Morgante
- Bonvicino CR and Moreira MAM (2001) Molecular phylogeny of the genus Oryzomys (Rodentia, Sigmodontinae) based on cytochrome-b DNA sequences. Mol Phylogenet Evol 18:282-292.
- Castro EC (1989) Ocorrência e caracterização cromossômica de roedores Akodontinos do Rio Grande do Sul. Dissertação de Mestrado, Universidade Federal do Rio Grande do Sul, Porto Alegre.
- Christoff AU, Fagundes V, Sbalqueiro IJ, Mattevi MS and Y Yonenaga-Yassuda Y (2000) Description of a new species of Akodon (Rodentia, Sigmodontinae) from Southern Brazil. J Mammalogy 81:838-851.
- Christoff AU (1997) Contribuição à sistemática das espécies do gênero Akodon (Rodentia, Sigmodontinae) do leste do Brasil: Estudos anatômicos, citogenéticos e de distribuição geográfica. Tese de Doutorado, Instituto de Biociências, Universidade de São Paulo, São Paulo.
- Da Silva MNF and Patton JL (1998) Molecular phylogeography and the evolution and conservation of Amazonian mammals. Mol Ecol 7:475-486.
- Fagundes V and Yonenaga-Yassuda Y (1998) Evolutionary conservation of the whole homologous chromosome arms in the Akodont rodents Bolomys and Akodon (Muridae, Sigmodontinae): Maintenance of interstitial telomeric segments (ITBs) in recent event of centric fusion. Chromosome Res 6:643-648.
- Fagundes V, Christoff AU and Yonenaga-Yassuda Y (1998) Extraordinary chromosomal polymorphism with 28 different karyotypes in the neotropical species of Akodon cursor (Muridae, Sigmodontinae), one of the smallest diploid numbers in rodents (2n = 16, 15 and 14). Hereditas 129:263-274.
- Fagundes V, Vianna-Morgante AM and Yonenaga-Yassuda Y (1997) Telomeric sequences localization and G-banding patterns in the identification of a polymorphic chromosomal rearrangement in the rodent Akodon cursor (2n = 14, 15 and 16). Chromosome Res 5:228-232.
- Garcia LF (1999) Molecular phylogenetics of neotropical oryzomyine rodents (Muridae, Sigmodontinae). Ph.D. Thesis, University of California, Berkeley.
- Geise L, Canavez FC and Seuánez HN (1998) Comparative karyology in Akodon (Rodentia, Sigmodontinae) from Southwestern Brazil. J Hered 89:158-163.
- Geise L, Cerqueira R and Seuánez HN (1996) Karyological characterization of a new population of Akodon lindberghi (Rodentia, Sigmodontinae) in Minas Gerais state (Brazil). Caryologia 49:57-63.
- Geise L, Smith MF and Patton JL (2001) Diversification in the genus Akodon (Rodentia, Sigmodontinae) in southeastern America: Mitochondrial DNA sequences analysis. J Mammalogy 82:92-101.
- Kasahara S and Yonenaga-Yassuda Y (1984) A progress report of cytogenetic data on Brazilian rodents. Rev Bras Genét 7:509-533.
- Kimura M (1980) A simple method for estimating evolutionary rate of base substitution through comparative studies of nucleotide sequences. J Mol Evol 16:111-120.
- Liascovich RC and Reig OA (1989) Low chromosomal number in Akodon cursor montensis Thomas, and karyologic confirmation of Akodon serrensis Thomas in Misiones, Argentina. J Mammalogy 70:391-395.
- Maia V and Langguth A (1981) New karyotypes of Brazilian Akodont with notes on taxonomy. Zeitschr Säugetierkunde 46:241-249.
- Myers P, Lundrigan B and Tucker PK (1995) Molecular phylogenetics of oryzomyine rodents: The genus Oligoryzomys Mol Phylogenet Evol 4:372-382.
- Rieger TT, Langguth A and Wienmer TA (1995) Allozymic characterization and evolutionary relationships in the Brazilian Akodon cursor species group (Rodentia, Cricetidae). Biochem Genet 33:283-295.
- Sbalqueiro IJ and Nascimento AP (1996) Occurrence of Akodon cursor (Rodentia, Cricetidae) with 14, 15 and 16 chromosome karyotypes in the same geographic area in South Brazil. Braz J Genet 19:565-569.
- Seabright M (1971) A rapid banding technique for human chromosomes. Lancet 2:971-972.
- Silva MJJ and Yonenaga-Yassuda Y (1998) Karyotype and chromosomal polymorphism of an undescribed Akodon from Central Brazil, a species with the lowest diploid chromosome number in rodents. Cytogenet Cell Genet 81:46-50.
- Smith MF and Patton JL (1999) Phylogenetic relationships and the radiation of sigmodontine rodents in South America: Evidence from cytochrome b. J Mammal Evol 6:89-128.
- Smith MF and Patton JL (1993) The diversification of South American murid rodents: Evidence from mitochondrial DNA sequence data for the akodontine tribe. Biol J Linnean Soc 50:149-177.
- Spotorno OA and Fernandez R (1976) Chromosome stability in southern Akodon (Rodentia, Cricetidae). Mammalian Chromosome Newsletter 17:13-14.
- Svartman M and Almeida EJC (1994) The karyotype of Akodon lindberghi Hershkovitz, 1990 (Cricetidae, Rodentia) from Central Brazil. Rev Bras Genét 17:963-972.
- Walsh PS, Metzger DA and Higuchi R (1991) Chelex 100 as a medium for simple extraction of DNA for PCR-based typing from forensic material. Biotechniques 10:506-513.
- Yonenaga Y (1972) Chromosomal polymorphism in the rodent Akodon arviculoides ssp. (2n = 14) resulting from two pericentric inversions. Cytogenetics 11:448-499.
- Yonenaga Y (1975) Karyotypes and chromosome polymorphisms in Brazilian rodents. Caryologia 28:269-286.
- Yonenaga-Yassuda Y (1979) New karyotypes and somatic and germ-cell banding in Akodon arviculoides (Rodentia, Cricetidae). Cytogenet Cell Genet 23:241-249.
- Yonenaga Y, Frota-Pessoa O, Kasahara S and Almeida EJC (1976) Cytogenetic studies on Brazilian rodents. Ciência & Cultura 28:202-211.
- Yonenaga-Yassuda Y, Pereira LA, Armada JL and L'Abbate M (1987) Chromosomal polymorphism in Akodon reinhardti Langguth, 1975 (Rodentia, Cricetidae). Rev Bras Genét 10:199-208.
Send correspondence to
Publication Dates
-
Publication in this collection
01 Sept 2006 -
Date of issue
2006
History
-
Received
28 July 2005 -
Accepted
29 Dec 2005