ABSTRACT
Introduction:
Several recent randomized clinical trials have evaluated hypofractionated regimens against conventionally fractionated EBRT and shown similar effectiveness with conflicting toxicity results. The current view regarding hypofractionation compared to conventional EBRT among North American genitourinary experts for management of prostate cancer has not been investigated.
Materials and Methods:
A survey was distributed to 88 practicing North American GU physicians serving on decision - making committees of cooperative group research organizations. Questions pertained to opinions regarding the default EBRT dose and fractionation for a hypothetical example of a favorable intermediate - risk prostate cancer (Gleason 3 + 4). Treatment recommendations were correlated with practice patterns using Fisher's exact test.
Results:
Forty - two respondents (48%) completed the survey. We excluded from analysis two respondents who selected radical hypofractionation with 5 – 12 fractions as a preferred treatment modality. Among the 40 analyzed respondents, 23 (57.5%) recommend conventional fractionation and 17 (42.5%) recommended moderate hypofractionation. No demographic factors were found to be associated with preference for a fractionation regimen. Support for brachytherapy as a first choice treatment modality for low - risk prostate cancer was borderline significantly associated with support for moderate hypofractionated EBRT treatment modality (p = 0.089).
Conclusions:
There is an almost equal split among North American GU expert radiation oncologists regarding the appropriateness to consider moderately hypofractionated EBRT as a new standard of care in management of patients with prostate cancer. Physicians who embrace brachytherapy may be more inclined to support moderate hypofractionated regimen for EBRT. It is unclear whether reports with longer follow-ups will impact this balance, or whether national care and reimbursement policies will drive the clinical decisions. In the day and age of patient - centered care delivery, patients should receive an objective recommendation based on available clinical evidence. The stark division among GU experts may influence the design of future clinical trials utilizing EBRT for patients with prostate cancer.
Keywords:
Prostatic Neoplasms; Dose Hypofractionation; Neoplasm Grading
INTRODUCTION
The standard eight-to-nine week course of conventional external beam radiation therapy (EBRT) for prostate cancer although effective, disrupts patients’ normal lives, causes financial toxicity to patients and places a significant financial strain on the healthcare system. For these reasons, hypofractionated radiation therapy (RT), which involves larger radiation doses administered over an overall shorter time period, has increased in popularity, and has been established in other disease sites, such as breast cancer, bone metastases, bladder cancer, glioblastoma and non - small cell lung cancer (11. Deshmukh AA, Shirvani SM, Lal L, Swint JM, Cantor SB, Smith BD, et al. Cost-effectiveness Analysis Comparing Conventional, Hypofractionated, and Intraoperative Radiotherapy for Early-Stage Breast Cancer. J Natl Cancer Inst. 2017;109.
2. Pichon B, Campion L, Delpon G, Thillays F, Carrie C, Cellier P, et al. High-Dose Hypofractionated Radiation Therapy for Noncompressive Vertebral Metastases in Combination With Zoledronate: A Phase 1 Study. Int J Radiat Oncol Biol Phys. 2016;96:840-7.
3. Hafeez S, McDonald F, Lalondrelle S, McNair H, Warren-Oseni K, Jones K, et al. Clinical Outcomes of Image Guided Adaptive Hypofractionated Weekly Radiation Therapy for Bladder Cancer in Patients Unsuitable for Radical Treatment. Int J Radiat Oncol Biol Phys. 2017;98:115-22. Erratum in: Int J Radiat Oncol Biol Phys. 2018;100:532-3.
4. Malmström A, Grønberg BH, Marosi C, Stupp R, Frappaz D, Schultz H, et al. Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomised, phase 3 trial. Lancet Oncol. 2012;13:916-26.-55. Walraven I, van den Heuvel M, van Diessen J, Schaake E, Uyterlinde W, Aerts J, et al. Long-term follow-up of patients with locally advanced non-small cell lung cancer receiving concurrent hypofractionated chemoradiotherapy with or without cetuximab. Radiother Oncol. 2016;118:442-6.). Four randomized clinical trials have compared moderately fractionated regimens to conventionally fractionated RT in prostate cancer (Table-1) (66. Catton CN, Lukka H, Gu CS, Martin JM, Supiot S, Chung PWM, et al. Randomized Trial of a Hypofractionated Radiation Regimen for the Treatment of Localized Prostate Cancer. J Clin Oncol. 2017;35:1884-90.
7. Lee WR, Dignam JJ, Amin MB, Bruner DW, Low D, Swanson GP, et al. Randomized Phase III Noninferiority Study Comparing Two Radiotherapy Fractionation Schedules in Patients With Low-Risk Prostate Cancer. J Clin Oncol. 2016;34:2325-32.
8. Dearnaley D, Syndikus I, Mossop H, Khoo V, Birtle A, Bloomfield D, et al. Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol. 2016;17:1047-60. Erratum in: Lancet Oncol. 2016;17:e321.
9. Incrocci L, Wortel RC, Alemayehu WG, Aluwini S, Schimmel E, Krol S, et al. Hypofractionated versus conventionally fractionated radiotherapy for patients with localised prostate cancer (HYPRO): final efficacy results from a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2016;17:1061-9.
10. Aluwini S, Pos F, Schimmel E, van Lin E, Krol S, van der Toorn PP, et al. Hypofractionated versus conventionally fractionated radiotherapy for patients with prostate cancer (HYPRO): acute toxicity results from a randomised non-inferiority phase 3 trial. Lancet Oncol. 2015;16:274-83. Erratum in: Lancet Oncol. 2015;16:e105.-1111. Aluwini S, Pos F, Schimmel E, Krol S, van der Toorn PP, de Jager H, et al. Hypofractionated versus conventionally fractionated radiotherapy for patients with prostate cancer (HYPRO): late toxicity results from a randomised, non-inferiority, phase 3 trial. Lancet Oncol. 2016;17:464-74.). With 5-years of follow-up, none revealed inferiority of hypofractionation regarding the treatment outcomes, and the toxicity reports are contradictory, with no overwhelming and reproducible toxicity associated with a moderately hypofractionated regimens using 2.5 to 3 Gy per fraction. We sought to determine the current view of moderate hypofractionation among North American genitourinary (GU) radiation oncology experts due to their influence in shaping the direction of national guidelines.
Summary of the four randomized clinical trials comparing hypofractionation (H-RT) with conventional fractionation (C-RT) for prostate cancer (OS = overall survival; DFS = disease-free survival; RFS = relapse-free survival; GU = genitourinary; GI = gastrointestinal; CI = confidence interval).
MATERIALS AND METHODS
Survey design and deployment
The survey was designed to assess the opinions of GU experts on the default EBRT dose and fractionation for a hypothetical patient with a favorable - intermediate risk prostate cancer who would require by most current conventions EBRT to prostate alone without prophylactic irradiation of pelvic lymph nodes. Three fractionation schemes were offered as choices: conventional fractionation (78 Gy in 2 Gy fractions, 79.2 Gy in 1.8 Gy fractions or equivalent), moderate hypofractionation (70 Gy in 2.5 Gy fractions or equivalent), or SBRT / radical hypofractionation (5 – 12 fractions or equivalent). The study was approved by IRB and electronically sent to 88 North American GU oncology physicians, who serve on cooperative group research organizations such as NRG Oncology. The survey was designed and hosted by Research Electronic Data Capture (REDCap), and contained screening questions to ensure respondents were currently practicing, not in training, and specializing in GU oncology (1212. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377-81.). A copy of the survey is available in the Appendix 1.
Statistical analysis
Based on responses, participants were categorized as “supporters” or “opponents” of moderate hypofractionation. For the purposes of this study, only responders choosing conventional fractionation or moderate hypofractionation were included. Fisher's exact test was used to determine whether treatment recommendations were correlated with practice patterns. R (R version 3.3.3 (2017-03-06)) was used for all data analysis. Statistical significance was set at p < 0.05.
RESULTS
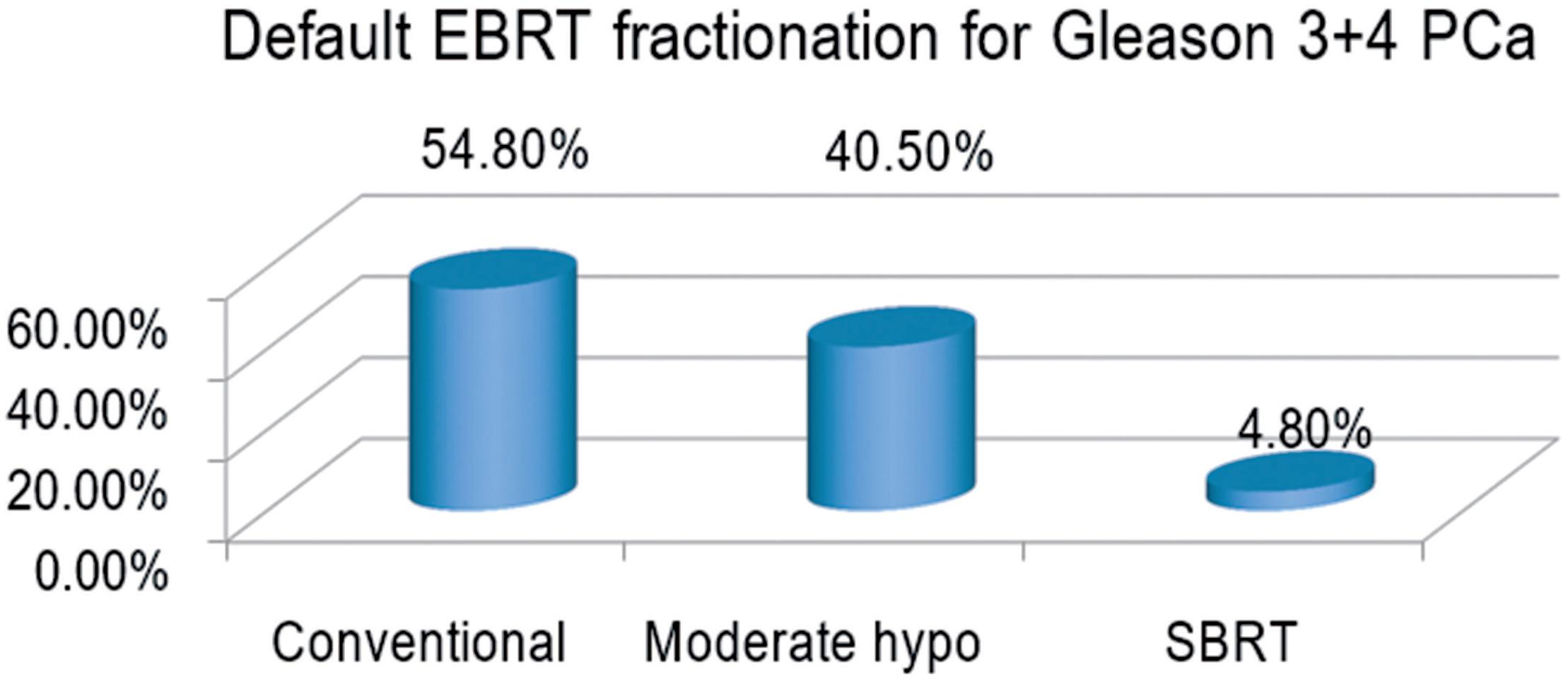
Forty - two of the 88 radiation oncologists completed the survey, of whom 40 (95.2%) recommended either conventional fractionation or moderate hypofractionation; two (4.8%) recommended stereotactic body radiation therapy (SBRT) (Figure-1) and were excluded from the analysis. Of 40 analyzable respondents, 23 (57.5%) recommended conventional fractionation and 17 (42.5%) recommended moderate hypofractionation.
Default External Beam Radiation Therapy Fractionation used by North American genitourinary oncology expert radiation oncologists for treatment of a hypothetical patient with a favorable intermediate risk Prostate Cancer (Gleason 3+4).
PCa = prostate cancer; hypo = hypofractionation
No demographic factors (years in practice, geographic location of residency, geographic location of practice, monthly patient volume, practice type) as well as other clinical positions (active surveillance recommendation preference, brachytherapy boost advocacy, self-identification as an expert brachytherapist, likelihood of considering stereotactic body RT for oligometastatic disease, likelihood of prophylactically irradiating pelvic lymph nodes, support of advanced imaging techniques) were significantly associated with support of moderate hypofractionation. Only the choice of brachytherapy as a preferred treatment option for patients with low - risk prostate cancer approached significance for recommendation of hypofractionation (p = 0.089) (Table-2).
Association between clinical practice recommendations and choice of default dose/fractionation for Gleason 3+4 prostate adenocarcinoma.
DISCUSSION
Biological considerations of a markedly lower alpha / beta ratio of prostate cancer in comparison to surrounding normal tissues led researchers to clinical investigation of hypofractionated regimens in management of patients with prostate cancer with EBRT (1313. Hegemann NS, Guckenberger M, Belka C, Ganswindt U, Manapov F, Li M. Hypofractionated radiotherapy for prostate cancer. Radiat Oncol. 2014;9:275.). Four large international randomized clinical trials have established non - inferiority of moderate hypofractionation (2.5 – 3 Gy per fraction), with varying toxicity results, some supporting conventional, others hypofractionated regimens, but none reporting overwhelming toxicity within the 5 - years of a follow-up period (Table-1) (66. Catton CN, Lukka H, Gu CS, Martin JM, Supiot S, Chung PWM, et al. Randomized Trial of a Hypofractionated Radiation Regimen for the Treatment of Localized Prostate Cancer. J Clin Oncol. 2017;35:1884-90.
7. Lee WR, Dignam JJ, Amin MB, Bruner DW, Low D, Swanson GP, et al. Randomized Phase III Noninferiority Study Comparing Two Radiotherapy Fractionation Schedules in Patients With Low-Risk Prostate Cancer. J Clin Oncol. 2016;34:2325-32.
8. Dearnaley D, Syndikus I, Mossop H, Khoo V, Birtle A, Bloomfield D, et al. Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol. 2016;17:1047-60. Erratum in: Lancet Oncol. 2016;17:e321.
9. Incrocci L, Wortel RC, Alemayehu WG, Aluwini S, Schimmel E, Krol S, et al. Hypofractionated versus conventionally fractionated radiotherapy for patients with localised prostate cancer (HYPRO): final efficacy results from a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2016;17:1061-9.
10. Aluwini S, Pos F, Schimmel E, van Lin E, Krol S, van der Toorn PP, et al. Hypofractionated versus conventionally fractionated radiotherapy for patients with prostate cancer (HYPRO): acute toxicity results from a randomised non-inferiority phase 3 trial. Lancet Oncol. 2015;16:274-83. Erratum in: Lancet Oncol. 2015;16:e105.-1111. Aluwini S, Pos F, Schimmel E, Krol S, van der Toorn PP, de Jager H, et al. Hypofractionated versus conventionally fractionated radiotherapy for patients with prostate cancer (HYPRO): late toxicity results from a randomised, non-inferiority, phase 3 trial. Lancet Oncol. 2016;17:464-74.).
The degree of acceptance / rejection of treatment modalities in North America is to a significant extent shaped by opinions of leading academic physicians who define and periodically update national treatment guidelines, author consensus statements and shape the future clinical trial protocols. Because of this influence, we sought to determine the acceptance of hypofractionation for prostate cancer among North American GU radiation oncology experts (1414. Nass SJ, Balogh E, Mendelsohn J. A National Cancer Clinical Trials Network: recommendations from the Institute of Medicine. Am J Ther. 2011;18:382-91.).
The results of this study indicate that hypofractionated EBRT, defined as 70 Gy in 2.5 Gy fractions or an equivalent regiment, has made significant inroads among North American GU experts in the treatment of prostate cancer, as more than 40% of experts recommended hypofractionated EBRT as their preferred EBRT treatment modality. Nevertheless, 55% of experts still consider conventionally fractionated EBRT as an unchallenged standard of care. Physicians who embrace a shorter treatment modality (brachytherapy), despite possible increase in acute toxicity - also tend to support hypofractionated EBRT. The relatively even duality regarding conventional versus hypofractionated treatment recommendation for intermediate - risk prostate cancer despite the four randomized trials already published on this topic (66. Catton CN, Lukka H, Gu CS, Martin JM, Supiot S, Chung PWM, et al. Randomized Trial of a Hypofractionated Radiation Regimen for the Treatment of Localized Prostate Cancer. J Clin Oncol. 2017;35:1884-90.
7. Lee WR, Dignam JJ, Amin MB, Bruner DW, Low D, Swanson GP, et al. Randomized Phase III Noninferiority Study Comparing Two Radiotherapy Fractionation Schedules in Patients With Low-Risk Prostate Cancer. J Clin Oncol. 2016;34:2325-32.
8. Dearnaley D, Syndikus I, Mossop H, Khoo V, Birtle A, Bloomfield D, et al. Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol. 2016;17:1047-60. Erratum in: Lancet Oncol. 2016;17:e321.-99. Incrocci L, Wortel RC, Alemayehu WG, Aluwini S, Schimmel E, Krol S, et al. Hypofractionated versus conventionally fractionated radiotherapy for patients with localised prostate cancer (HYPRO): final efficacy results from a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2016;17:1061-9.) speaks to the issue that randomized trials do not necessarily change the standard of care, particularly in the United States, and a significantly longer follow-up is required; this duality is reflected in the most updated clinically localized prostate cancer guidelines published jointly by the American Urological Association, American Society for Radiation Oncology (ASTRO), and the Society of Urologic Oncology (1515. Sanda MG, Cadeddu JA, Kirkby E, Chen RC, Crispino T, Fontanarosa J, et al. Clinically Localized Prostate Cancer: AUA/ASTRO/SUO Guideline. Part I: Risk Stratification, Shared Decision Making, and Care Options. J Urol. 2018;199:683-90., 1616. Sanda MG, Cadeddu JA, Kirkby E, Chen RC, Crispino T, Fontanarosa J, et al. Clinically Localized Prostate Cancer: AUA/ASTRO/SUO Guideline. Part II: Recommended Approaches and Details of Specific Care Options. J Urol. 2018;199:990-7.). Hypofractionation in breast cancer similarly was adopted in other countries much sooner than in the United States, where ASTRO consensus statements, educational sessions and even direct advertisement to patients regarding hypofractionated options and their non - inferiority, led to final acceptance of hypofractionation as a new standard of care. It is unclear whether reimbursement system in the U.S. is partially responsible for a slower update of shorter treatment courses. Limitations of this study are relatively small sample size, despite an impressive (but still below fifty percent) response rate, inability to capture a full range of options due to multiple choice format, and a lack of granularity in addressing the impact of racial demographic of patients being treated (1717. McClelland S 3rd, Sandler KA, Degnin C, Chen Y, Mitin T. Active Surveillance for Low and Intermediate Risk Prostate Cancer: Opinions of North American Genitourinary Oncology Expert Radiation Oncologists. Clin Genitourin Cancer. 2018;16:e323-5.). Furthermore, the absence of decade - long toxicity and outcome data comparing conventional versus moderate hypofractionation provides an uncertainty of outcomes beyond the five years of currently published results (66. Catton CN, Lukka H, Gu CS, Martin JM, Supiot S, Chung PWM, et al. Randomized Trial of a Hypofractionated Radiation Regimen for the Treatment of Localized Prostate Cancer. J Clin Oncol. 2017;35:1884-90.
7. Lee WR, Dignam JJ, Amin MB, Bruner DW, Low D, Swanson GP, et al. Randomized Phase III Noninferiority Study Comparing Two Radiotherapy Fractionation Schedules in Patients With Low-Risk Prostate Cancer. J Clin Oncol. 2016;34:2325-32.
8. Dearnaley D, Syndikus I, Mossop H, Khoo V, Birtle A, Bloomfield D, et al. Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol. 2016;17:1047-60. Erratum in: Lancet Oncol. 2016;17:e321.
9. Incrocci L, Wortel RC, Alemayehu WG, Aluwini S, Schimmel E, Krol S, et al. Hypofractionated versus conventionally fractionated radiotherapy for patients with localised prostate cancer (HYPRO): final efficacy results from a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2016;17:1061-9.
10. Aluwini S, Pos F, Schimmel E, van Lin E, Krol S, van der Toorn PP, et al. Hypofractionated versus conventionally fractionated radiotherapy for patients with prostate cancer (HYPRO): acute toxicity results from a randomised non-inferiority phase 3 trial. Lancet Oncol. 2015;16:274-83. Erratum in: Lancet Oncol. 2015;16:e105.-1111. Aluwini S, Pos F, Schimmel E, Krol S, van der Toorn PP, de Jager H, et al. Hypofractionated versus conventionally fractionated radiotherapy for patients with prostate cancer (HYPRO): late toxicity results from a randomised, non-inferiority, phase 3 trial. Lancet Oncol. 2016;17:464-74.).
In conclusion, there is currently a nearly even split between radiation oncology experts in North America recommending conventionally fractionated vs moderately hypofractionated EBRT for patients with prostate cancer, based on dramatically different interpretation of results of 4 randomized clinical trials. Longer follow-up of these trials may impact the balance, while national care and reimbursement policies may influence the accepted standard of care.
REFERENCES
-
1Deshmukh AA, Shirvani SM, Lal L, Swint JM, Cantor SB, Smith BD, et al. Cost-effectiveness Analysis Comparing Conventional, Hypofractionated, and Intraoperative Radiotherapy for Early-Stage Breast Cancer. J Natl Cancer Inst. 2017;109.
-
2Pichon B, Campion L, Delpon G, Thillays F, Carrie C, Cellier P, et al. High-Dose Hypofractionated Radiation Therapy for Noncompressive Vertebral Metastases in Combination With Zoledronate: A Phase 1 Study. Int J Radiat Oncol Biol Phys. 2016;96:840-7.
-
3Hafeez S, McDonald F, Lalondrelle S, McNair H, Warren-Oseni K, Jones K, et al. Clinical Outcomes of Image Guided Adaptive Hypofractionated Weekly Radiation Therapy for Bladder Cancer in Patients Unsuitable for Radical Treatment. Int J Radiat Oncol Biol Phys. 2017;98:115-22. Erratum in: Int J Radiat Oncol Biol Phys. 2018;100:532-3.
-
4Malmström A, Grønberg BH, Marosi C, Stupp R, Frappaz D, Schultz H, et al. Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomised, phase 3 trial. Lancet Oncol. 2012;13:916-26.
-
5Walraven I, van den Heuvel M, van Diessen J, Schaake E, Uyterlinde W, Aerts J, et al. Long-term follow-up of patients with locally advanced non-small cell lung cancer receiving concurrent hypofractionated chemoradiotherapy with or without cetuximab. Radiother Oncol. 2016;118:442-6.
-
6Catton CN, Lukka H, Gu CS, Martin JM, Supiot S, Chung PWM, et al. Randomized Trial of a Hypofractionated Radiation Regimen for the Treatment of Localized Prostate Cancer. J Clin Oncol. 2017;35:1884-90.
-
7Lee WR, Dignam JJ, Amin MB, Bruner DW, Low D, Swanson GP, et al. Randomized Phase III Noninferiority Study Comparing Two Radiotherapy Fractionation Schedules in Patients With Low-Risk Prostate Cancer. J Clin Oncol. 2016;34:2325-32.
-
8Dearnaley D, Syndikus I, Mossop H, Khoo V, Birtle A, Bloomfield D, et al. Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol. 2016;17:1047-60. Erratum in: Lancet Oncol. 2016;17:e321.
-
9Incrocci L, Wortel RC, Alemayehu WG, Aluwini S, Schimmel E, Krol S, et al. Hypofractionated versus conventionally fractionated radiotherapy for patients with localised prostate cancer (HYPRO): final efficacy results from a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2016;17:1061-9.
-
10Aluwini S, Pos F, Schimmel E, van Lin E, Krol S, van der Toorn PP, et al. Hypofractionated versus conventionally fractionated radiotherapy for patients with prostate cancer (HYPRO): acute toxicity results from a randomised non-inferiority phase 3 trial. Lancet Oncol. 2015;16:274-83. Erratum in: Lancet Oncol. 2015;16:e105.
-
11Aluwini S, Pos F, Schimmel E, Krol S, van der Toorn PP, de Jager H, et al. Hypofractionated versus conventionally fractionated radiotherapy for patients with prostate cancer (HYPRO): late toxicity results from a randomised, non-inferiority, phase 3 trial. Lancet Oncol. 2016;17:464-74.
-
12Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377-81.
-
13Hegemann NS, Guckenberger M, Belka C, Ganswindt U, Manapov F, Li M. Hypofractionated radiotherapy for prostate cancer. Radiat Oncol. 2014;9:275.
-
14Nass SJ, Balogh E, Mendelsohn J. A National Cancer Clinical Trials Network: recommendations from the Institute of Medicine. Am J Ther. 2011;18:382-91.
-
15Sanda MG, Cadeddu JA, Kirkby E, Chen RC, Crispino T, Fontanarosa J, et al. Clinically Localized Prostate Cancer: AUA/ASTRO/SUO Guideline. Part I: Risk Stratification, Shared Decision Making, and Care Options. J Urol. 2018;199:683-90.
-
16Sanda MG, Cadeddu JA, Kirkby E, Chen RC, Crispino T, Fontanarosa J, et al. Clinically Localized Prostate Cancer: AUA/ASTRO/SUO Guideline. Part II: Recommended Approaches and Details of Specific Care Options. J Urol. 2018;199:990-7.
-
17McClelland S 3rd, Sandler KA, Degnin C, Chen Y, Mitin T. Active Surveillance for Low and Intermediate Risk Prostate Cancer: Opinions of North American Genitourinary Oncology Expert Radiation Oncologists. Clin Genitourin Cancer. 2018;16:e323-5.
Publication Dates
-
Publication in this collection
27 May 2019 -
Date of issue
2019
History
-
Received
17 Apr 2018 -
Accepted
13 Sept 2018 -
Published
30 Oct 2018