Abstracts
A convenient pathway to obtain 3beta-hydroxy-6-oxo-5alpha-steroids from 3beta-acetoxy delta5-steroids is reported; the methodology was applied to the synthesis of laxogenin (7), substance that behaves as a plant growth hormone. This is an alternative way to produce an important functionality found in many examples of naturally occurring steroids. The developed procedure uses inexpensive reagents and can be carried out in four steps. The oxidizing and acidic steps used in this methodology did not affect the labile spiroketal side chain present in diosgenin (16).
laxogenin; diastereoselective epoxidation; 3beta-hydroxy-6-oxo-5alpha-steroids
Uma rota conveniente para a síntese de 3beta-hidroxi-6-oxo-5alfa-esteroides a partir de delta5-esteroides é descrita tendo sido aplicada para a síntese da laxogenina, substância que apresenta atividade como hormônio de crescimento vegetal. O método é uma alternativa para instalar este grupo funcional importante encontrado em esteróides naturais. O processo descrito utiliza reagentes baratos e pode ser executado em quatro etapas. As etapas de oxidação e de tratamento ácido não afetam a cadeia lateral espirocetálica presente na diosgenina (16).
ARTICLE
A convenient procedure for the synthesis of 3b-hydroxy-6-oxo-5a-steroids. Application to the synthesis of laxogenin
Martín A. Iglesias-ArteagaI,* * e-mail: martin.iglesias@servidor.unam.mx, jsandova@siu.buap.mx ; Eva M. Símuta-LopezII; Sergio Xochihua-MorenoII; Omar Viñas-BravoII; Sara Montiel SmithII; Socorro Meza ReyesII; Jesús Sandoval-RamírezII,* * e-mail: martin.iglesias@servidor.unam.mx, jsandova@siu.buap.mx
IDepartamento de Química Orgánica, Facultad de Química, Universidad Nacional Autónoma de México, Ciudad Universitaria, 04510 México - D. F., México
IIFacultad de Ciencias Químicas, Benemérita Universidad Autónoma de Puebla, Ciudad Universitaria, 72570 Puebla - Pue., México
ABSTRACT
A convenient pathway to obtain 3b-hydroxy-6-oxo-5a-steroids from 3b-acetoxy D5-steroids is reported; the methodology was applied to the synthesis of laxogenin (7), substance that behaves as a plant growth hormone. This is an alternative way to produce an important functionality found in many examples of naturally occurring steroids. The developed procedure uses inexpensive reagents and can be carried out in four steps. The oxidizing and acidic steps used in this methodology did not affect the labile spiroketal side chain present in diosgenin (16).
Keywords: laxogenin, diastereoselective epoxidation, 3b-hydroxy-6-oxo-5a-steroids
RESUMO
Uma rota conveniente para a síntese de 3b-hidroxi-6-oxo-5a-esteroides a partir de D5-esteroides é descrita tendo sido aplicada para a síntese da laxogenina, substância que apresenta atividade como hormônio de crescimento vegetal. O método é uma alternativa para instalar este grupo funcional importante encontrado em esteróides naturais. O processo descrito utiliza reagentes baratos e pode ser executado em quatro etapas. As etapas de oxidação e de tratamento ácido não afetam a cadeia lateral espirocetálica presente na diosgenina (16).
Introduction
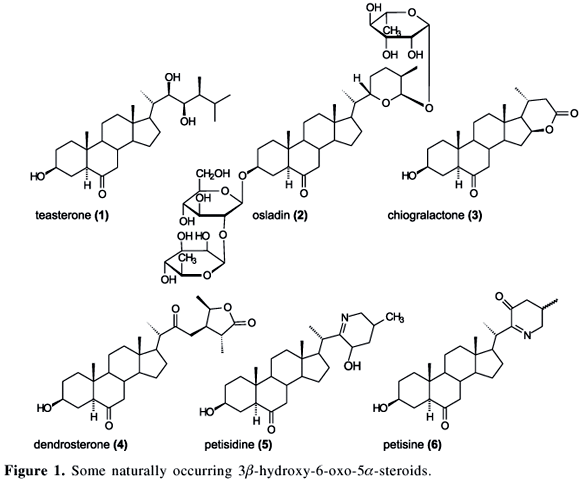
The 3b-hydroxy-6-oxo moiety is present in different naturally occurring steroids.1 The brassinosteroid teasterone (1),2 the steroid glycoside osladin (2),3 lactones as chiogralactone (3)4 and dendrosterone (4),5 and steroidal alkaloids like petisidine (5)6 and petisine (6)7 are some examples of naturally occurring steroids bearing such functionality (Figure 1).
Recently, we have synthesized laxogenin (7), a steroidal sapogenin isolated from Smilax sieboldi8 and its C-23 substituted derivatives 8 and 9 and reported that they have shown plant growth promoting activity similar of that of brassinosteroids (Figure 2).8 In that report, a protocol based on Brown's hydroboration for the introduction of the 3b-hydroxy-6-oxo moiety was developed.
The 5b,6b-epoxy moiety has been previously used for the preparation of 6-oxosteroids. In particular, Henbest and Wrigley reported9 that treatment of 5,6b-epoxy-5b-cholestan-3b-ol acetate with BF3·Et2O led to the corresponding 3b-acetoxy-5-fluoro-6b-hydroxy-5a-steroid which was oxidized to 3b-acetoxy-5-fluoro-5a-cholestan-6-one using Jones reagent. More recently, an extension of this procedure to stigmasterol has been used to obtain brassinosteroid analogues bearing the 5a-fluoro-6-oxo moiety.9
Owing our interest on steroids bearing such functionality, and after some reports on the highly stereoselective b-epoxidation of D5-steroids using biphasic systems involving potassium permanganate and metal salts,10 we envisaged the b-epoxidation of the C5-C6 double bond followed by the regioselective oxirane ring opening as the key steps for the introduction of an oxygen atom at position C-6. This led us to an alternative protocol for the synthesis of 3b-hydroxy-6-oxo steroids.
Results and Discussion
Treatment of cholesteryl acetate, in a mixture of CH2Cl2, tert-butyl alcohol and H2O, with KMnO4 and Fe2(SO4)3 resulted in the highly diasteroselective b-epoxidation of the C5-C6 double bond; only traces of the a-epoxide could be detected in the 1H NMR spectra. Regioselective opening of the b-oxirane ring with aq. HBr led to the bromohydrin 11 as sole product.
Treatment of the bromohydrin 12 with Jones reagent, supported on silica gel, led the bromoketone 13 (Scheme 1). In this way, manipulation of the reaction was rapidly effectuated and permitted a better treatment for wastes.
We also studied this three-reaction sequence without isolation of the intermediate epoxide 11 and bromohydrin 12; this resulted in a very fast and convenient protocol for the conversion of cholesteryl acetate into the bromoketone 13 (75% overall yield for the consecutive three steps). Treatment of 13 with zinc in refluxing acetic acid yielded the acetylated ketone 14, which on saponification afforded the desired 3b-hydroxy-5a-cholestan-6-one (15).
The same one-pot synthetic sequence was applied to diosgenin acetate (16) to produce laxogenin (7). Under these strong oxidizing and acidic media, the labile spiroketal side chain of diosgenin resulted unchanged (Scheme 2).
Experimental
NMR spectra were registered in CDCl3 on a Varian Mercury spectrometer at 400 MHz for 1H or 100 MHz for 13C. Chemical shifts (d) are expressed on ppm downfield from TMS. Melting points were obtained on a Gallenkamp MFB 595 apparatus and were not corrected.
5,6b-Epoxy-5b-cholestan-3b-ol acetate (11)
KMnO4 (2 g) and Fe2(SO4)3.nH2 O (1 g) were finely grounded in a mortar, H2O (0.2 mL) was added and the mixture was placed in a round bottom flask containing CH2Cl2 (5 mL). A solution of cholesteryl acetate (10) (428 mg, 1 mmol) in CH2Cl2 (5 mL) was added followed by addition of tert-butyl alcohol (0.5 mL). After 20 min of stirring at room temperature, the mixture was filtered through a pad of celite, and eluted with 30 mL of CH2Cl2. The crude filtrate was washed with H2O (5x15) mL, dried (anhydrous Na2SO4) and evaporated to afford 427 mg (96%) of the desired epoxide 11; mp 110-111 ºC, lit.11 111-112 ºC; 1H NMR d 4.76 (m, H-3), 3.07 (d. J 2.4 Hz, H-6), 2.03 (s, CH3COO-3), 1.0 (s, CH3-19), 0.89 (d, J21-20 6.8 Hz, CH3-21), 0.86 (d, J 6.4 Hz, CH3-26 and CH3-27), 0.64 (s, CH3-18); 13C NMR d 29.76 C-1, 27.25 C-2, 71.28 C-3, 38.02 C-4, 62.47 C-5, 63.55 C-6, 36.71 C-7, 32.49 C-8, 56.13 C-9, 35.03 C-10, 21.99 C-11, 39.78 C-12, 42.28 C-13, 50.97 C-14, 24.23 C-15, 28.21 C-16, 56.13 C-17, 11.85 C-18, 17.11 C-19, 35.76 C-20, 18.7 C-21, 36.15 C-22, 23.85 C-23, 39.51 C-24, 28.06 C-25, 22.64 C-26, 22.90 C-27, 21.41 CH3COO-3, 170.24 CH3COO-3.
5-Bromo-5a-cholestan-3b,6b-diol 3-acetate (12)
A solution of the epoxide 11 (445 mg, 1 mmol) in CH2Cl2 (15 mL) was vigorously shaken for 5 min in a separatory funnel with 5 mL of 48% HBr. The organic layer was washed with H2O (5x 15 mL), dried (anh. Na2SO4) and evaporated to afford 510 mg (97%) of the bromohydrin 12; mp 174-175 ºC, lit.12 177-179 ºC; 1H NMR d 5.47 (m, H-3), 4.18 (s, H-6), 2.03 (s, CH3COO-3), 1.31 (s, CH3-19), 0.90 (d, J21-20 6.6 Hz, CH3-21), 0.86 (d, J 6.4 Hz, CH3-26 and CH3-27), 0.67 (s, CH3-18); 13C NMR d 35.16 C-1, 26.43 C-2, 72.17 C-3, 38.42 C-4, 86.73 C-5, 75.68 C-6, 34.62 C-7, 30.64 C-8, 47.44 C-9, 41.30 C-10, 21.39 C-11, 39.78 C-12, 42.72 C-13, 55.73 C-14, 24.16 C-15, 28.29 C-16, 56.10 C-17, 12.32 C-18, 18.15 C-19, 35.83 C-20, 18.78 C-21, 36.18 C-22, 23.90 C-23, 39.51 C-24, 28.10 C-25, 22.67 C-26, 22.93 C-27, 21.50 CH3COO-3, 170.30 CH3COO-3.
3b-Acetoxy-5-bromo-5a-cholestan-6-one (13)
A mixture of Jones reagent (2.5 mL) and silica gel (5 g) was stirred until an homogeneous orange powder was formed. CH2Cl2 (15 mL) was added followed by the addition of a solution of the bromohydrin 12 (526 mg, 1 mmol) in CH2Cl2 (10 mL); the mixture was stirred for 20 min, filtered through an small pad of silica gel and the eluent was evaporated to afford 414 mg (79%) of the bromoketone 13; mp 157-158 ºC (decomp.), lit.13 162 ºC (decomposition); 1H NMR d 5.32 (m, H-3), 3.15 (dd, J7ax-7eq, 7ax-8ax 14.85, 12.1 Hz, H-7a), 2.38 (ddd, J4eq-4ax 14.3, J4eq-3ax 4.2, J4eq-2eq 1.5 Hz, H-4eq), 2.28 (dd, J7ax-7eq 15, J7eq-8ax 5.5 Hz, H-7e), 2.03 (s, CH3COO-3), 0.99 (s, CH3-19), 0.90 (d, J21-20 6.2 Hz, CH3-21), 0.86 and 0.85 (d, J 6.6 Hz, CH3- 26 and 27), 0.65 (s, CH3-18); 13C NMR d 30.43 C-1, 26.09 C-2, 70.92 C-3, 34.84 C-4, 79.61 C-5, 203.67 C-6, 40.45 C-7, 36.24 C-8, 47.26 C-9, 42.69 C-10, 21.79 C-11, 39.51 C-12, 43.03 C-13, 55.93 C-14, 23.84 C-15, 28.10 C-16, 56.16 C-17, 12.18 C-18, 14.59 C-19, 35.71 C-20, 18.72 C-21, 36.10 C-22, 23.84 C-23, 39.28 C-24, 28.07 C-25, 22.65 C-26, 22.90 C-27, 21.36 CH3COO-3, 170.04 CH3COO-3.
3b-Acetoxy-5-bromo-5a-cholestan-6-one (13) via a three steps procedure: b-epoxidation, oxirane-opening, alcohol-oxidation
KMnO4 (6 g) and Fe2(SO4)3.nH2 O (3 g) were finely grounded in a mortar, H2O (0.6 mL) was added and the mixture was placed in a round bottom flask containing CH2Cl2 (45 mL). A solution of cholesteryl acetate (10) (1.284 g, 3 mmol) in CH2Cl2 (45 mL) was added followed by addition of tert-butyl alcohol (1.5 mL). After 20 min of stirring at room temperature, the mixture was filtered through a pad of celite and eluted with 30 mL of CH2Cl2. The crude filtrate was washed with H2O (5x25 mL) and vigorously shaken for 5 min in a separatory funnel with 15 mL of 48% HBr, washed with H2O (5x 30 mL) and added to a stirred mixture of silica gel-supported Jones Reagent (prepared from 2.5 mL of Jones reagent and 5 g silica gel as described for the oxidation of 12) and CH2Cl2 (30 mL). The mixture was stirred for 20 min, filtered through a small pad of silica gel and the eluent was evaporated to afford 1.178 mg (75%) of the bromoketone 13, identical as described above.
3b-Acetoxy-5a-cholestan-6-one (14)
A mixture of the bromoketone (524, 1 mmol), zinc powder (262 mg, 4 mmol) and acetic acid (10 mL) was stirred under reflux for 2 h. AcOEt (50 mL) was added and the mixture was washed with saturated NaCl solution (5x15 mL), 10% NaHCO3 solution (3x20 mL) and H2O (5x15 mL). The organic layer was dried with Na2SO4 and evaporated to afford 436 mg (98%) of the acetylated ketone 14; mp 126-127 ºC, lit.14 128-129 ºC; 1H NMR d 4.67 (m, H-3), 2.03 (s, CH3COO-3), 0.91 (d, J21-20 6.6 Hz, CH3-21), 0.87 and 0.86 (d, J 6.6 Hz, CH3-26 and CH3-27), 0.77 (s, CH3-19), 0.66 (s, CH3-18); 13C NMR d 36.32 C-1, 26.75 C-2, 72.78 C-3, 26.04 C-4, 56.01 C-5, 210.40 C-6, 46.58 C-7, 37.85 C-8, 53.73 C-9, 40.87 C-10, 21.28 C-11, 39.38 C-12, 42.89 C-13, 56.59 C-14, 23.89 C-15, 27.97 C-16, 56.38 C-17, 11.94 C-18, 12.96 C-19, 35.62 C-20, 18.56 C-21, 36.00 C-22, 23.73 C-23, 39.38 C-24, 27.93 C-25, 22.48 C-26, 22.74 C-27, 21.39 CH3COO-3, 170.56 CH3COO-3.
3b-Hydroxy-5a-cholestan-6-one (15)
A mixture of the acetylated ketone 14 (445 mg, 1 mmol), KOH (0.5 g) and MeOH (50 mL) was gently warmed for 15 min, H2O (15 mL) was added, most of the MeOH was evaporated and the mixture was extracted with AcOEt (2x20 mL). The organic layer was washed with brine (3x15 mL) and H2O, dried with MgSO4 and evaporated to afford 412 mg (98%) of the hydroxylated ketone 15; mp 141-142 ºC, lit.15 142-143 ºC; 1H NMR d 3.57 (m, H-3), 0.91 (d, J21-20 6.6 Hz, CH3-21), 0.87 and 0.86 (d, J 6.6 Hz, CH3-26 and CH3-27), 0.75 (s, CH3-19), 0.66 (s, CH3-18); 13C NMR d36.65 C-1, 30.61 C-2, 70.44 C-3, 29.97 C-4, 56.72 C-5, 210.87 C-6, 46.70 C-7, 37.92 C-8, 53.83 C-9, 40.96 C-10, 21.53 C-11, 39.44 C-12, 42.93 C-13, 56.66 C-14, 23.99 C-15, 28.03 C-16, 56.02 C-17, 12.06 C-18, 13.21 C-19, 35.68 C-20, 18.67 C-21, 36.07 C-22, 23.84 C-23, 39.44 C-24, 28.02 C-25, 22.60 C-26, 22.86 C-27.
(25R)-3b-Acetoxy-5-bromo-5a-spirostan-6-one (17)
Application of the described one-pot b-epoxidation, oxirane-opening, alcohol-oxidation procedure to diosgenin acetate (16) (1.370 g, 3 mmol) afforded 1.208 mg (73%) of the bromoketone 17; mp 229-230 ºC (petroleum ether/AcOEt), lit.16 230-233 ºC; 1H NMR d 5.31 (m, H-3), 4.42 (ddd, J16-17 J16-15 7.3 Hz, H-16), 3.48 (ddd, J26ax-26eq 11, J26eq-25ax 4.8, J26eq-24eq 1.8 Hz, H-26eq), 3.59 (dd, J26ax-26eq J26ax-25ax 11 Hz, H-26ax), 3.18 (dd, J7ax-7eq 11.7, J7ax-8ax 15 Hz, H-7a), 2.40 (ddd, J4ax-4eq 14.7, J4eq-3ax 5.3, J4eq-2eq 1.8 Hz, H-4a), 2.31 (dd, J7ax-7eq 15, J7eq-8ax 5.1 Hz, H-7b), 2.03 (s, CH3COO-3), 1.01 (s, CH3-19), 0.97 (d, J20-21 7 Hz, CH3-21), 0.79 (d, J25-27 7 Hz, CH3-27), 0.76 (s, CH3-18); 13C NMR d 30.40 C-1, 36.05 C-2, 70.82 C-3, 34.80 C-4, 79.37 C-5, 203.21 C-6, 40.50 C-7, 35.74 C-8, 47.29 C-9, 42.68 C-10, 21.62 C-11, 39.25 C-12, 40.99 C-13, 55.88 C-14, 31.38 C-15, 80.34 C-16, 61.90 C-17, 16.55 C-18, 14.68 C-19, 41.60 C-20, 14.58 C.21, 109.14 C-22, 31.44 C-23, 28.80 C-24, 30.29 C-25, 66.83 C-26, 17.22 C-27, 170.02 CH3COO-3, 21.35 CH3COO-3
(25R)-3b-Acetoxy-5a-spirostan-6-one (18)
1.103 g (2 mmol) of the bromoketone 17 were reduced as described for 13 to afford 756 mg (80%) of the ketone 20; mp 223-224º (petroleum ether/AcOEt), lit.16 222-224 ºC; 1H NMR d 4.66 (m, H-3), 4.41 (m, H-16), 3.47 (ddd, J26ax-26eq 11, J26eq-25ax 4.2, J26eq-24eq 1.8 Hz, H-26eq), 3.36 (dd, J26ax-26e J 26ax-25ax 11 Hz, H-26ax.), 2.03 (s, CH3COO-3), 0.97 (d, J21-20 6.97 Hz, CH3-21), 0.79 (d, J27-25 6.97 Hz, CH3-27), 0.78 (s, CH3-19), 0.78 (s, CH3-18). 13C NMR d 36.35 C-1, 26.84 C-2, 72.66 C-3, 26.16 C-4, 56.35 C-5, 209.63 C-6, 46.68 C-7, 37.38 C-8, 53.71 C-9, 40.95 C-10, 21.36 C-11, 39.44 C-12, 40.92 C-13, 56.42 C-14, 31.58 C-15, 80.32 C-16, 61.96 C-17, 16.49 C-18, 13.18 C-19, 41.60 C-20, 14.56 C-21, 109.14 C-22, 31.34 C-23, 28.77 C-24, 30.29 C-25, 66.81 C-26, 17.21 C-27, 170.31 CH3COO-3, 21.41 CH3COO-3.
(25R)-3b-Hydroxy-5a-spirostan-6-one, laxogenin (7)
473 mg (1 mmol) of the ketone 18 were hydrolyzed as described for 14 to afford 422 mg (98%) of laxogenin (7); mp 210-211 ºC (petroleum ether/AcOEt), [a]D25 - 83 (c 1.1, CHCl3); lit.17 210-212 ºC; [a]D25- 86 (c = 1.0, CHCl3); 1H NMR d 4.41 (m, H-16), 3.57 (m, H-3), 3.47 (ddd, J26ax-26eq 11, J26e-25ax 4.2 and J26eq-24eq 1.8 Hz, H-26e), 3.36 (dd, J26ax-26eq and J26ax-25ax 11 Hz, H-26ax.), 0.97 (d, J21-20 6.97 Hz, CH3-21), 0.79 (d, J27-25 6.97 Hz, CH3-27), 0.78 (s, CH3-19), 0.78 (s, CH3-18); 13C NMR d 36.59 C-1, 30.56 C-2, 70.27 C-3, 29.91 C-4, 56.70 C-5, 210.39 C-6, 46.68 C-7, 37.33 C-8, 53.79 C-9, 40.90 C-10, 21.36 C-11, 39.46 C-12, 40.90 C-13, 56.39 C-14, 31.53 C-15, 80.31 C-16, 61.91 C-17, 16.43 C-18, 13.26 C-19, 41.55 C-20, 14.50 C-21, 109.11 C-22, 31.29 C-23, 28.71 C-24, 30.21 C-25, 66.75 C-26, 17.14 C-27.
Conclusions
A convenient procedure to generate 3b-hydroxy-6-oxo-5a-steroids from D5-unsaturated steroids has been accomplished through a four steps process; the three first steps can be effectuated in a one-pot manner. Each step has been performed obtaining a good overall yield.
Acknowledgments
We are indebted to CONACyT, Mexico, for financial support via grant 35235-E and the scholarships granted to S.X.M and O.V.B. We also want to acknowledge Professor Benjamín Ruiz for laboratory facilities at the Facultad de Química of UNAM.
References
1. Hill, R. A.; Makin, H. L. J.; Kirk, D. N.; Murphy, G. M., eds.; Dictionary of Steroids, Chapman and Hall: London, 1991.
2. Adam, G.; Marquardt, V.; Phytochemistry 1986, 25, 1787.
3. Jizba, J.; Herout, V.; Collect. Czech. Chem. Commun. 1967, 32, 2867.
4. Takeda, K.; Shimaoka, A.; Iwasaki, M.; Minato, H.; Chem. Pharm. Bull. 1965, 13, 691.
5. Behr, D.; Berg, J. E.; Karlsson, B.; Leander, K.; Pilotti, A. M.; Wiehager, A. C.; Acta Chem. Scand. (B) 1975, 29, 401.
6. Nakahatov, I.; Nabiev, A.; Shakirov, R.; Yusonov, S.; Khim. Prir. Soedin 1983, 6, 747. (CA 100: 188749)
7. Nakahatov, I.; Nabiev, A.; Shakirov, R.; Khim. Prir. Soedin. 1981, 4, 616. (CA 96: 65672x)
8. Okanishi, T.; Akahor, A.; Yasuda, F.; Chem. Pharm. Bull. 1965, 13, 545; Iglesias-Arteaga, M. A.; Perez Gil R.; Perez Martinez C. S.; Coll Manchado, F.; J. Chem. Soc., Perkin Trans. 1 2001, 261.
9. Henbest, H. B.; Wrigley, T. I.; J. Chem. Soc. 1957, 4765; Ramírez, J. A.; Gros, E. G.; Galagovsky, L. R.; Tetrahedron 2000, 56, 6171.
10. Syamala, M. S.; Das, J.; Baskaran, S.; Chandrasekaran, S.; J. Org. Chem. 1992, 57, 1928; Parish, E. J.; Li, H.; Li, S.; Synth. Commun. 1995, 25, 927; Hanson, J. R.; Hitchock, P. B.; Liman, M. D.; Nagaratnam, S.; Manickavasagar, R.; J. Chem. Res. (S) 1995, 220; Parish, E. J.; Li, S.; J. Chem. Res. (S) 1996, 288; Parish, E. J.; Li, S.; J. Org. Chem. 1996, 61, 5665; Salvador, J. A. R.; Sáe Melo, M. L.; Campos Neves, A. S.; Tetrahedron Lett. 1996, 37, 687; Salvador, J. A. R.; Hanson, J. R.; J. Chem. Res. (S) 2002, 576. The authors became aware that, after submission of the present paper, a different and convenient method for b-epoxidation of D5-steroids using Co(II) salts and molecular oxygen had been published: Silvestre, S. M.; Salvador, J. A. R.; Clark, J. H.; J. Mol. Catal. A: Chemical 2004, 219, 143.
11. Chakravorty, P. N.; Levin, R.; J. Am. Chem. Soc. 1942, 64, 2317.
12. James, D. R.; Shoope. C. W.; J. Chem. Soc. 1954, 4224.
13. Heilbron, I. M.; Jones, E. H. R.; Spring, F. S.; J. Chem. Soc. 1937, 801.
14. Fonken, G. J.; Miles, H. M.; J. Org. Chem. 1963, 28, 2432.
15. Dodson, R. G.; Riegel, B.; J. Org. Chem. 1948, 13, 424.
16. Bowers, A.; Denot, E.; Cuéllar Ibáñez, L.; Cabezas, M. E.; Ringold, H. J.; J. Org. Chem. 1962, 27, 1862.
17. Elks, J. In Rodd's Chemistry of Carbon Compounds; Coffey, S., ed., Elsevier Publ. Co.: Amsterdam, 1971, ch. 18.
Received: June 24, 2004
Published on the web: March 30, 2005
- 1. Hill, R. A.; Makin, H. L. J.; Kirk, D. N.; Murphy, G. M., eds.; Dictionary of Steroids, Chapman and Hall: London, 1991.
- 2. Adam, G.; Marquardt, V.; Phytochemistry 1986, 25, 1787.
- 3. Jizba, J.; Herout, V.; Collect. Czech. Chem. Commun 1967, 32, 2867.
- 4. Takeda, K.; Shimaoka, A.; Iwasaki, M.; Minato, H.; Chem. Pharm. Bull. 1965, 13, 691.
- 5. Behr, D.; Berg, J. E.; Karlsson, B.; Leander, K.; Pilotti, A. M.; Wiehager, A. C.; Acta Chem. Scand (B) 1975, 29, 401.
- 6. Nakahatov, I.; Nabiev, A.; Shakirov, R.; Yusonov, S.; Khim. Prir. Soedin 1983, 6, 747. (CA 100: 188749)
- 7. Nakahatov, I.; Nabiev, A.; Shakirov, R.; Khim. Prir. Soedin 1981, 4, 616. (CA 96: 65672x)
- 8. Okanishi, T.; Akahor, A.; Yasuda, F.; Chem. Pharm. Bull. 1965, 13, 545;
- Iglesias-Arteaga, M. A.; Perez Gil R.; Perez Martinez C. S.; Coll Manchado, F.; J. Chem. Soc., Perkin Trans. 1 2001, 261.
- 9. Henbest, H. B.; Wrigley, T. I.; J. Chem. Soc 1957, 4765;
- Ramírez, J. A.; Gros, E. G.; Galagovsky, L. R.; Tetrahedron 2000, 56, 6171.
- 10. Syamala, M. S.; Das, J.; Baskaran, S.; Chandrasekaran, S.; J. Org. Chem 1992, 57, 1928;
- Parish, E. J.; Li, H.; Li, S.; Synth. Commun. 1995, 25, 927;
- Hanson, J. R.; Hitchock, P. B.; Liman, M. D.; Nagaratnam, S.; Manickavasagar, R.; J. Chem. Res. (S) 1995, 220;
- Parish, E. J.; Li, S.; J. Chem. Res. (S) 1996, 288;
- Parish, E. J.; Li, S.; J. Org. Chem. 1996, 61, 5665;
- Salvador, J. A. R.; Sáe Melo, M. L.; Campos Neves, A. S.; Tetrahedron Lett 1996, 37, 687;
- Salvador, J. A. R.; Hanson, J. R.; J. Chem. Res. (S) 2002, 576.
- The authors became aware that, after submission of the present paper, a different and convenient method for b-epoxidation of D5-steroids using Co(II) salts and molecular oxygen had been published: Silvestre, S. M.; Salvador, J. A. R.; Clark, J. H.; J. Mol. Catal. A: Chemical 2004, 219, 143.
- 11. Chakravorty, P. N.; Levin, R.; J. Am. Chem. Soc 1942, 64, 2317.
- 12. James, D. R.; Shoope. C. W.; J. Chem. Soc. 1954, 4224.
- 13. Heilbron, I. M.; Jones, E. H. R.; Spring, F. S.; J. Chem. Soc. 1937, 801.
- 14. Fonken, G. J.; Miles, H. M.; J. Org. Chem. 1963, 28, 2432.
- 15. Dodson, R. G.; Riegel, B.; J. Org. Chem. 1948, 13, 424.
- 16. Bowers, A.; Denot, E.; Cuéllar Ibáñez, L.; Cabezas, M. E.; Ringold, H. J.; J. Org. Chem. 1962, 27, 1862.
- 17. Elks, J. In Rodd's Chemistry of Carbon Compounds; Coffey, S., ed., Elsevier Publ. Co.: Amsterdam, 1971, ch. 18.
Publication Dates
-
Publication in this collection
18 July 2005 -
Date of issue
June 2005
History
-
Accepted
30 Mar 2005 -
Received
24 June 2004