Abstract
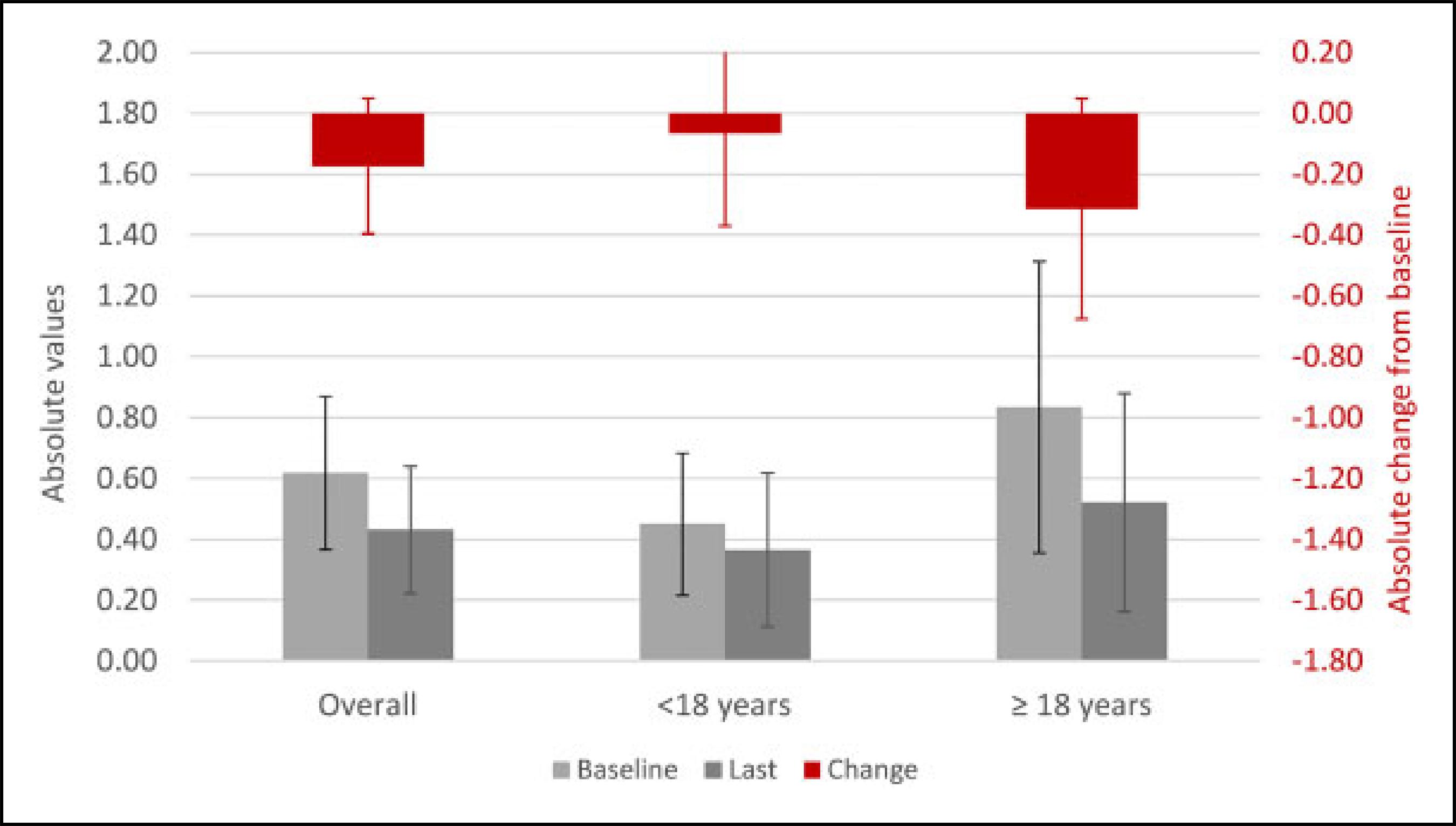
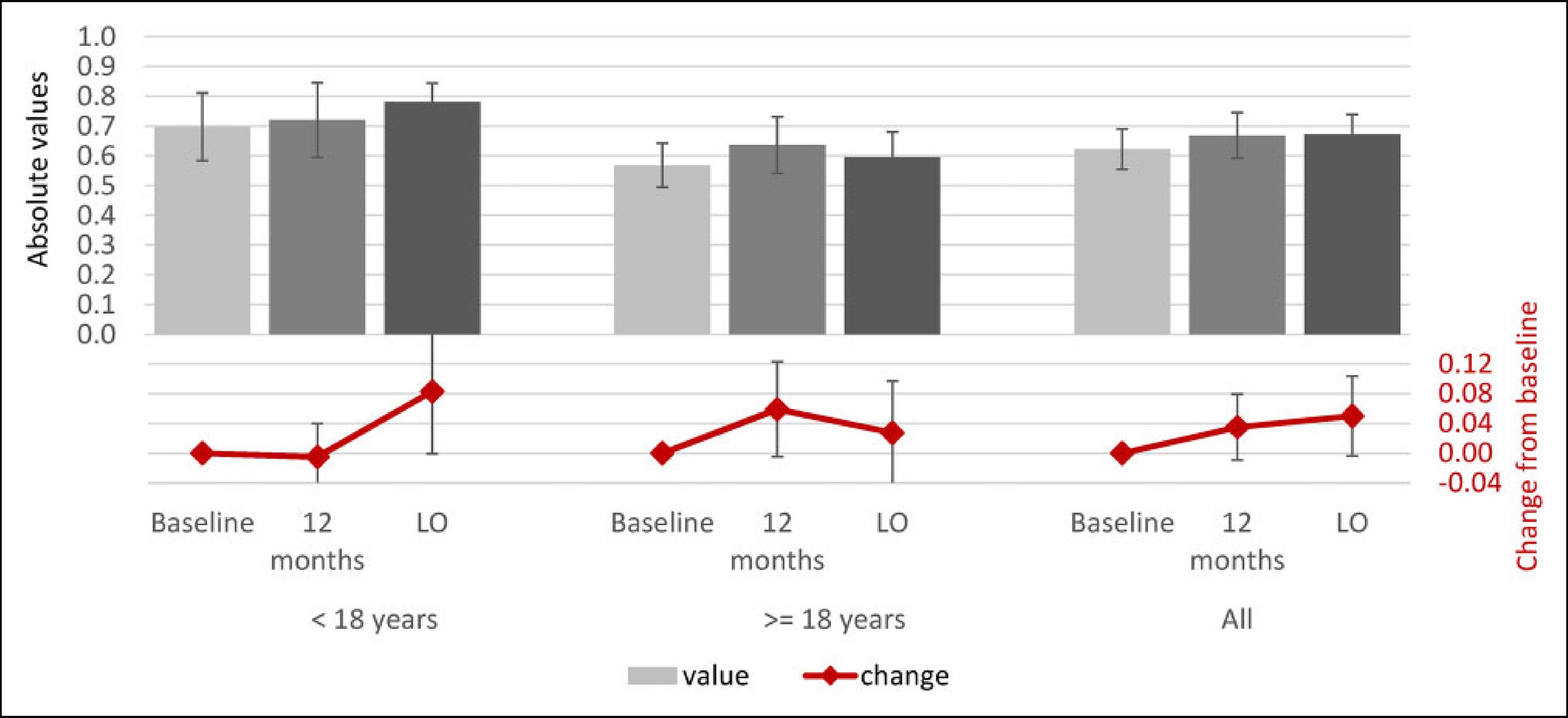
Alpha-mannosidosis, a rare lysosomal storage disorder caused by deficiency of the lysosomal enzyme alpha-mannosidase, results in accumulation of mannose-rich glycoproteins in the tissues and sequelae leading to intellectual disability, ataxia, impaired hearing and speech, recurrent infections, skeletal abnormalities, muscular pain, and weakness. This study aimed to investigate disability, pain, and overall health using the Childhood Health Assessment Questionnaire (CHAQ) and the EuroQol 5 Dimension-5 Level Questionnaire (EQ-5D-5L) in patients with alpha-mannosidosis participating in rhLAMAN-10, a phase III open-label, clinical trial of velmanase alfa, a recombinanthumanlysosomalalpha-mannosidase. Long-termprognosesformost patients withuntreatedalpha-mannosidosisarepoor due to progressive neuromuscular, skeletal, and intellectual deterioration, leading to increased dependence in mobility and activities of daily living and increased caregiver and health-care burden. Long-term CHAQ and EQ-5D-5L data highlight improvement trends in health-related quality of life and a reduction in disability and pain in patients receiving up to 48 months of velmanase alfa treatment.
Keywords
alpha-mannosidosis; recombinant human alpha-mannosidase; CHAQ; EQ-5D-5L; HRQoL; disability