Abstracts
OBJECTIVE: To evaluate spontaneous release of superoxide anion by peripheral blood granulocytes of atopic patients with uncontrolled asthma undergoing glucocorticoid therapy and of healthy subjects. METHODS: We studied 32 patients, aged 6 to 18 (mean 12.04), and 29 healthy subjects as a comparative group. Patients were grouped according to the forced expiratory vital capacity in the first second. Group I, forced expiratory vital capacity in the first second of between 60 and 80%, had 19 patients, and group II, forced expiratory vital capacity in the first second = 60%, had 13 patients. Spontaneous superoxide release by granulocytes was measured by a spectrophotometer method based on superoxide dismutase, before and after oral prednisone and beclomethasone, budesonide or fluticasone inhaled therapy. Statistical analyses were performed using ANOVA, Wilcoxon and Tukey tests. RESULTS: Comparing the superoxide anion release by granulocytes of asthmatic patients and healthy subjects, we observed a higher release by cells of the uncontrolled patient group II (p < 0.05). Evaluating the superoxide release by cells of asthmatic patients before and after steroid therapy, a significant decrease was found only in patient group I. CONCLUSION: The impact of corticosteroids on inflammatory modulation occurred in the uncontrolled asthmatics with forced expiratory vital capacity in the first second between 60 and 80%. In those with forced expiratory vital capacity in the first second of = 60%, this finding was not observed. Further studies are necessary to evaluate the effect of this finding on asthmatic patients.
Corticosteroids; superoxide radical; granulocytes; asthma; children; adolescents
OBJETIVO: Avaliar a liberação espontânea de ânion superóxido por granulócitos de sangue periférico de pacientes com asma crônica não-controlada antes e após corticoterapia e de indivíduos sadios. MÉTODOS: Foram estudados 32 pacientes entre 6 e 18 anos (média 12,04 anos) e 29 indivíduos sadios como grupo de comparação. Os pacientes foram agrupados de acordo com o volume expiratório forçado no primeiro segundo: grupo I, volume expiratório forçado no primeiro segundo entre 60 e 80%, 19 pacientes; e grupo II, volume expiratório forçado no primeiro segundo = 60%, 13 pacientes. A liberação espontânea de superóxido por granulócitos, medida por espectrofotometria utilizando superóxido dismutase, foi avaliada nos pacientes antes e após o tratamento com prednisona por via oral e beclometasona, budesonida ou fluticasona administradas por via inalatória. Na análise estatística foram utilizados os testes de análise de variância, Tukey e de Wilcoxon. RESULTADOS: Comparando-se a liberação de ânion superóxido por granulócitos dos pacientes asmáticos e indivíduos sadios observamos que a liberação foi maior nos asmáticos não-controlados do grupo II (p < 0,05). Avaliando-se a liberação de superóxido pelas células dos pacientes antes e após a terapia com corticosteroide uma diminuição significativa foi observada apenas no grupo I. CONCLUSÃO: O impacto dos glicocorticoides sobre a modulação da inflamação ocorreu nos indivíduos asmáticos não-controlados com volume expiratório forçado no primeiro segundo entre 60 e 80%. Naqueles com volume expiratório forçado no primeiro segundo = 60 não foi observada essa modulação, havendo necessidade de mais estudos para avaliar o impacto de tal achado nos pacientes asmáticos.
Corticosteroides; radical superóxido; granulócitos; asma; criança; adolescente
ORIGINAL ARTICLE
Assessment of inflammation based on the release of oxygen radicals by granulocytes in chronic uncontrolled asthma
Cristina F. SartorelliI; Jussara RehderII; Antonio Condino NetoIII; Maria Marluce S. VilelaIV
IMestre, Pediatria, Curso de Pós-Graduação em Saúde da Criança e do Adolescente, Centro de Investigação em Pediatria (CIPED), Faculdade de Ciências Médicas, Universidade Estadual de Campinas (UNICAMP), Campinas, SP, Brazil. Professora assistente, Departamento de Pediatria, Faculdade de Medicina de Jundiaí (FMJ), Jundiaí, SP, Brazil
IIBióloga, CIPED, Faculdade de Ciências Médicas, UNICAMP, Campinas, SP, Brazil
IIIProfessor associado, Departamento de Pediatria, Faculdade de Ciências Médicas, UNICAMP, Campinas, SP, Brazil
IVProfessora titular, Departamento de Pediatria, CIPED, Faculdade de Ciências Médicas, UNICAMP, Campinas, SP, Brazil
Correspondence
ABSTRACT
OBJECTIVE: To evaluate spontaneous release of superoxide anion by peripheral blood granulocytes of atopic patients with uncontrolled asthma undergoing glucocorticoid therapy and of healthy subjects.
METHODS: We studied 32 patients, aged 6 to 18 (mean 12.04), and 29 healthy subjects as a comparative group. Patients were grouped according to the forced expiratory vital capacity in the first second. Group I, forced expiratory vital capacity in the first second of between 60 and 80%, had 19 patients, and group II, forced expiratory vital capacity in the first second = 60%, had 13 patients. Spontaneous superoxide release by granulocytes was measured by a spectrophotometer method based on superoxide dismutase, before and after oral prednisone and beclomethasone, budesonide or fluticasone inhaled therapy. Statistical analyses were performed using ANOVA, Wilcoxon and Tukey tests.
RESULTS: Comparing the superoxide anion release by granulocytes of asthmatic patients and healthy subjects, we observed a higher release by cells of the uncontrolled patient group II (p < 0.05). Evaluating the superoxide release by cells of asthmatic patients before and after steroid therapy, a significant decrease was found only in patient group I.
CONCLUSION: The impact of corticosteroids on inflammatory modulation occurred in the uncontrolled asthmatics with forced expiratory vital capacity in the first second between 60 and 80%. In those with forced expiratory vital capacity in the first second of = 60%, this finding was not observed. Further studies are necessary to evaluate the effect of this finding on asthmatic patients.
Keywords:Corticosteroids, superoxide radical, granulocytes, asthma, children, adolescents.
Introduction
Asthma is a chronic inflammatory disease with multiple phenotypes. Airway inflammation in allergic asthma is mediated by T helper cells type 2 (Th2) specific effectors for environmental harmless proteins, aeroallergens.1 In Brazil, in spite of the reduction trend, the prevalence of asthmatic symptoms remains as one of the highest rates in Latin America.2
The frequent exposition to aeroallergens keeps the Th2 memory cells active and leads to chronic dysfunction of the immune system, resulting in persistent inflammatory process. The activation of eosinophils, basophils, neutrophils and monocytes and their interaction with resident cells cause the release of proinflammatory pharmacological and immunological mediators, including reactive oxygen species (ROS).3 Since early childhood, the ontogeny of the leukocyte NADPH oxidase system, superoxide anion synthesis and microbicidal activity, is similar to that of an adult individual.4 Oxygen radicals lead to acute injury of cells and tissues by means of lipid peroxidation, protein oxidation, and the release of endogenous mediators, mainly the arachidonic acid metabolites. Oxygen radicals also activate the transcription nuclear factor ? B (NF-?B) and the activator protein-1 (AP-1), stressing the inflammatory response.5
Previous studies have demonstrated that peripheral blood eosinophils,6 neutrophils7 and monocytes,8 as well as airway leukocytes of asthmatic patients release large amounts of ROS, mainly when the disease is exacerbated.9 Direct correlation between the production of superoxide anion by peripheral blood neutrophils and severity, evolution and duration of the disease has also been demonstrated.7
Although inflammation is the main pathophysiological characteristic of asthma, the conventional methods used to classify the disease and assess response to treatment do not include a direct measurement of inflammation. However, especially during childhood, the management of asthmatic patients based solely on symptoms and pulmonary function might be a mistake, since the perception of symptoms and its correlation with the pulmonary function can vary.10,11 In addition to these aspects, it is important to consider that the alterations in the pulmonary function might be permanent due to remodeling of bronchial mucosa. Thus, the evaluation of pulmonary function is insufficient to assess the therapeutic response.12 Therefore, the necessity of using an inflammation marker during evaluation and follow-up of the asthmatic patient becomes evident.
The invasive nature of lung biopsy and bronchoalveolar lavage makes these methods unacceptable for the assessment of inflammation. In the analysis of spontaneous or saline solution induced sputum, the number of eosinophils and the levels of eosinophil cationic protein, leukotriene E4 and RANTES are increased in patients with uncontrolled asthma, but they improve after treatment.13 Although this method is promising, it is difficult to be conducted. Currently, the measurement of the level of ROS14 and the fraction of nitric oxide in exhaled air (FeNO) are being used as non-invasive methods to assess airway inflammation.15
The objectives of our study were: to compare superoxide anion release by peripheral blood granulocytes of atopic patients with uncontrolled asthma, classified according to the degree of airway obstruction determined by the forced expiratory vital capacity (FEV1) in the first second, with healthy individuals, and to investigate the therapeutic effects of oral and inhaled glucocorticoids on superoxide anion release of these groups of patients.
Methods
Subjects
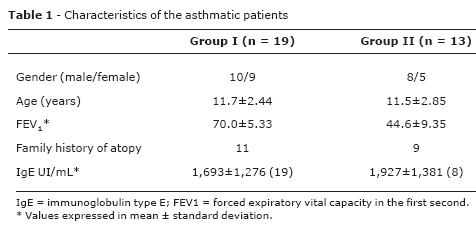
Patients were selected at the Pediatric Allergy and Immunology Outpatient Clinic of Hospital de Clínicas of Universidade Estadual de Campinas (UNICAMP), state of São Paulo, Brazil. The study was conducted from March 2001 to July 2004. Initially, we selected 41 atopic individuals with persistent asthma classified as mild in 18 subjects, moderate in 17 and severe in six patients. The inclusion criteria were suffering from uncontrolled asthma characterized by the presence of symptoms and frequent exacerbations, baseline FEV1 < 80%, limited physical activity and usual need of beta2-agonists.1 Due to that, nine individuals with mild persistent asthma were excluded from the study. Patients' age ranged from 6 to 18 years old (mean ± standard deviation 12.04±2.47 years). The diagnosis of asthma was established for each patient based on symptoms and reversibility of airway obstruction.1 Atopy was characterized by family history of rhinitis, asthma or atopic dermatitis, immediate reading skin tests positive for domestic aeroallergens and high levels of serum IgE. For the skin test, we used allergenic extracts (IPI-ASAC from Brazil) for: Dermatophagoides pteronyssinus, Dermatophagoides farinae, Blomia tropicalis, Blatella germânica, Canis familiaris, Felis domesticus, fungus I and II, dog epithelium, cat epithelium, histamine and negative control. During the study, there was not clinical evidence of asthma triggered by infections. FEV1 was used to classify the patients in two groups: group I, comprising 19 patients with FEV1 between 60 and 80% of expected; and group II, comprising 13 patients with FEV1 = 60% of expected. The control group included 29 healthy individuals aged 25 to 40 years old without clinical history of atopy.
The treatment with systemic and inhaled corticosteroids complied with the recommendations of the Global Initiative for Asthma (GINA).1 All patients received a course of 2 mg/kg/day of oral prednisone, with maximum of 60 mg associated with the use of short-acting beta2-agonists, during 7 days and, then they kept receiving inhaled corticosteroids. When necessary, during the study, patients received short-acting and long-acting beta2-agonists. The assessment using spirometry and superoxide anion release by peripheral blood granulocytes of patients was performed immediately before and after treatment with oral corticosteroids and simultaneously to inhaled corticoidsteroids. Ten (52%) patients of group I were assessed after 1 week of therapy with prednisone and nine (48%) patients were assessed after inhaling corticosteroid for a mean period of 2.4 months. Nine (70%) patients of group II were assessed after 1 week of therapy with prednisone and four (30%) patients were assessed after inhaling corticosteroid for a mean period of 4.7 months. In group I, a moderate dose of budesonide was prescribed for one patient and a high dose was prescribed for three individuals; a high dose of fluticasone propionate was prescribed for two subjects and three individuals received high doses of beclomethasone dipropionate. Four patients from group II received a high dose of budesonide.
Parents or patients' guardians signed a written consent form in the beginning of the study, and the study protocol was approved by the Research Ethics Committee of the School of Medicine in accordance with the Helsinki Declaration.
Isolation of granulocytes
Granulocytes were isolated from samples of 10 mL of peripheral blood by means of Ficoll-Hypaque centrifugation (Hystopaque®1119).16 Cells were washed three time using Hanks' balanced salt solution, and the final count of granulocytes was adjusted for 2x107 cells mL-1. Cell viability, measured by Trypan blue exclusion test, was higher than 90%.
Production of superoxide anion
Spontaneous release of superoxide was assessed using the spectrophotometric method based on the inhibition of cytochrome-C reduction by superoxide-dismutase according to McCord & Fridovich,17 previously modified.18 On the day the experiment was conducted, granulocytes were incubated in Hanks' balanced salt solution containing cytochrome-C (50 µM), and superoxide-dismutase (60 U/mL) was added to the tubes. The optical absorbance (550 nm) of the supernatant was measured at 0, 5, 15, 25, 45 and 60 minutes starting at the beginning of the experiment. The amount of superoxide anion was calculated using extinction coefficients of 21,100 M-1 cm-1, and these coefficients were expressed in nanomols (nmol) of superoxide by 106 cells.
Statistical analysis
Repeated measures analysis of variance (ANOVA) was done by means of an initial test conducted to check if there were differences between the groups. Tukey test was used to compare the amount of superoxide released by healthy individuals' cells with both group of patients, before and after treatment. Wilcoxon test for related samples was used to compare patients' superoxide anion release and values of FEV1 before and after treatment with corticosteroids. The results of the kinetic study of superoxide release were expressed in mean ± standard deviation. For the analyses, we considered p < 0.05 as statistically significant.19
Results
Table 1 shows the characteristics of asthmatic children and adolescents and the control group. The control group comprised 29 individuals, 17 males and 12 females. Their mean age was 30.4±2.63 years old.
Mean FEV1 of asthmatic patients before corticotherapy was 70±5.3% for group I and 44.62±9.4% for group II. The differences in the mean FEV1 before and after corticotherapy were 14.5% for group I (p = 0.002 Wilcoxon test) and 28% for group II (p = 0.011 Wilcoxon test) (Figure 1).
The immediate reading skin test with aeroallergens was positive for antigens of Dermatophagoides pteronyssinus in all patients, followed by Dermatophagoides farinae with a positive rate of 80% in both groups and positive Blomia tropicalis in 8 (42%) patients from group I and 5 (38%) patients from group II. Blatella germânica, Canis familiaris, Felis domesticus, and fungus I had a positive rate of 20% in both groups.
Increase in the spontaneous release of superoxide by granulocytes of uncontrolled asthmatics from group II
The initial assessment of spontaneous release of superoxide anion by granulocytes of asthmatics and controls demonstrated that there was a significant difference among the groups (p = 0.007, ANOVA). The comparison between superoxide anion release by cells of healthy and asthmatic individuals from group I and II separately showed that, before corticotherapy, asthmatics from group II (FEV1 = 60%) released significantly larger amounts of superoxide anion at 25, 45 and 60 minutes of incubation (p < 0.05 Tukey test) (Figure 2). After treatment with corticosteroids, the values of superoxide anion released by asthmatics from group II and controls were similar.
There was no statistical difference between the spontaneous release of superoxide anion by asthmatics from group I (60% < FEV1 < 80%) and healthy individuals before and after corticotherapy.
Reduction of spontaneous release of superoxide anion by granulocytes of asthmatics from group I after corticotherapy
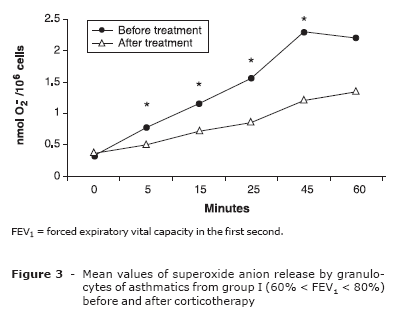
The comparison of the spontaneous release of superoxide anion by granulocytes of asthmatics from group I (60% < FEV1 < 80%), before and after corticotherapy (Wilcoxon test for related samples), showed a significant reduction at 15 (p = 0.027), 25 (p = 0.001), 45 (p = 0.001) and 60 (p = 0.001) minutes of incubation (Figure 3). For patients from group II (FEV1 = 60%), after corticotherapy, there was a non-significant reduction of superoxide release.
Discussion
The increase in superoxide anion release by granulocytes of peripheral blood of uncontrolled asthmatics we found in the present study confirms our previous results.20 The correlation between the severity of the asthmatic disease, the exaggerated production of ROS by inflammatory cells and a lack of balance between oxidant and antioxidant systems has been reported by several authors.6,14 Typical alterations of asthma, such as epithelial damage, bronchial hyperreactivity and increase in the expectoration of sputum, can be the result of the action of ROS, confirming their participation in the pathophysiology of asthma.5
Glucocorticoids are the most powerful anti-inflammatories for the treatment of chronic asthma. However, the effect of corticotherapy on the markers of airway inflammation can vary. Majori et al. demonstrated that monocytes of asthmatic patients treated with oral or inhaled corticosteroid released less superoxide anion than monocytes of patients who were not treated. Nevertheless, in the cortico-dependent patients this difference was not found.21 In a study involving patients with difficult asthma, undergoing treatment with oral corticosteroid, Stirling et al. found a subgroup of patients that kept high FeNO levels and unfavorable clinical evolution.22 In patients with moderate asthma, La Grutta et al. evidenced a correlation between high levels of granulocyte-macrophage colony-stimulating factor (GM-CSF), IL-8, NF-?B and FeNO with more exacerbations and decrease in FEV1, showing the persistence of inflammation in spite of the use of high doses of corticosteroids.23
After corticotherapy, although the patients from group I (60% < FEV1 < 80%) had clinical improvement and significant decrease in superoxide anion release, only 64% of the individuals reached normal values of FEV1. This incomplete reversibility of airway obstruction was also found by Bisgaard, who analyzed the effect of long-term inhaled corticotherapy on the pulmonary function.24 One might assume that such result is caused by the presence of airway remodeling, where the action of corticosteroids is questionable12 and, also, the fast positive effect of corticosteroids on the inflammatory markers and the delayed effect on the pulmonary function.25
Another important aspect of the present study was the absence of the modulator effect of corticotherapy on superoxide anion release in patients with higher degree of airway obstruction (FEV1 = 60%). This result may evidence the diversity of the inflammatory patterns of bronchial mucosa, eosinophilic, neutrophilic, mixed or pauci-granular, identified in chronic asthmatic patients, which have an influence on the response to corticosteroids.26
Several studies involving children have shown correlation between FeNO, eosinophilic inflammation in the peripheral blood, and induced sputum assessed by bronchoalveolar lavage and endobronchial biopsy.27,28 Such results prompted the joint publication by the American Thoracic Society and the European Respiratory Society of the guideline on FeNO measurement in children, confirming the need to assess the inflammation.29
We confirmed previous results21-25,30 that glucocorticoids contribute to the restoration of the normal oxidative state of peripheral blood granulocytes and reduce airway obstruction in patients with FEV1 between 60 and 80%. On the other hand, granulocytes of asthmatics with FEV1 = 60% do not achieve their normal oxidative state, although there is increase in the FEV1 and improvement of symptoms. Our results show that there is a subgroup of patients with chronic uncontrolled asthma that can have improvement of symptoms and reduced bronchial obstruction after corticotherapy and keep high levels of superoxide anion release by granulocytes. In this subgroup of patients, the individuals have persistent inflammation and, therefore, there is need of other therapeutic measures to achieve better prognosis. Further studies with larger samples and long-term clinical follow-up of patients with persistent uncontrolled asthma and FEV1 = 60% are needed to assess the definite impact of such findings on the evolution of the disease.
References
- 1. Global Initiative for Asthma [site na internet]. Global strategy for asthma management and prevention. Revised 2006. http://www.ginasthma.com/Guidelineitem.asp??l1=2&l2=1&intId=60 Acesso: 29/03/2006.
- 2. Solé D, Melo KC, Camelo-Nunes IC, Freitas LS, Britto M, Rosário NA, et al. Changes in the prevalence of asthma and allergic diseases among Brazilian schoolchildren (13-14 years old): comparison between ISAAC Phases One and Three. J Trop Pediatr. 2007;53:13-21.
- 3. Barnes PJ. Immunology of asthma and chronic obstructive pulmonary disease. Nat Rev Immunol. 2008;8:183-92.
- 4. Speer CP, Ambruso DR, Grimsley J, Johnston RB Jr. Oxidative metabolism in cord blood monocytes and monocyte-derived macrophages. Infect Immun. 1985;50:919-21.
- 5. Sugiura H, Ichinose M. Oxidative and nitrative stress in bronchial asthma. Antioxid Redox Signal. 2008;10:785-97.
- 6. Chanez JP, Dent G, Yukawa T, Barnes PJ, Chung KF. Generation of oxygen free radicals from blood eosinophils from asthma patients after stimulation with PAF or phorbol ester. Eur Resp J. 1990;3:1002-7.
- 7. Kanazawa H, Kurihara N, Hirata K, Takeda T. The role of free radicals in airway obstruction in asthmatic patients. Chest. 1991;100:1319-22.
- 8. Vachier I, Damon M, Le Doucen C, de Paulet AC, Chanez P, Michel FB, et al. Increased oxygen species generation in blood monocytes of asthmatic patients. Am Rev Respir Dis. 1992;146:1161-6.
- 9. Jarjour NN, Busse WW, Calhoun WJ. Enhanced production of oxygen radicals in nocturnal asthma. Am Rev Respir Dis. 1992;146:905-11.
- 10. Yawn BP, Brenneman SK, Allen-Ramey FC, Cabana MD, Markson LE. Assessment of asthma severity and asthma control in children. Pediatrics. 2006;118:322-9.
- 11. Fonseca AC, Fonseca MT, Rodrigues ME, Lasmar LM, Camargos PA. Peak expiratory flow monitoring in asthmatic children. J Pediatr (Rio J). 2006;82:465-9.
- 12. James AL, Wenzel S. Clinical relevance of airway remodelling in airway diseases. Eur Respir J. 2007;30:134-55.
- 13. Romagnoli M, Vachier I, Tarodo de la Fuente P, Meziane H, Chavis C, Bousquet J, et al. Eosinophilic inflammation in sputum of poorly controlled asthmatics. Eur Respir J. 2002;20:1370-7.
- 14. Nadeem A, Raj HG, Chabra SK. Increased oxidative stress in acute exacerbations of asthma. J Asthma. 2005;42:45-50.
- 15. Gogate S, Katial R. Pediatric biomarkers in asthma: exhaled nitric oxide, sputum eosinophils and leukotriene E4. Curr Opin Allergy Clin Immunol. 2008;8:154-7.
- 16. Boyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968: 97:77-89.
- 17. McCord JM, Fridovich I. Superoxide dismutase. An enzymatic function for erythrocuprein (hemocuprein). J Biol Chem. 1969;244:6044-55.
- 18. Condino-Neto A, Newburger PE. NADPH oxidase activity and cytochrome b558 content of human Epstein-Barr-virus-transformed B lymphocytes correlate with expression of genes encoding components of the oxidase system. Arch Biochem Biophys. 1998;360:158-64.
- 19. Montgomery DC. Design and analysis of experiments. 6th ed. New York, NY: John Wiley & Sons; 2004.
- 20. Marçal LE, Rehder J, Newburger PE, Condino-Neto A. Superoxide release and cellular glutathione peroxidase activity in leukocytes from children with persistent asthma. Braz J Med Biol Res. 2004;37:1607-13.
- 21. Majori M, Vachier I, Godard P, Farce M, Bousquet J, Chanez P. Superoxide anion production by monocytes of corticosteroid-treated asthmatic patients. Eur Resp J. 1998;11:133-8.
- 22. Stirling RG, Kharitonov SA, Campbell D, Robinson DS, Durham SR, Chung KF, et al. Increase in exhaled nitric oxide levels in patients with difficult asthma and correlation with symptoms and disease severity despite treatment with oral and inhaled corticosteroids. Asthma and Allergy Group. Thorax. 1998;53:1030-4.
- 23. La Grutta S, Gagliardo R, Mirabella F, Pajno GB, Bonsignore G, Bousquet J, et al. Clinical and biological heterogeneity in children with moderate asthma. Am J Respir Crit Care Med. 2003;167:1490-5.
- 24. Bisgaard H. Use of inhaled corticosteroids in pediatric asthma. Pediatr Pulmonol Suppl. 1997;15:27-33.
- 25. Kharitonov SA, Barnes PJ. Effects of corticosteroids on noninvasive biomarkers on inflammation in asthma and chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2004;1:191-9.
- 26. Green RH, Brightling CE, Bradding P. The reclassification of asthma based on subphenotypes. Curr Opin Allergy Clin Immunol. 2007;7:43-50.
- 27. Strunk RC, Szefler SJ, Phillips BR, Zeiger RS, Chinchilli VM, Larsen G, et al. Relationship of exhaled nitric oxide to clinical and inflammatory markers of persistent asthma in children. J Allergy Clin Immunol. 2003;112:883-92.
- 28. Pontin J, Blaylock MG, Walsh GM, Turner SW. Sputum eosinophil apoptotic rate is positively correlated to exhaled nitric oxide in children. Pediatr Pulmonol. 2008;43:1130-4.
- 29. Baraldi E, de Jongste JC; European Respiratory Society; American Thoracic Society. Measurement of exhaled nitric oxide in children, 2001. Eur Respir J. 2002;20:223-37.
- 30. Sadowska AM, Klebe B, Germonpré P, De Backer WA. Glucocorticosteroids as antioxidants in treatment of asthma and COPD. New application for and old medication? Steroids. 2007;72:1-6.
Correspondência:
Publication Dates
-
Publication in this collection
13 Apr 2009 -
Date of issue
Apr 2009
History
-
Accepted
04 Feb 2009 -
Received
06 Oct 2008