Abstract
Leishmaniasis, a zoonosis of worldwide distribution, presents a significant impact on immunosupressed patients. This study aimed to evaluate Leishmania chagasi infection in BALB/c mice immunosuppressed with dexamethasone. Spleen cells stimulated or not with L. chagasi were cultured for cytokine quantification (IFN-γ, IL-2, IL-4 and IL-10) by sandwich ELISA. Parasite loads in the spleen and liver were determined by means of culture microtitration. Immunosuppressed groups showed statistically lower spleen weight and CD4-cell percentage in blood on the day of infection and produced Th1 and Th2 cytokines on other days of the study. The other infected groups, weather immunosupressed or not, also produced Th1 and Th2 cytokines. Parasite loads in the spleen and liver were not statistically different among the groups. It was concluded that L. chagasi infection was not affected by dexamethasone-induced immunosuppression, probably due the reversible effect of the treatment.
visceral leishmaniasis; immunosuppression; immunopathology; mice; interleukins
ORIGINAL PAPER
Cell-mediated immune response to Leishmania chagasi experimental infection of BALB/c immunosuppressed mice
Machado JGI; Hoffmann JLI; Krause VLKI; da Silva AVI; Dias-Melicio LAII; Langoni HI
IZoonosis Research Center, NUPEZO, Veterinary Medicine and Animal Husbandry School, São Paulo State University, UNESP, Botucatu, São Paulo State, Brazil
IIDepartment of Microbiology and Immunology, Botucatu Biosciences Institute, São Paulo State University, UNESP, Botucatu, São Paulo State, Brazil
Correspondence to Correspondence to: Juliana Giantomassi Machado Departamento de Higiene Veterinária e Saúde Pública Faculdade de Medicina Veterinária e Zootecnia, UNESP Distrito de Rubião Júnior, s/n Botucatu, SP, 18618-000, Brasil Phone: +55 14 38116270. Fax: +55 14 38116075 Email: julianamachado2002@yahoo.com.br
ABSTRACT
Leishmaniasis, a zoonosis of worldwide distribution, presents a significant impact on immunosupressed patients. This study aimed to evaluate Leishmania chagasi infection in BALB/c mice immunosuppressed with dexamethasone. Spleen cells stimulated or not with L. chagasi were cultured for cytokine quantification (IFN-γ, IL-2, IL-4 and IL-10) by sandwich ELISA. Parasite loads in the spleen and liver were determined by means of culture microtitration. Immunosuppressed groups showed statistically lower spleen weight and CD4-cell percentage in blood on the day of infection and produced Th1 and Th2 cytokines on other days of the study. The other infected groups, weather immunosupressed or not, also produced Th1 and Th2 cytokines. Parasite loads in the spleen and liver were not statistically different among the groups. It was concluded that L. chagasi infection was not affected by dexamethasone-induced immunosuppression, probably due the reversible effect of the treatment.
Key words: visceral leishmaniasis, immunosuppression, immunopathology, mice, interleukins.
INTRODUCTION
Leishmaniasis is caused by trypanosomatidae protozoans of the genus Leishmania. Diseases caused by these agents are severe and distributed worldwide, with incidence growing by two million cases a year and 350 million individuals at risk of infection in 88 countries (1). Ninety percent of visceral leishmaniasis cases occur in India, Bangladesh, Nepal, Sudan and Brazil (2).
Leishmania may cause cutaneous or visceral diseases and are important pathogens for immunocompromised hosts (3). Co-infection with HIV and Leishmania has been reported in more than 25 countries, mainly in the Mediterranean region and, to a lesser extent, in Equatorial Africa, Asia and South America (4-7). Despite the innumerable studies on human and canine visceral leishmaniasis, many questions have yet to be answered (8). One of these points involves the immune response of a host immunosupressed and infected with Leishmania chagasi. Given the importance of visceral leishmaniasis co-infection in immunosuppressed patients, the objective of the present study was to analyze the immunopathological aspects related to cytokine production and parasite load in the spleen and liver of BALB/c mice immunosuppressed with dexamethasone (DXM) until the moment of infection with L. chagasi.
MATERIALS AND METHODS
This study was approved by the Ethics Committee of the Veterinary Medicine and Animal Husbandry School, UNESP, São Paulo state, Brazil.
Animals
Eighty male, 5 week-old, BALB/c mice - from the Multidisciplinary Center for Biological Investigation (CEMIB) at State University of Campinas (UNICAMP), São Paulo state, Brazil - were used. Animals were kept in polypropylene boxes, fed commercial pelleted feed and had free access to water. Boxes were placed in an Alesco ventilated rack system (model ALE 99002-001, Brazil), provided with an uninterruptible power supply, in a room with screened doors and windows in the Department of Veterinary Hygiene and Public Health at the Veterinary Medicine and Animal Husbandry School, UNESP.
Animals were divided into four groups: Group I - control animals; Group II - animals immunosuppressed until day 0 and not infected; Group III - normal animals infected on day 0 and Group IV - animals immunosuppressed until day 0 and infected on day 0. Groups II and IV were immunosuppressed with 6.65 mg/kg dexamethasone sodium phosphate (Decadron®, Aché Laboratórios Farmacêuticos, Brazil) once a day by the intraperitoneal route, for 22 days (9). Groups I and III were inoculated with water by injection using the same route and frequency as in the immunosuppression protocol. On day 0, immunosuppression was withdrawn in groups II and IV, and groups III and IV were infected.
Four animals of each group were euthanized on days 0, 3, 7, 14 and 21. The spleen was removed and weighed; spleen cells were cultured, and parasite loads of spleen and liver were determined for each animal.
Parasite Strain and Infection Protocol
Amastigote forms of Leishmania chagasi strain M6445 were used. Strains were supplied by the Laboratory of Protozoology, Institute of Tropical Medicine, School of Medicine, University of São Paulo (USP). The strain was maintained in golden hamsters (Mesocricetus auratus) that were kept in the Zoonosis Research Center of the Veterinary Medicine and Animal Husbandry School, UNESP, and replicated every two months.
Infection of groups III and IV was carried out according to the protocol by Stauber et al. (10). L. chagasi (strain M6445) amastigotes were obtained by differential centrifugation of spleens of infected hamsters, and 107 parasites were inoculated in the retro-orbital sinus of mice anesthetized by intraperitoneal administration of 100 mg/kg ketamine (Cetamin®, Laboratório Syntec do Brasil, Brazil) and 10 mg/kg xylazine (Anasedan®, Vetbrands Saúde Animal, Brazil) (11).
Flow Cytometry
On day 0, blood samples of immunosuppressed (II and IV) and non-immunosuppressed (I and III) animals were collected and submitted to flow cytometry in order to evaluate the immunosuppressive protocol. Anti-mouse CD4 antibodies conjugated with FITC (rat IgG2a, H129,19) and anti-mouse CD8 antibodies conjugated with PE (rat IgG2a, 53-6.7) (BD Pharmingen, USA) were used for labeling CD4 and CD8 cells, respectively. A BD FACSCalibur® (BD Biosciences, USA) multipurpose flow cytometer was used in the analysis of the samples at the Flow Cytometry Laboratory in the blood center at the Botucatu Medical School Hospital, UNESP.
Parasite Load
Parasite load was quantified by means of culture microtitration (9). Spleen and liver fragments were weighed and then macerated with 4 mL of Schneider drosophila medium, supplemented with 20% inactivated bovine fetal serum, 10,000 UI/mL penicillin and 10 mg/mL streptomycin (Cultilab, Brazil). Microtitration plates were prepared under sterile conditions and each sample was analyzed in quadruplicate. The macerate was submitted to serial fourfold dilutions, from 1/4 to 1/4,194,304. Plates were incubated for eight days at 28ºC, and read in an inverted microscope at 200x magnification. Dilutions were considered to be positive when at least one live parasite was observed. Counts were transformed into geometric means and used in the formula that determined parasite load (9).
Cytokine Quantification
IL-2, IFN-γ, IL-4 and IL-10 were quantified in the supernatant of total spleen cell cultures in 48-well plates containing 5 x 106 cells/mL stimulated or not with L. chagasi-sonicated antigen (10 µg/mL). Cultures were incubated at 37ºC, in 5% CO2 for 48 hours. The supernatant was maintained at -80°C until analyzed by Duoset® ELISA Development System according to the manufacturer's instructions (R&D Systems, USA).
Statistical Analysis
Variance analysis was used to assess differences in cytokine production; Student's t test was used to determine percentage differences of CD4 and CD8 cells and in parasite loads of spleen and liver; Tukey's test was used to determine spleen weight differences among the different groups (12).
RESULTS
Although the study involved two trials, the results presented herein are based on the more representative one.
Spleen Weight
According to Figure 1, mean spleen weight of immunosuppressed groups II and IV on day 0, (31.50 ± 4.87 and 27.00 ± 1.22 mg, respectively) was significantly lower than that of non-immunosuppressed groups I and III (119.00 ± 10.42 and 110.25 ± 4.09 mg, respectively) (p < 0.05). On day 3, mean spleen weight of Group III (non-immunosuppressed and infected on day 0) was statistically greater than that of the other groups. On day 7, groups III and IV (immunosuppressed and infected, and only infected, respectively) were not statistically different from each other but presented greater mean spleen weight than those of the other two non-infected groups. However, results of Group II (immunosuppressed and not infected) were significantly lower than those of the other groups. On days 14 and 21, Group II was not statistically different from the control group (I), but was different from the infected groups (III and IV) (p < 0.05).
Flow Cytometry
The comparison between immunosuppressed (groups II and IV) and non-immunosuppressed (groups I and III) animals showed that the percentages of CD8 and CD4 cells differed statistically (p > 0.05). On day 0, immunosuppressed animals showed greater mean percentage of CD8 cells and lower mean percentage of CD4 cells than non-immunossupressed animals (p > 0.05).
Cytokine Quantification
Figure 2 (A, B and C) shows cytokine kinetics in non-stimulated spleen cell cultures. IL-4 was not detected in any of the groups.
IFN-γ concentrations in immunosuppressed groups (II and IV) were statistically greater than those of non-immunosuppressed groups (I and III), on days 7 and 14. This cytokine was also detected in the infected groups (III and IV); in the immunosuppressed and infected group (IV), its concentration was statistically greater than in the other groups on days 14 and 21 (p < 0.05). Infected groups (III and IV) showed greater IFN-γ production on day 21 (Figure 2 - A).
IL-10 concentration in immunosuppressed groups (II and IV) was statistically greater than in non-immunosuppressed groups (I and III) on days 3 and 7 (p < 0.05). This cytokine was also detected in the infected groups (III and IV). However, its production was greater on days 3 and 7 than on days 14 and 21 (Figure 2 - B).
IL-2 concentration in immunosuppressed groups (II and IV) was statistically greater than in non-immunosuppressed groups (I and III) on day 7. In the infected, non-immunosuppressed group (III), this cytokine was detected only on day 7, and in the immunosuppressed infected group (IV), production on day 21 was statistically greater than in all the other groups (p < 0.05) (Figure 2 - C).
Different letters (a, b and c) above the columns indicate significant differences between the groups, for the same time, as determined by the variance analysis, considering á = 0.05.
Figure 3 (A, B and C) shows cytokine production in spleen cell cultures stimulated with L. chagasi antigen. IL-4 was not detected in any of the groups.
According to Figure 3 - A, IFN-γ concentration in cell cultures of non-infected groups (I and II) stimulated with specific antigen was not greater than endogenous production (Figure 2 - A), while IFN-γ production in the immunosuppressed and infected group (IV) was statistically greater than that of the other groups at all the other times (p < 0.05).
According to Figure 3 - C, IL-2 concentration detected in cell cultures of the non-infected groups (I and II) stimulated with specific antigen was not greater than endogenous production (Figure 2 - C). This cytokine was detected in the infected groups (III and IV) at all moments studied, with the immunosuppressed and infected group (IV) showing statistically greater production than the other groups on days 3 and 21 (p < 0.05).
Different letters (a,b and c) above the columns indicate significant differences between the groups, for the same time, as determined by the variance analysis, considering á = 0.05.
Parasite Load
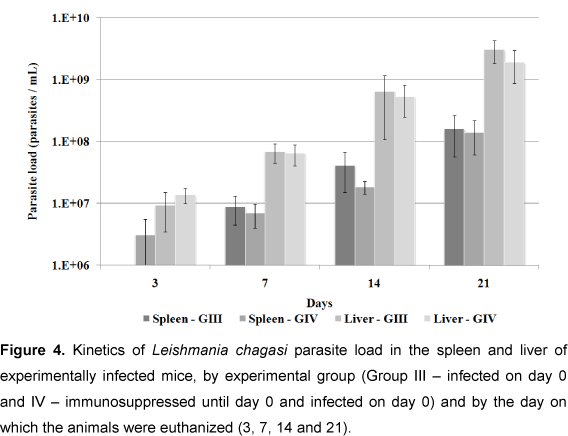
Figure 4 shows parasite loads in spleen and liver of the infected groups (groups III and IV). No statistical difference was observed between the organs in these groups (p < 0.05), but parasite load was greater in the liver than in the spleen at all moments tested.
DISCUSSION
Spleen weight in dexamethazone-immunosuppressed animals (groups II and IV) was statistically lower than in non-immunosuppressed animals (groups I and III). These results were similar to those by Lebrec et al. (13) and Keil et al. (14), who observed a statistically significant, dose-dependent decrease in spleen weight after DXM administration. Miller and Schaefer (15) reported decreases in spleen and thymus size greater than 80% followed by increase in spleen size when DXM treatment was concluded, as occurred in the present study (Figure 1). This fact suggests either a partial recovery of spleen cells sensitive to DXM or a reversible immunosuppression model (16, 17). Infected groups showed increased spleen weight, while splenomegaly was probably caused by infection with viscerotropic leishmania species, as observed by Melby et al. (18) and Engwerd et al. (19).
According to Finamor et al. (20), glucocorticoid treatment interferes in the circulation of immune cells, promotes apoptosis of lymphoid cells, and decreases the number of peripheral lymphocytes, mainly T cells. On day 0 of the present study, there was a decrease in CD4 cells and an increase in CD8 cells in immunosuppressed animals. Similar findings were reported by Miller and Schaefer (15), who observed that immunosuppression caused by DXM decreased the production of CD4 lymphocytes, with a gradual increase of CD8 lymphocytes after a slight decrease. Gangneux et al. (21) observed that the percentage of CD4 and CD8 cells in the spleen of mice treated with glucocorticoids did not show statistical differences in relation to the control group, in contrast to the findings of the present study.
Figure 2 (A, B and C) shows that cytokine production was greater in immunosuppressed groups (II and IV) at almost all moments. Glucocorticoid treatment may either cause selective immunosuppression of Th1 and a change towards Th2-mediated humoral immunity, or may favor the production of Th2 cytokines, which may explain the increase in IL-10 production in immunosuppressed groups (22-24). Krouwels et al. (25) observed that inhibition of IL-4 and IL-5 production was greater than that of IFN-γ production, favoring the Th1 profile. In the present study, dexamethasone administered until day 0 probably favored production of two cytokines, Th1 (IL-2 and IFN-γ) and Th2 (IL-10), but according to Rousseau et al. (16), the effect of DXM on Th1 and Th2 profiles was not clear.
In the present study, the cytokine profiles of both Th1 (IL-2 and IFN-γ) and Th2 (IL-10) were detected in non-stimulated cell cultures of infected groups (III and IV) (Figure 2 - A, B and C). These results were similar to those by Miralles et al. (26) and Rolão et al. (27), who could not observe a distinct production pattern for Th1 and Th2 cytokines after Leishmania infantum infection.
IFN-γ was detected in the infected groups (III and IV) at all moments, peaking on day 21 in both groups (Figure 2 - A). However, even this increased cytokine concentration did not determine decreased parasite loads (Figure 4), in contrast to the findings of Rolão et al. (27), who observed high levels of IFN-γ together with reduced parasite loads. On the other hand, Ansari et al. (28) stated that, in spite of the high levels of IFN-γ observed during infection, host control of the infection may fail due to inadequate IFN-γ response. The increase in IFN-γ on day 21 suggests that infection was starting to be controlled, which is shown by some studies to occur approximately four weeks after infection (19, 29, 30).
IL-10 was detected at all times in the infected groups (III and IV). However, this cytokine's concentration was greater on the first days (3 and 7) than on days 14 and 21 (Figure 2 - B), similar to the findings by Melby et al. (30). These authors observed that at the onset of L. infantum infection, IL-10 and TGF-β production were more intense and contributed to the establishment of the infection.
In cell cultures stimulated with L. chagasi-sonicated antigen, IFN-γ was detected in both infected groups (III and IV) on days 7, 14 and 21. These results differ from the findings by Murray et al. (29), who showed that spleen cells of mice infected with visceral Leishmania failed to produce IFN-γ and IL-2 in the first weeks of infection. However, according to Rousseau et al. (31), these cells were able to respond to any specific antigen by producing IFN-γ in the chronic phase (70 days after infection).
In relation to cytokine production, IL-4 was absent both in non-stimulated and stimulated spleen cell cultures. According to Goto and Lindoso (32), IL-4 is not involved in susceptibility to visceral leishmania, while this cytokine has been found by other authors to lack any significant role in murine models infected with this organism (21, 26, 30).
Parasite loads in spleen and liver tissue of the infected groups (III and IV) were similar at the majority of observational moments. However, on day 3, loads in group IV (immunosuppressed) were greater than in group III (non-immunosuppressed), and were more evident in the spleen, but without any statistical difference (p < 0.05). Group IV appeared to be still affected by DXM treatment, as corroborated by Rousseau et al. (16), who observed an augmented parasite load increased during the chronic phase of L. infantum infection in spleen tissue of DXM-treated mice; and by Gangneux et al. (21), who showed that mice treated with DXM and pentoxifiline showed significant increases in parasite loads in spleen and liver compared with the control group. In the present study, parasite loads in these two groups were similar at day 3 (Figure 4).
Parasite loads in the liver of both infected groups were greater than in the spleen, at all moments, a finding similar to those of Rousseau et al. (31) and Honoré et al. (33). However, Rousseau et al. (31) showed that parasite load decreased in the liver, but increased in the spleen. Apparently, the liver is the focus for parasite multiplication while the spleen functions as a shelter for its prolonged persistency (34). Rolão et al. (27) suggested that the spleen was more susceptible to L. infantum infection than the liver.
The finding herein that spleen weight was lower in the immunosuppressed group than in the control and uninfected groups at the end of immunosuppressive treatment suggests that immunosuppression caused by dexamethasone until day 0 was reversible because by the end of treatment animals had apparently recovered from immunosuppression. Furthermore, at the last observation moment of the study, mean spleen weight of the immunosuppressed uninfected group was statistically similar to that of Group I (control group). Another parameter that demonstrates this possible reversible immunosuppression was cytokine production in non-stimulated cultures. On days 3, 7, 14 and 21 after dexamethasone treatment ended, none of the cytokines evaluated (Th1 and Th2) showed decreased production in the immunosuppressed group (II) compared with the control group (I); instead, Th1 and Th2 production was increased. In the group immunosuppressed until day 0 then infected (Group IV), immunosuppression did not cause an exacerbation in L. chagasi infection because the parasite loads in this group and in the group that was only infected (III) did not differ statistically. It was concluded that L. chagasi infection was not affected by dexamethasone-induced immunosuppression, probably due the reversible effect of the treatment.
ACKNOWLEDGEMENTS
We would like to thank The State of São Paulo Research Foundation (FAPESP) for the masters degree grants, process numbers 2004/14153-4 and 2004/14762-0, and Prof. Dr. Paula Tavolaro, for helping with the English version of the text.
Received: May 4, 2009
Accepted: August 17, 2009
Abstract published online: August 24, 2009
Full paper published online: February 28, 2010
Conflicts of interest: There is no conflict.
Financial source: FAPESP processes n. 2004/14153-4 and n. 2004/14762-0.
- 1. Lukes J, Maurício IL, Schonian G, Dujardin JC, Soteriadou K, Dedet JP, et al. Evolutionary and geographical history of the Leishmania donovani complex with a revision of current taxonomy. Proc Natl Acad Sci USA . 2007;104(22):9375-80.
- 2. Murray HW, Berman JD, Davies CR, Saravia NG. Advances in leishmaniasis. The Lancet. 2005;366(9496):1561-77.
- 3. Ferreira MS, Borges AS. Some aspects of protozoan infections in immunocompromised patients - a review. Mem Inst Oswaldo Cruz. 2002;97(4):443-57.
- 4. Alvar J. Leishmaniasis and AIDS co-infection: the Spanish example. Parasitol Today. 1994;10(1):160-3.
- 5. Dedet JP, Lambert M, Pratlong F. Leishmanioses in infection par le virus de l'immunodéficience humaine. Presse Med. 1995;24(22):1036-40.
- 6. Grandoni L, Scalone A, Gramiccia M, Troiani M. Epidemiological surveillance of leishmaniasis in HIV-1 infected individuals in Italy. AIDS. 1996;10(7):785-91.
- 7. Borges AS, Machado AA, Ferreira MS, Figueiredo JFC, Silva GF, Cimerman S, et al. Concomitância de leishmanioses e infecção pelos vírus da imunodeficiência humana (HIV): estudo de quatro casos. Rev Soc Bras Med Trop. 1999;32(6):713-9.
- 8. Camargo LB, Langoni H. Impact of leishmaniasis on public health. J Venom Anim Toxins incl Trop Dis. 2006;12(4):527-48.
- 9. Buffet PA, Sulahian A, Garin YJF, Nassar N, Derouin F. Culture microtitration: a sensitive method for quantifying Leishmania infantum in tissues of infected mice. Antimicrob Agents Chemother. 1995;39(9):2167-8.
- 10. Stauber LA, Franchino EM, Grun J. An eight-day method for screening compounds against Leishmania donovani in the golden hamsters. J Eukaryot Microbiol. 2007 5(4):269-73.
- 11. Curl JL, Peters LL. Ketamine hydrochloride and xylazine hydrochloride anesthesia in the golden hamster (Mesocricetus auratus). Lab Anim. 1983;17(4):290-3.
- 12. Triola MF. Introdução à estatística. 9th ed. Rio de Janeiro: LTC; 2005. 440 p.
- 13. Lebrec H, Blot C, Pequet S, Roger R, Bohuon C, Pallardy M. Immunotoxicological investigation using pharmaceutical drugs: in vivo evaluation of immune effects. Fundam Appl Toxicol. 1994;23(2):159-68.
- 14. Keil DE, Luebke RW, Pruett SB. Differences in the effects of dexamethasone on macrophage nitrite production: dependence on exposure regimen (in vivo or in vitro) and activation stimuli. Int J Immunopharmacol. 1995;17(3):157-66.
- 15. Miller TA, Schaefer FW. Changes in mouse circulating leukocyte numbers in C57BL/6 mice immunosuppressed with dexamethasone for Crypstosporidium parvum oocyst production. Vet Parasitol. 2007;149(1):147-57.
- 16. Rousseau D, Suffia I, Ferrua B, Philip P, Le Fichoux Y, Kubar JL. Prolonged administration of dexamethasone induces limited reactivation of visceral leishmaniasis in chronically infected BALB/c mice. Eur Cytokine Netw. 1998;9(4):655-62.
- 17. Kunicka JE, Talle MA, Denhardt GH, Brown M, Prince LA, Goldstein G. Immunosuppression by glucocorticoides: inhibition of production of multiple lymphokines by in vivo administration of dexamethasone. Cell Immunol. 1993;149(1):39-49.
- 18. Melby PC, Yang YZ, Cheng J, Zhao W. Regional differences in the cellular immune response to experimental cutaneous or visceral infection with Leishmania donovani Infect Immun. 1998;66(1):18-27.
- 19. Engwerda CR, Ato M, Kaye PM. Macrophages, pathology and parasite persistence in experimental visceral leishmaniasis. Trends Parasitol. 2004;20(11):524-30.
- 20. Finamor LP, Finamor Jr F, Muccioli C. Corticoterapia e uveítes. Arquiv Bras Oftalmol. 2002;65(1):483-6.
- 21. Gangneux JP, Chau F, Sulahian A, Derouin F, Garin YJ. Effects of immunosuppressive therapy on murine Leishmania infantum visceral leishmaniasis. Eur Cytokine Netw. 1999;10(4):557-9.
- 22. Elenkov IJ. Glucocorticoids and TH1/TH2 balance. Ann N Y Acad Sci. 2004;1024(1):138-46.
- 23. Franchimont D, Louis E, Dewe W, Martens H, Vrindts-Gevaert Y, De Groote D, et al. Effects of dexamethasone on the profile of cytokine secretion in human whole blood cell cultures. Regul Pept. 1998;73(1):59-65.
- 24. Ramírez F, Fowell DJ, Puklavec M, Simmonds S, Mason D. Glucocorticoids promote a TH2 cytokine response by CD4+ T cells in vitro J Immunol. 1996;156(7) 2406-12.
- 25. Krouwels FH, van der Heijden JF, Lutter R, van Neerven RJ, Jansen HM, Out TA. Glucocorticosteroids affect functions of airway-and blood-derived human T-cell clones, favoring the TH1 profile through two mechanisms. Am J Respir Cell Mol Biol. 1996;14(4):388-97.
- 26. Miralles GD, Stoeckle MY, McDermott DF, Finkelman FD, Murray HW. TH1 and TH2 cell-associated cytokines in experimental visceral leishmaniasis. Infect Immun. 1994;62(3):1058-63.
- 27. Rolão N, Cortes S, Gomes-Pereira S, Campino L. Leishmania infantum: mixed T-helper-1/T-helper-2 immune response in experimentally infected BALB/c mice. Exp Parasitol. 2007;115(3):270-6.
- 28. Ansari NA, Saluja S, Salotra P. Elevated levels of interferon-γ, interleukin-10, and interleukin-6 during active disease in Indian kala azar. Clin Immunol. 2006;119(3):339-45.
- 29. Murray HW, Stern JJ, Welte K, Rubin BY, Carriero SM, Nathan CF. Experimental visceral leishmaniasis: production of interleukin 2 and interferon-γ, tissue immune reaction, and response to treatment with interleukin 2 and interferon-γ. J Immunol. 1987;138(7):2290-7.
- 30. Melby PC, Tabares A, Restrepo BI, Cardona AE, McGuff HS, Teale JM. Leishmania donovani: evolution and architecture of the splenic cellular immune response related to control of infection. Exp Parasitol. 2001;99(1):17-25.
- 31. Rousseau D, Le Fichoux Y, Stien X, Suffia I, Ferrua B, Kubar J. Progression of visceral leishmaniasis due to Leishmania infantum in BALB/c mice is markedly slowed by prior infection with Trichinella spiralis Infect Immun. 1997;65(12):4978-83.
- 32. Goto H, Lindoso JAL. Immunity and immunossupression in experimental visceral leishmaniasis. Braz J Med Biol Res. 2004;37(1):615-23.
- 33. Honoré S, Garin YJ, Sulahian A, Gangneux JP, Derouin F. Influence of the host and parasite strain in a mouse model of visceral Leishmania infantum infection. FEMS Immunol Med Microbiol. 1998;21(3):231-9.
- 34. Wilson ME, Jeronimo SMB, Pearson RD. Immunopathogenesis of infection with the visceralizing Leishmania species. Microb Pathog. 2005;38(4):147-60.
Publication Dates
-
Publication in this collection
18 Mar 2010 -
Date of issue
2010
History
-
Received
04 May 2009 -
Accepted
17 Aug 2009